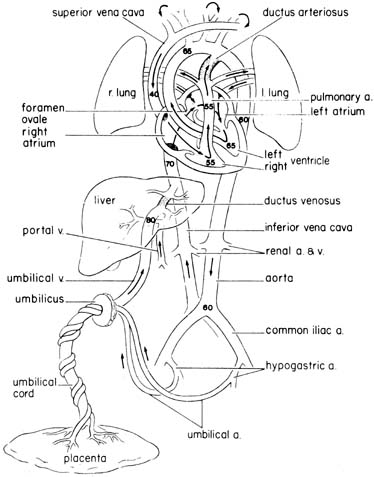
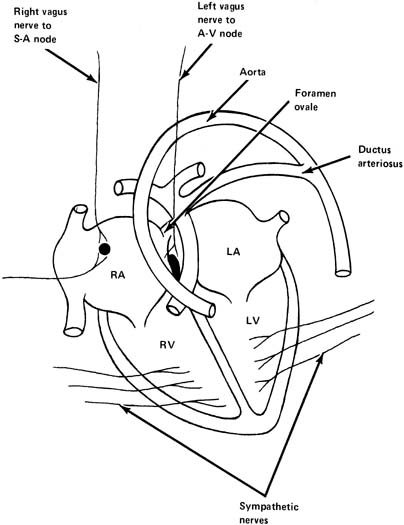
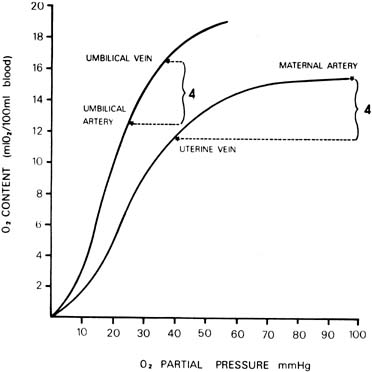
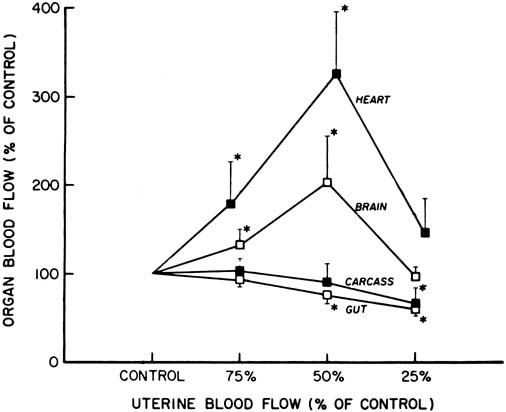
Parasympathetic Nervous System The parasympathetic nervous system consists primarily of the vagus nerve (tenth
cranial nerve), which originates in the medulla oblongata. Fibers
from this nerve supply the SA node and also the atrioventricular
node (AV) (see Fig. 4). Stimulation of the vagus nerve or injection of its mediator, acetylcholine, results
in a decrease in heart rate in a normal fetus because
of vagal influence on the SA node, decreasing its rate of firing
and decreasing the rate of transmission of impulses from atrium to ventricle. In
a similar fashion, blocking of this nerve in a normal fetus
with a substance that blocks the effects of acetylcholine (e.g., atropine) causes an increase in the fetal heart rate of approximately 20 beats/min
at term. This demonstrates that there is normally
a constant vagal tone on the fetal heart rate, tending to decrease it
from its normal intrinsic rate. The vagus nerve is also responsible for transmission of impulses causing
beat-to-beat variability of fetal heart rate. Blocking
the vagus nerve with atropine results in a disappearance of this variability. Hence, it
has been postulated that two vagal influences impinge
on the heart: a tonic influence tending to decrease its rate and an
oscillatory influence that results in fetal heart rate variability. The vagal tone is not necessarily constant. Its influence increases with
gestational age. In fetal sheep, vagal activity increases as much as
four-fold during acute hypoxia or experimentally produced fetal
growth restriction. Sympathetic Nervous System Sympathetic nerves are widely distributed in the myocardium at term (see Fig. 4). Stimulation of the sympathetic nerves will release norepinephrine
and cause an increase in fetal heart rate and also an increase in the
vigor of cardiac contractions. These result in an increase in cardiac
output. The sympathetic nerves are a reserve mechanism to improve the
pumping activity of the heart during intermittent stressful situations. There
is normally a tonic sympathetic influence on the heart. When the β-adrenergic receptor blocker propranolol is administered
to a normal fetus, the fetal heart rate will decrease approximately 10 beats/min. There is, however, only a small decrease in fetal
heart rate variability after blocking the sympathetic nerves. α-Adrenergic receptors also are found in a fetus at term. When
they are stimulated with methoxamine, there is an increase in blood
pressure and a decrease in the kidney and carcass blood flow. The opposite
is observed after a-adrenergic blockers as phentolamine or
phenoxybenzamine.8 Several factors cause the parasympathetic and sympathetic nervous systems
to increase their activity. Baroreceptors In the walls of the arch of the aorta and in the carotid sinus at the junction
of the internal and external carotid arteries are found small
stretch receptors that are sensitive to increases in blood pressure (Fig. 5). When blood pressure increases, impulses are sent from these receptors
by way of the vagus and glossopharyngeal nerve to the midbrain, resulting
in further impulses through the vagus nerve to the heart, tending
to slow it. This is an extremely rapid response, being noted with
almost the first increase of blood pressure. It is a protective stabilizing
function by the body attempting to lower blood pressure by decreasing
heart rate and cardiac output when blood pressure is increasing. 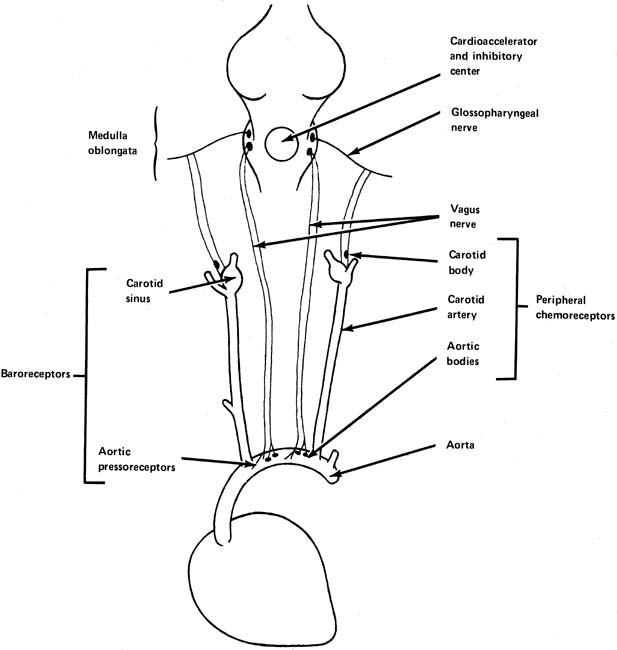 Fig. 5. The peripheral chemoreceptors and baroreceptors and their input to the
cardiac integrating center in the medulla oblongata. (Parer JT:
Physiological regulation of fetal heart rate.
J Obstet Gynecol Neonatal Nurs
5: 265,
1976. Fig. 5. The peripheral chemoreceptors and baroreceptors and their input to the
cardiac integrating center in the medulla oblongata. (Parer JT:
Physiological regulation of fetal heart rate.
J Obstet Gynecol Neonatal Nurs
5: 265,
1976.
|
Chemoreceptors Chemoreceptors are found in both the peripheral and central nervous systems. They
have their most dramatic effects on the regulation of respiration, but
they are still important in the control of the circulation. The
peripheral chemoreceptors are found in the carotid and aortic bodies (see Fig. 5). Like the pressoreceptors, they are found in the arch of the aorta
and in the area of the carotid sinus. The central chemoreceptors, found
in the medulla oblongata, respond to changes in the oxygen and carbon
dioxide tensions in blood or cerebrospinal fluid perfusing this area. In adults, when oxygen in the arterial blood perfusing the central chemoreceptors
is decreased or the carbon dioxide content is increased, there
is ordinarily a reflex tachycardia. There is also a substantial arterial
blood pressure increase, which is extremely pronounced with increases
in carbon dioxide concentration. Both of these effects, that is, tachycardia
and an increase in blood pressure, are thought to be protective
in attempting to circulate more blood through the affected areas
to bring about a decrease in carbon dioxide tension or an increase
in oxygen-selective hypoxia or hypercapnia of the peripheral chemoreceptors
alone in the adult produces a bradycardia, in contrast to
the tachycardia and hypertension seen with central hypoxia or hypercapnia. The peripheral chemoreceptors seem to be highly important in the cardiovascular
responses to hypoxia. The interaction of baroreceptor and chemoreceptor
systems in a fetus is not well understood, although the net
result of hypoxia in a fetus is bradycardia and hypertension. During
basal conditions, they seem to contribute to stabilize heart rate and
blood pressure.9 Central Nervous System It has been established that in adults there are influences on heart rate
from the higher centers of the brain (Fig. 6). Heart rate can be increased by various emotional stimuli such as
fear or sexual arousal. Observations of fetal lambs and monkeys have
shown that the electroencephalogram or electro-oculogram shows
increased activity at times in association with variability of the heart
rate and body movements. At other times, apparently when the fetus
is sleeping, activity slows, and the fetal heart rate variability decreases, suggesting
an association between these two factors and central
nervous system activity. 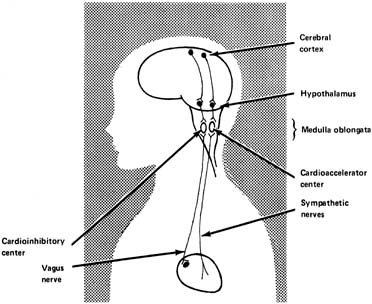 Fig. 6. Schematic depiction of regulation of heart rate from the higher centers. (Parer JT:
Physiological regulation of fetal heart rate.
J Obstet Gynecol Neonatal Nurs
5:265,
1976.) Fig. 6. Schematic depiction of regulation of heart rate from the higher centers. (Parer JT:
Physiological regulation of fetal heart rate.
J Obstet Gynecol Neonatal Nurs
5:265,
1976.)
|
The hypothalamus is thought to be the area of dispatch of nerve impulses
produced by physical expressions of emotion, including acceleration
of the heart rate and elevation of the blood pressure. It has been shown
in fetal lambs that stimulating an electrode in the hypothalamus causes
the fetal heart rate to increase, at least initially, followed by
a decrease, probably because of the baroflex mentioned earlier. The increase
in blood pressure and heart rate appears to be mediated by the
sympathetic nerves. The medulla oblongata contains the vasomotor center, an integrative center
where the net input results in either cardioacceleration or cardiodeceleration. It
is probably in these centers that the net result of numerous
central and peripheral inputs is processed to generate irregular
oscillatory vagal impulses, giving rise to FHR variability. Humoral Regulation ADRENAL MEDULLA The fetal adrenal medulla produces epinephrine and norepinephrine in response to stressful situations (e.g., hypoxia). Both of these substances act on the heart and cardiovascular
system in a way similar to sympathetic stimulation. That is, they
produce a faster heart rate, greater force of contraction of the heart, and
an increased arterial blood pressure. However, it is not clear
whether catecholamines exert a regulatory function in a resting fetus, at
least in sheep.10 RENIN-ANGIOTENSIN SYSTEM Angiotensin II seems to play a role in fetal circulatory regulation at
rest, but its main activity is observed during hemorrhagic stress on a
fetus. VASOPRESSIN Vasopressin has been shown to affect the distribution of blood flow in
fetal sheep. However, this is probably only operative during hypoxia and
possibly other stressful situations. PROSTAGLANDINS Arachidonic acid metabolites are found in high concentrations in the fetal
circulation and in many tissues. Their main role seems to be in the
regulation of umbilical blood flow as well as in maintaining the patency
of the ductus arteriosus during fetal life. Other hormones such as a-melanocyte-stimulating hormone (a-MSH), atrial natriuretic hormone, neuropeptide Y, thyrotropin-releasing hormone (TRH), cortisol, and metabolites
such as adenosine have also been described to be present in the fetus and to participate
in the circulatory function regulation, but their overall quantitative
importance in the human is still not determined. Blood Volume Control CAPILLARY FLUID SHIFT In adults, when the blood pressure of the body is elevated by excessive
blood volume, some fluid moves out of the capillaries into interstitial
spaces, thereby decreasing the blood volume back toward normal. Conversely, if
an adult loses blood through hemorrhage, some fluid shifts
out of the interstitial spaces into the circulation, thereby increasing
the blood volume back toward normal. There is normally a delicate balance
between the pressure inside and outside the capillaries. This mechanism
to regulate blood pressure is slower than the almost instantaneous
regulation found with the reflex mechanisms discussed earlier. Its
role in a fetus is imperfectly understood, but studies performed on
sheep show that a fetus appears to be able to keep its blood volume closer
to normal than an adult after reductions or expansions of volume.11 INTRAPLACENTAL PRESSURES Fluid moves down hydrostatic pressure gradients and also in response to
osmotic pressure gradients. The actual values of these factors within
the placental site where fetal and maternal blood closely approximate
are controversial. It seems likely, however, that some delicate balancing
mechanisms within the placental site prevent rapid fluid shifts between
mother and fetus. As noted earlier, maternal arterial blood pressure
is much higher (approximately 100 mmHg) than that of a
fetus (approximately 55 mmHg); hence, some compensatory mechanism
must be present to equalize the effective pressures at the exchange
points. Imbalances may be responsible for the hydrops encountered
in some cases of Rh isoimmunization and extreme fetal tachycardia. |