Rapidly developing soft tissue infections are potentially lethal infections
of the subcutaneous tissue, muscle, or myometrium, most commonly
caused by clostridia or group A streptococci. Patients with these rapidly
developing infections usually have a history and physical examination
similar to patients with other causes of postpartum infection. These
infections commonly masquerade as postpartum endometritis, and patients
may have the usual risk factors for infection, including prolonged
labor or rupture of membranes, although many others will have no particular
risk factors. However, two clinical features suggest a more serious
infection: the severity of clinical manifestations and indications
of septic shock. Patients with these infections invariably exhibit
marked systemic signs and symptoms, including severe pain out of proportion
to that usually expected, prostration, and, at times, disorientation
initially or later in the course of infection. Temperature may be
high (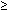 40°C), leukocytosis may be marked (>30,000), and hemoconcentration
may occur as large shifts of fluid leak from the vascular compartment
infected area. These patients frequently develop the features of particularly
severe infection, including adult respiratory distress syndrome, disseminated
intravascular coagulopathy, or renal shutdown. Signs
of septic shock often eventually develop. 40°C), leukocytosis may be marked (>30,000), and hemoconcentration
may occur as large shifts of fluid leak from the vascular compartment
infected area. These patients frequently develop the features of particularly
severe infection, including adult respiratory distress syndrome, disseminated
intravascular coagulopathy, or renal shutdown. Signs
of septic shock often eventually develop. A relatively high (approximately 20%) bacteremic rate occurs with postpartum
endometritis.6 However, few otherwise healthy postpartum women with uncomplicated postpartum
endometritis or uncomplicated postpartum infection develop septic
shock. Even in young patients without underlying disease, the mortality
rate remains between 20% and 30% when septic shock occurs.17 Patients in septic shock require immediate resuscitation with fluids, respiratory
support, circulatory support, and antibiotics. Intensive care
monitoring becomes mandatory. The differential diagnosis includes
amniotic fluid embolism, pulmonary embolism, cardiogenic shock (from drugs, cardiac
disease, or aortic dissection), and diabetic ketoacidosis. Although
unusual, rupture of an abscess with release of gram-negative
anaerobic and aerobic bacteria must also be considered if septic shock
occurs. The causative organism and the infectious source must be identified
with certainty using physical examination, diagnostic tests, and
appropriately obtained cultures. Poor prognostic signs include leukopenia, severe
disseminated intravascular coagulopathy, high levels
of lactate, and adult respiratory distress syndrome. Successful outcome
usually depends on surgical removal of the source of infection. Virtually
all postpartum septic shock develops from a source of infection
that is amenable to surgical drainage or removal. Therefore it becomes
the duty of the surgeon-obstetrician to absolutely exclude the possibility
of surgically treatable infection when signs of severe infection
occur. The surgeon must not relinquish this responsibility when the patient
with shock is transferred to an intensive care specialist. Fluid
resuscitation often restores the blood pressure to normal, giving a
false sense of security. Thus, unless it can be established that the infection
originates from the lung, kidney, or heart or from another site
outside the pelvis, wound exploration, an examination under anesthesia, curettage, or
exploratory laparotomy may be necessary for diagnosis
and treatment. Surgery should be performed as soon as possible after
restoration of adequate circulation. Surgery that becomes necessary
despite a deteriorating circulatory function is hazardous but occasionally
lifesaving if a defined focus of infection exists. Endotracheal intubation
with minimal balanced general anesthesia is preferable to regional
anesthesia. Some causes of rapidly developing infection or shock are more common than
others. Among these, myonecrosis caused by either Clostridium perfringens or other clostridia, including C. sordellii, is high on the list of possibilities, particularly if an unusual amount
of tissue trauma has resulted from a difficult forceps delivery or
during cesarean section. Other evidence of clostridial infection includes
the classic signs of severe pain, hemolysis, renal shutdown, and gas
formation. Other toxin-producing organisms, such as group A streptococci, or
other organisms, including E. coli, can produce rapidly developing cellulitis without myonecrosis. Bacterial
toxins usually produce necrosis in the area of cellulitis, and the
combination of tissue necrosis and the systemic release of toxins causes
unusually severe manifestations. Necrosis can occur in subcutaneous
tissue, striated muscle, or myometrial tissue. Patients with necrosis
exhibit progressive local infection and the alarming systemic signs
previously noted. The source of infection may be particularly difficult
to locate for physical or imaging examination if group A streptococci
or clostridia produce deep striated muscle or uterine muscle necrosis. Necrotizing
fasciitis from either an abdominal or an episiotomy wound
is another cause of rapidly developing infection and shock that will
be covered in more detail below. Immediate antibiotic therapy must cover gram-negative facultative and anaerobic
bacteria and clostridia, in particular. Three antibiotics are
initially indicated for patients with septic shock or the severe manifestations
of infection described above. Either an aminoglycoside or a
first-generation cephalosporin is chosen to inhibit facultative aerobic
bacteria. Clindamycin, imipenem-cilastatin, or metronidazole is given
to inhibit anaerobic bacteria, and either penicillin or ampicillin
is used for its effect on clostridia and for its synergistic action with
aminoglycosides against enterococci. However, it cannot be overemphasized
that antibiotic therapy alone in these serious infections is almost
never sufficient to control sepsis, and surgical exploration is usually
required (Table 5). Failure to recognize myonecrosis or necrotic cellulitis often proves
fatal for patients with these serious infections. Antibiotics alone are
without effect in necrotic tissue. TABLE 5. Therapy for Rapidly Developing Soft Tissue Infections and Necrotizing
Fasciitis
Three antibiotics - Choice of one to inhibit gram-negative and gram-positive facultative aerobic
bacteria
Gentamicin
Tobramycin
Amikacin
- Choice of one to inhibit anaerobic bacteria
Clindamycin
Imipenem-cilastatin
Metronidazole
- Choice of one to inhibit enterococci and clostridia
Penicillin
Ampicillin
Surgical removal of infected tissue
Obvious cellulitis of the subcutaneous tissue needs to be explored with
the patient under general anesthesia, as described for necrotizing fasciitis. Tissue
culture and Gram stain are required to identify the responsible
organisms. Frozen tissue section may be required to identify
necrosis.18 Necrotic tissue needs to be removed to the point of viability. Debridement
serves to remove both necrotic tissue and the source of bacterial
toxins. The involved area may be small in cases of early necrotizing
cellulitis or extensive when infection has gone uncontrolled. Clostridial
myonecrosis can involve muscles in the uterus or elsewhere in the
pelvic diaphragm or sacral area. Exploratory laparotomy can be lifesaving for patients with unchecked bacteremia
and uncontrolled intra-abdominal sepsis. Laparotomy should be
particularly considered when no apparent source of infection is evident. Obviously
infected or necrotic structures need to be removed, whereas
normal organs and tissue should be conserved. Removal or drainage
of an abscess can be undertaken without removal of the uterus or fallopian
tubes, if these organs are not extensively infected. The extensively
infected uterus is usually pale, yellow, or obviously necrotic, often
with extensive thrombosis of ovarian and adnexal vessels. When these
findings are present and patients have increasingly severe and life-threatening
disseminated coagulopathy or respiratory distress, hysterectomy
can be lifesaving. The uterus usually contains areas of frank necrosis
or micro-abscesses in the myometrium. Life-threatening coagulopathy
or respiratory distress from sepsis slowly resolves with removal
of infected tissue. On the other hand, there are rare circumstances in which ascites and edematous
bowel, uterus, and adnexae are present without obviously infected
tissue. Considerable clinical judgment is required. We have had these
rare cases that have progressive life-threatening coagulopathy and
adult respiratory distress at maximal levels of oxygen and positive end-expiratory
pressure in which the uterus contained only normal necrotic
deciduae. Resolution of these life-threatening signs began immediately
although slowly following hysterectomy. It is likely that in these
rare circumstances the sepsis that began the coagulopathy and respiratory
distress was successfully treated with antibiotics but the normal
necrosis of deciduae continued to release enough thromboplastin or other
factors to maintain the vicious cycle of coagulopathy and respiratory
distress. | 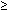 ;25,000)
;25,000)