Assessment of First-Trimester Complications First-trimester ultrasonography can be of significant value in predicting
the outcome in patients with bleeding in early pregnancy. Except
in the unusual circumstance of a combined pregnancy (incidence 1 in 12,000 to 30,000), the finding of a pregnancy within the
uterus excludes an ectopic pregnancy. This distinction, however, is not
always clear. The normal gestational sac has a well-defined, echogenic
border. In ectopic gestation, decidua and blood may distend
the uterine cavity, and the ultrasound image can mimic a gestational sac, resulting
in the so-called pseudogestational sac.16 These entities often can be distinguished by ultrasound; in a pseudogestational
sac, the echogenic rim usually is absent, ill defined, or not
centrally positioned in the uterus.17 In questionable cases, serial growth of the sac can be assessed. In a
normal pregnancy, the gestational sac should grow at least .6 mm daily. 18 Using a quantitative assay of human chorionic gonadotrophin (hCG) with
ultrasound improves diagnostic accuracy. Even with, β-hCG
values as low as 750 mIU/mL, a gestational sac can be visualized.19 If the level is below this value, in a clinically stable patient, serial
HCG values can be followed. In a normal early pregnancy, the β-hCG
level should increase at least two-fold in 72 hours. In practice, a clinical problem often faced is differentiation of a threatened
abortion from an ectopic pregnancy. In both these conditions, a
subnormal increase in β-hCG usually is seen. Ultrasound findings
in a pregnancy destined to abort include a poorly defined gestational
sac, a large yolk sac (6 mm or greater in size), a low
site of sac location in the uterus, or an empty gestational sac at 8 weeks' gestational
age (the blighted ovum). The only absolute assurance that a pregnancy is intrauterine is the finding
of a fetal pole within the uterine cavity. The endovaginal ultrasound
can be useful in this clinical setting because the fetal pole can
be seen at 6 weeks. In a normal pregnancy, the fetal pole should be visible
if the gestational sac is 25 mm or larger in diameter. The presence of a fetal pole with demonstrable cardiac activity is reassuring
and greatly decreases the likelihood of spontaneous abortion. In
an ultrasonically normal gestation without bleeding at 8 to 9 weeks, there
is a 3% chance of subsequent pregnancy loss.20 If bleeding is present, this chance increases to approximately 13%.21 Also included in the differential diagnosis of vaginal bleeding in pregnancy
is the hydatid mole, which has a characteristic ultrasound appearance (Fig. 6). 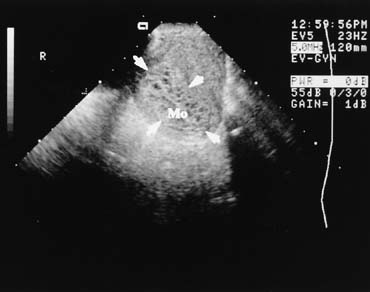 Fig. 6. Endovaginal scanning provides superior resolution of the nonpregnant or
very early pregnant pelvic organs. This is a midline endovaginal view
of an anteflexed uterus filled with clusters of tiny anechoic areas and
is the image of a hydatidiform mole (Mo). The outline of
the mole is indicated (arrowheads). The transducer in the vaginal
fornix is at the top of the image, the maternal abdominal wall is
to the left, and the cul-de-sac would be to the right. Fig. 6. Endovaginal scanning provides superior resolution of the nonpregnant or
very early pregnant pelvic organs. This is a midline endovaginal view
of an anteflexed uterus filled with clusters of tiny anechoic areas and
is the image of a hydatidiform mole (Mo). The outline of
the mole is indicated (arrowheads). The transducer in the vaginal
fornix is at the top of the image, the maternal abdominal wall is
to the left, and the cul-de-sac would be to the right.
|
Evaluation of Abnormal Biochemical Testing For two decades, AFP testing has been offered to obstetric patients in
the United States. Many practitioners are now using multiple markers, which
may include AFP, hCG, estriol, and inhibin. These biochemical markers
are used as a screening test for fetal anatomic abnormalities and
Down syndrome. Fetal abnormalities associated with elevated AFP levels
include fetal death, spina bifida, anencephaly, abdominal wall defect
such as omphalocele and gastroschisis, and renal abnormalities. Low
AFP levels have been associated with Down syndrome and other chromosomal
abnormalities. Because AFP levels and the other biochemical markers
vary with gestational age, the interpretation of these tests demands
accurate clinical dating. The first step in evaluation of a patient with
an abnormal serum AFP level is ultrasound. Because maternal serum AFP levels normally increase with advancing gestational
age, an apparent elevation might result from an error in dates. Twins
also cause an elevation of AFP levels. In a basic ultrasound examination
to evaluate elevated maternal serum AFP levels, as many as
one third of patients are shown to have incorrect dates, multiple gestation, or
fetal death. If the dates are correct and the elevated maternal
serum AFP level is confirmed, referral for a high-detail ultrasound
examination is appropriate. This should be performed by a sonologist
with experience in diagnosing fetal anomalies by ultrasound. Detection
of these anomalies is discussed later in the sections on clinical
applications. In patients who have elevated AFP levels and normal high-detail
ultrasound examinations, determination must be made about whether amniocentesis
is appropriate. The information from the ultrasound can then
be used when counseling the patient. In the past, amniocentesis was recommended
for determination of amniotic fluid AFP and karyotype. High-detail
ultrasound now can detect up to 95% of neural tube
defects, so the risk of missing spina bifida is low. It is appropriate
to inform the patient regarding the risk of an invasive procedure
such as amniocentesis as well as about the very low risk of a normal high-detail
ultrasound missing spina bifida.22 In patients who have low AFP levels or abnormal biochemical screen results, ultrasound
can play an important role in interpretation and diagnosis.23 An inaccurate gestational age may explain up to half of cases of apparently
low maternal serum AFP values. Usually, if the ultrasound gestational
age is within 2 weeks of that determined by the last menstrual period, the
gestational age used for interpretation should not be changed. Ultrasound
is less accurate for determination of chromosomal abnormalities, such
as Down syndrome, than it is for determination of neural
tube defects. Benacerraf and colleagues24 reported the finding of occipital nuchal skin thickening in approximately
half of fetuses with trisomy 21; these data subsequently were confirmed
by other investigators.25 Other abnormalities seen with increased incidence in fetuses with Down
syndrome include short femurs, short humeri, dilation of the fetal renal
pelvis, ventriculomegaly, and fetal cardiac abnormalities. Combining
multiple minor dysmorphic features into an aneuploidy scoring system
may prove useful both in the detection of abnormal fetuses and in the
reduction of risk. 26 There has been considerable interest recently in using ultrasound in combination
with biochemical markers (PAPP A, free β-hCG) to
diagnose Down syndrome in the first trimester of pregnancy. The
same sonographic finding seen in second trimester fetuses with Down
syndrome, increased nuchal skin thickness, can also be seen in the first
trimester. At 10 to 14 weeks' gestation, if the distance between
the posterior fetal cervical spine and the overlying skin is greater
than 3 mm, the incidence of Down syndrome is increased.27,28 Absence of the fetal nasal bone in the first timester (at 11 or more
weeks' gestation) has been observed in fetuses with Down
syndrome. In one report, more than 70% of fetuses with Down syndrome
had an absent nasal bone, while this was observed in only .5% of
chromosomally normal fetuses.29 Evaluation of Fetal Growth Abnormalities Fetal growth can be evaluated by comparing individual dimensions to normative
data, by comparing various fetal dimensions to assess symmetry, or
by integrating selected measurements to produce an estimation of fetal
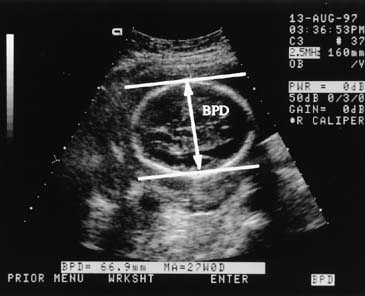
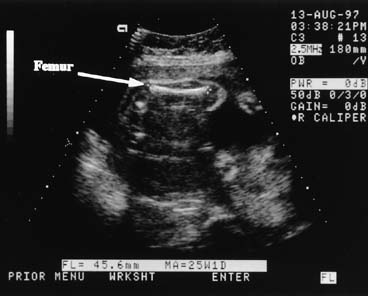
weight. Several investigators have derived equations useful for estimating weight, using
fetal dimensions, such as BPD, mean abdominal diameter, abdominal
circumference, and femur length. Warsof and colleagues30 first provided a clinically useful formula for weight estimation using
BPD and AC. Shepard and colleagues31 reported a second similar equation with slightly improved accuracy. Rose
and McCallum32 described a system using the sum of the BPD, mean abdominal diameter, and
femur length in centimeters that is relatively easy to use. The differences
between these and other published methods is small when compared
with the overall accuracy of fetal weight estimation. Generally, the
ultrasonic predicted weight is within 10% of the actual weight
in two thirds of cases and within 20% in 95% of cases. In the preterm fetus in which clinical estimation of fetal weight is notably
inaccurate, estimating fetal weight using ultrasound is useful. In
the term fetus, however, because of the greater actual error, ultrasound-estimated
fetal weight may be no more accurate than a clinical
weight estimation.33 Assessment of Third-Trimester Bleeding Ultrasound examination is useful in differentiating causes of third-trimester
bleeding. The placenta can be identified easily with the
real-time scanner. Placenta previa can be diagnosed with a high
degree of accuracy (Fig. 7). In cases of marginal previa, the examination should be performed
with both an empty and a full bladder for greatest accuracy. The internal
os may be located by identifying the bladder angle in a moderately
full bladder. 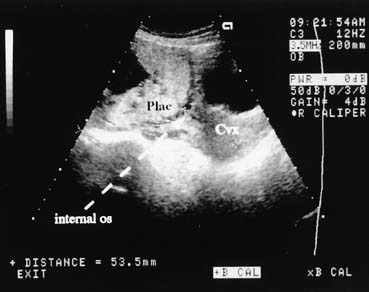 Fig. 7. This midline suprapubic view shows the cervix (Cvx) and the placental (Plac) implantation over the internal os. The maternal
bladder is seen as the black (anechoic) area above the cervix. Fig. 7. This midline suprapubic view shows the cervix (Cvx) and the placental (Plac) implantation over the internal os. The maternal
bladder is seen as the black (anechoic) area above the cervix.
|
Placenta previa is found in approximately 5% of second-trimester
scans performed for other indications. In marginal previa or central
previa (central insertion of the placenta over the cervix) seen
on a second-trimester scan, a follow-up scan
in the third trimester is indicated; many of these cases will have spontaneously
resolved on subsequent examination because of asymmetry in
uterine growth, not placental migration.34,35 The persistence of placenta previa depends on the gestational age at which
it is detected. For example if previa is detected at 15 to 19 weeks, it
persists in 12% of patients. If it is detected at 24 to 27 weeks, it
may persist in up to 50%.36 Other causes of third-trimester bleeding include abruptio placentae
and vasa previa. Although these conditions have been diagnosed with
ultrasound, they cannot be excluded confidently in most cases. A retroplacental
clot may be seen in some cases of abruptio,37 but ultrasound cannot exclude abruption, and the wise course in most cases
is to treat the patient as clinical circumstances dictate. With increasing gestational age, the placenta increases in echogenicity
because of increased fibrosis and calcium content. This feature of placental
maturation has led to a grading of placentas from immature (grade 0) to
mature (grade 3).38 In a grade 3 placenta, individual cotyledons are outlined by dense fibrotic
calcified septa. The correlation of a grade 3 placenta with fetal
pulmonary maturity is high. A grade 3 placenta can be observed in the
third trimester in a variety of high-risk fetal conditions (e.g., IUGR, chronic hypertension) but also can occur in normal pregnancy. Conversely, a
grade 3 placenta may not be seen in many normal patients
in labor at term. Clinical Applications Ultrasound estimation of fetal weight can be invaluable in many clinical
situations, such as obstetric management of the patient presenting with
labor and no previous prenatal care. Identification of fetal macrosomia also is of particular interest to the
clinician because this condition is associated with an increased risk
of a variety of obstetric complications. The accuracy of ultrasound
in predicting macrosomia depends on the clinical situation in which the
ultrasound is used.39 The sensitivity of ultrasound in predicting macrosomia is approximately 60%.40 As the prevalence of macrosomia increases, such as in the case of a mother
with diabetes, the accuracy of ultrasound in predicting macrosomia
increases. The percent error in sonographic estimation of fetal weight, however, is
similar in both diabetic and nondiabetic pregnancies.41 Some clinicians have advocated prophylactic cesarean section when fetal
macrosomia is found, to prevent brachial plexus injury. Any management
decisions must take all other clinical features into account, including
the estimated fetal weight presentation, progress in labor, clinical
pelvimetry, and obstetric history. Diagnosis of Intrauterine Growth Restriction If the gestational age in a pregnancy is confidently established, ultrasound
can provide an accurate diagnosis of IUGR. IUGR can result from
uteroplacental insufficiency, drug exposure, intrauterine infection, or
genetic factors.42 Symmetric IUGR, in which all fetal parameters are small for a given gestational
age, occurs with extrinsic conditions that are active early
in pregnancy (e.g., tobacco or alcohol abuse, congenital rubella). Symmetric IUGR also
can result from intrinsic conditions that limit fetal growth potential (e.g., chromosomal abnormalities). Symmetrically reduced growth also is
seen in the constitutionally small, normal fetus. Fetal growth may be normal until the late second or early third trimester, at
which time the limit of uteroplacental circulation in sustaining
fetal growth is reached. This results in asymmetric fetal growth, in
which the fetus adapts to the relative decrease in utero placental blood flow by redistributing cardiac output to favor the brain at the
expense of the muscles and abdominal viscera. For these reasons, the use of BPD alone can detect only half of IUGR cases. When
IUGR is suspected, ultrasound-estimated fetal weight
can detect up to 90% of affected infants.43,44 An estimated fetal weight below the 10th percentile for gestational age
is predictive of IUGR. When IUGR is suspected, the ultrasonographer
should look critically at amniotic fluid volume. Oligohydramnios, resulting
from decreased fetal urine production as blood is shunted away from
the fetal kidneys, commonly is associated with severe IUGR. Manning
and associates noted that when a 1-cm pocket of amniotic fluid
could not be found, IUGR was present in 90% of cases.45 The amniotic fluid index also has been described as a useful tool in the
evaluation of fluid volume.46 When the gestational age is not precisely known, assessment of fetal growth
over a 2-week interval is useful in distinguishing IUGR from
incorrectly dated pregnancies. In the third trimester, fetal weight
should increase approximately 300 grams in 2 weeks. Another gestational
age-independent assessment is the abdominal circumference-to-fetal
length or mean abdominal diameter-to-femur
length ratio.47 Reece and colleagues48 reported that the transverse fetal cerebellar diameter is unaffected by
IUGR; this additional parameter may help to discriminate IUGR from incorrectly
dated pregnancies. Evaluation of Multiple Gestations In evaluating multiple gestations, the ultrasonographer should note the
number and presentation of the fetuses and the position and number of
placentas. A membrane almost always can be seen between each fetus in
early gestation. Monoamniotic twins, which comprise only 3% of
all twin gestations, have no intervening membrane and are at much greater
risk of perinatal mortality than other twins. The thickness of the
membrane between twins may provide a clue about whether both chorion
and amnion are present, because a thicker membrane is present in dichorionic–diamniotic
twins.49 As is the case with ultrasound diagnosis of IUGR, discordance is better
predicted by estimated fetal weight calculation than by BPD alone.50 Discordance is determined by dividing the observed fetal weight difference
by the weight of the larger twin. Mild discordance (15% to 25% difference in fetal weights) occurs in approximately 20% of
twin pregnancies, and severe discordance (more
than 25% difference) occurs in approximately 5% of
twins.51 Discordant twins are at increased risk for perinatal mortality; the smaller
twin is at greatest risk antenatally. A reasonable approach to assess
growth in twin gestation is to perform serial estimated fetal weight
measurements on both twins every 4 weeks beginning at 20 weeks' gestation. If
discordance is present, or if intertwin weight differences
are increasing, weight determination is recommended more often. Detection of Fetal Malformations The ability of ultrasound to detect and characterize a broad array of fetal
malformations is well known. Ultrasound is best suited to the detection
of obstructive malformations or major distortions of surface anatomy. Each
ultrasound examination performed should comprise an anatomic
survey of the fetus, including cranial and intracranial structures, spinal
anatomy, a four-chamber cardiac view, the abdomen with
stomach and umbilicus, the bladder, and at least one long bone. Any suspicion
of abnormality should result in referral for a more detailed ultrasound
examination. A basic office ultrasound examination is adequate for most patients. A
targeted scan, performed by an ultrasonographer with experience in the
evaluation of fetal malformations, is performed when there is a suspected
fetal abnormality on a basic ultrasound examination or to evaluate
fetal anatomy when the patient is in a high-risk category based
on history, physical findings such as polyhydramnios, or laboratory
testing (e.g., AFP). Determination of fetal karyotype should be considered in any case in which
a malformation is ultrasonographically detected. Aneuploidy is discovered
in approximately 10% of cases of ultrasonographic dysmorphology. The
finding of a lethal karyotype could alter obstetric management
because fetal distress during labor often occurs in this setting, and
a cesarean section delivery might otherwise result. Additionally, the
finding of one structural malformation should prompt a more detailed
search for other anomalies.52 CENTRAL NERVOUS SYSTEM ANOMALIES The incidence of fetal open neural tube defects in the United States is 1 to 2 per 1000 births. Anencephaly, in which the cranial vault is absent, accounts
for approximately half of these. By locating the fetal
spine and moving the ultrasound transducer cephalad, the absence of a
cranial vault can be confirmed. In anencephaly, the fetal orbits should
be visible. The cranium should be visualized by 14 weeks' gestation. Spina bifida is more difficult to detect; the fetal spine must be meticulously
examined in three planes. Successful ultrasonographic detection
of spina bifida requires examination of both spinal and cranial anatomy. Experience
strongly suggests that intracranial abnormalities are
present in most all cases of spina bifida.22,53 Five cranial signs associated with spina bifida are frontoparietal notching (the lemon sign) (Fig. 8), mild hydrocephalus, a small-for-date BPD, abnormal
cerebellar hemispheres, and obliteration of the cisterna magna. The
finding of a normal fetal cerebellum and cisterna magna virtually excludes
spina bifida. This view is obtained by finding the axial plane of
the BPD and rotating the transducer to view the posterior fossa (Fig. 9). 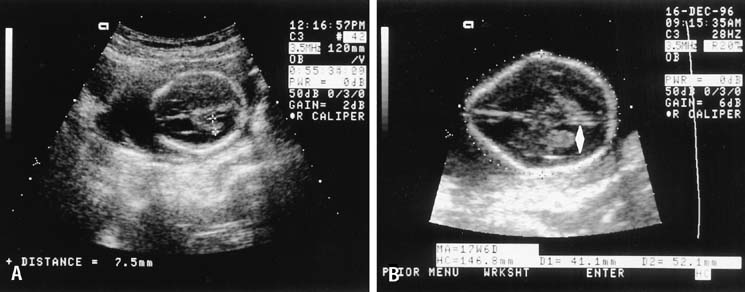 Fig. 8. A. From this occipitofrontal image, the normal smooth oval shape of the
fetal cranium is seen, and the atrium of the lateral ventricle may be
measured, as is performed here. Normally, the atrium is no more than 10 mm
in internal dimension, not including the walls. Compare both the
oval shape and the lateral ventricle with those seen in (B). B. Nearly the same scan plane as in (A), this view shows a distinct loss of the normal oval shape (the
lemon sign). The atrium of the lateral ventricle is dilated (double
arrowhead). These findings are typical of those seen
with fetal spina bifida. Fig. 8. A. From this occipitofrontal image, the normal smooth oval shape of the
fetal cranium is seen, and the atrium of the lateral ventricle may be
measured, as is performed here. Normally, the atrium is no more than 10 mm
in internal dimension, not including the walls. Compare both the
oval shape and the lateral ventricle with those seen in (B). B. Nearly the same scan plane as in (A), this view shows a distinct loss of the normal oval shape (the
lemon sign). The atrium of the lateral ventricle is dilated (double
arrowhead). These findings are typical of those seen
with fetal spina bifida.
|
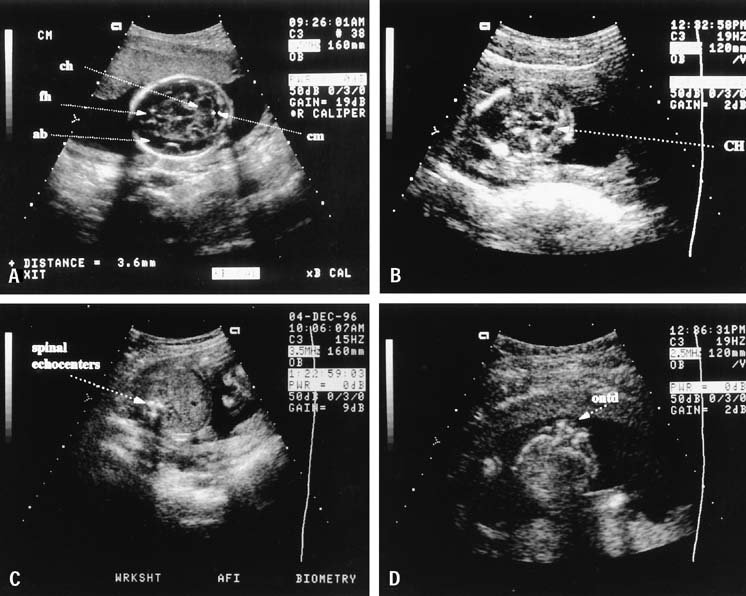 Fig. 9. A. Transaxial (transverse to the midline) view in the suboccipitobregmatic
scan plane showing normal posterior fossa anatomy. Normally, the
cerebellar hemispheres (ch) are circular, there is fluid
in the cisterna magna (cm), the frontal horns of the lateral
ventricles (fh) may be seen in the anterior area, and
the early fetal brain is anechoic (ab). Compare this view with (B). B. The posterior fossa of an infant with spina bifida shows compression
of the cerebellar hemispheres (CH) into what has been called
the banana sign and loss of the fluid in the cisterna magna. This is
representative of the Chiari deformation associated with spina bifida. C. The normal transverse fetal spine shows three echocenters nearly equidistant
from one another, as seen here. The soft tissue dorsal to these
echocenters should remain intact, and the relations of these echocenters
should remain unchanged throughout the spine. D. Transverse view of an open spina bifida defect (ontd), which
is seen as a loss of the integrity of the echocenters shown in (C). Fig. 9. A. Transaxial (transverse to the midline) view in the suboccipitobregmatic
scan plane showing normal posterior fossa anatomy. Normally, the
cerebellar hemispheres (ch) are circular, there is fluid
in the cisterna magna (cm), the frontal horns of the lateral
ventricles (fh) may be seen in the anterior area, and
the early fetal brain is anechoic (ab). Compare this view with (B). B. The posterior fossa of an infant with spina bifida shows compression
of the cerebellar hemispheres (CH) into what has been called
the banana sign and loss of the fluid in the cisterna magna. This is
representative of the Chiari deformation associated with spina bifida. C. The normal transverse fetal spine shows three echocenters nearly equidistant
from one another, as seen here. The soft tissue dorsal to these
echocenters should remain intact, and the relations of these echocenters
should remain unchanged throughout the spine. D. Transverse view of an open spina bifida defect (ontd), which
is seen as a loss of the integrity of the echocenters shown in (C).
|
Hydrocephalus is most sensitively detected by measurement of the width
of the lateral ventricle at the choroid plexus. A value of 10 mm or less
before 20 weeks' gestation is normal. In early hydrocephalus, the
choroid plexus may be displaced anteriorly and does not fill the atrium
of the ventricle. Early diagnosis is thus made on morphologic as
well as measurement criteria. In later gestation, the diagnosis may be
made by comparing the distance to the lateral aspect of the ventricle
width relative to the cranial hemisphere width. The normal ratio of
these measurements is dependent on fetal gestational age.54 CARDIOVASCULAR DISORDERS The four-chamber view of the fetal heart is obtained from a near
transverse midchest scan plane at the level of the heart (Fig. 10). The normal fetal heart is angled at 45° (±10 degrees [1 SD]) to the left of the anteroposterior midline.55 The anteroposterior midline passes through the left atrium and the right
ventricle. Asymmetry in chamber size, defects in the septum, or displacement
of the fetal heart should lead to referral for a more detailed
ultrasonographic evaluation (Fig. 11). In one study, screening ultrasound had an overall detection rate
of 75% for major congenital heart disease.56 A normal four-chamber view excludes many cardiac anomalies,57 but accuracy of anomaly detection can be improved by the addition of outflow
tract views. 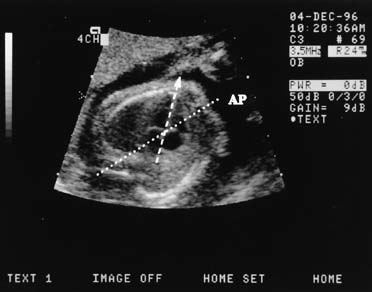 Fig. 10. The ideal scan plane to visualize the four-chamber heart is almost
perfectly parallel to the ribs, as shown here. Normally, the heart
is approximately one third of the area of the chest and is inclined to
the left at approximately 45 degrees to the midline. Here, the midline (AP) and
the cardiac axis (arrowhead on dashed line) intersect
at approximately the correct angle. The two ventricles
and the atria are seen clearly. Fig. 10. The ideal scan plane to visualize the four-chamber heart is almost
perfectly parallel to the ribs, as shown here. Normally, the heart
is approximately one third of the area of the chest and is inclined to
the left at approximately 45 degrees to the midline. Here, the midline (AP) and
the cardiac axis (arrowhead on dashed line) intersect
at approximately the correct angle. The two ventricles
and the atria are seen clearly.
|
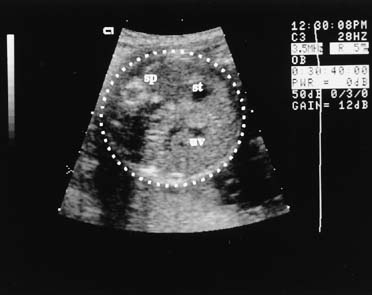
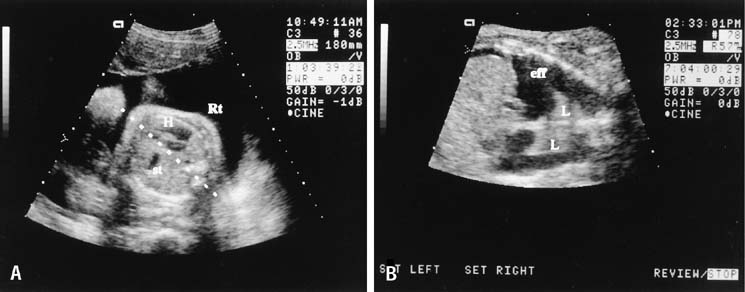 Fig. 11. A. In the case of the left-side diaphragmatic hernia seen here, the
heart (H) is displaced to the right of the midline (dashed
line), and the stomach (st) is seen in the chest
to the left of the heart. B. Congenital pleural effusions alter chest anatomy by separating the lung
from the adjacent tissue. Fluid reflects no echoes and appears black. In
this longitudinal view, the effusion (eff) fills the chest
and surrounds the lungs (L). A slight ascitic effusion
is seen above the liver to the left of the image. Fig. 11. A. In the case of the left-side diaphragmatic hernia seen here, the
heart (H) is displaced to the right of the midline (dashed
line), and the stomach (st) is seen in the chest
to the left of the heart. B. Congenital pleural effusions alter chest anatomy by separating the lung
from the adjacent tissue. Fluid reflects no echoes and appears black. In
this longitudinal view, the effusion (eff) fills the chest
and surrounds the lungs (L). A slight ascitic effusion
is seen above the liver to the left of the image.
|
GASTROINTESTINAL DISORDERS The fetal stomach can be seen as a fluid-filled structure on the
left side of the abdomen caudal to the four chamber view (see Fig. 5). The normal umbilical cord insertion can be seen in a transverse
section below the fetal stomach (Fig. 12). In this area, ventral wall defects of the fetus are seen, including
gastroschisis and omphalocele. Gastroschisis is an open defect in
the abdominal wall with evisceration of the abdominal organs that usually
occurs to the right of a normal cord insertion. There is no membrane
covering the eviscerated fetal bowel loops, which can be seen in the
amniotic fluid at the site of the defect (Fig. 13). Omphalocele, a failure of the embryonic gut to return into the
abdomen by 12 weeks' gestation, occurs at the cord insertion (Fig. 14) and usually is covered by a membrane consisting of peritoneum and
amnion.58 Omphalocele is associated with fetal chromosome abnormalities in one third
to one half of cases59 and should prompt a careful search for other fetal anomalies. IUGR commonly
is associated with both gastroschisis and omphalocele. 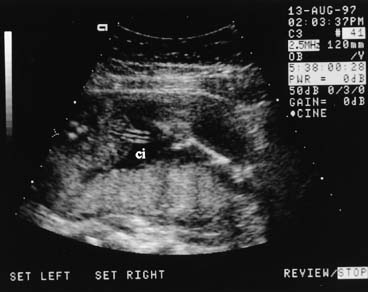 Fig. 12. Transverse abdominal view at the level of the umbilical insertion (ci) showing
the normal abrupt angulation. Fig. 12. Transverse abdominal view at the level of the umbilical insertion (ci) showing
the normal abrupt angulation.
|
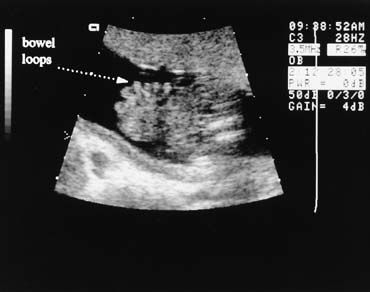 Fig. 13. Gastroschisis is diagnosed when free bowel loops are seen to exit the abdomen
through a defect above and to the right of a normal cord insertion. No
membrane covers these bowel loops. Fig. 13. Gastroschisis is diagnosed when free bowel loops are seen to exit the abdomen
through a defect above and to the right of a normal cord insertion. No
membrane covers these bowel loops.
|
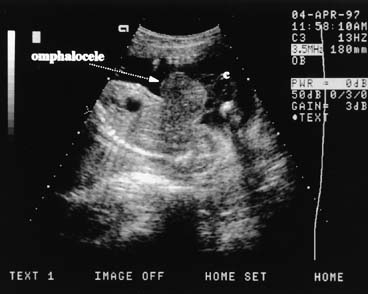 Fig. 14. This sagittal scan plane shows an upper omphalocele arising below the chest. The
cord (C) is seen to the right of the mass. An omphalocele
is covered by a bilayer membrane of amnion and peritoneum. Fig. 14. This sagittal scan plane shows an upper omphalocele arising below the chest. The
cord (C) is seen to the right of the mass. An omphalocele
is covered by a bilayer membrane of amnion and peritoneum.
|
The double-bubble sign, classically associated with duodenal obstruction, is not specific
for atresia but also may be seen with obstruction from malrotation or
an annular pancreas. RENAL DISORDERS The fetal kidneys can be seen on a transverse scan plane just below the
level of the stomach, in the dorsal paraspinal areas (Fig. 15). The fetal bladder is seen as a round fluid-filled structure
in the anterior midline of the fetal pelvis. The amniotic fluid volume
provides a clue to fetal renal function. In the fetus with absent
kidneys, renal obstruction, or nonfunctioning kidneys (renal dysplasia) after 20 weeks' gestation, the amniotic fluid volume
is markedly decreased. Before 20 weeks' gestation, however, the
amniotic fluid volume may be normal despite renal malformations. 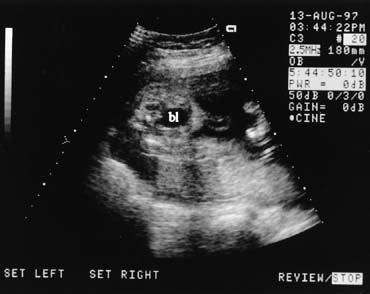 Fig. 15. Transverse scan of the lower pelvis showing the fetal bladder (bl). Cord
insertion is seen to the right of the bladder. Fig. 15. Transverse scan of the lower pelvis showing the fetal bladder (bl). Cord
insertion is seen to the right of the bladder.
|
Obstructive uropathy may be caused by posterior urethral valves, urethral
atresia, or persistent cloaca syndrome. Ultrasound findings include
a large bladder and enlarged ureters, which are seen as cystic convoluted
structures in the lower abdomen (Fig. 16). Ureteropelvic junction obstruction is suspected when an enlarged
fetal renal pelvis, without an enlarged bladder, is seen. 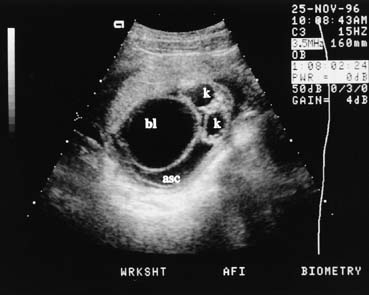 Fig. 16. In the case of the urethral atresia, as seen here in early pregnancy, the
large fetal bladder (bl), urinary ascites (asc), and
hydronephrotic kidneys (K) are all seen in the same transverse
image. Fig. 16. In the case of the urethral atresia, as seen here in early pregnancy, the
large fetal bladder (bl), urinary ascites (asc), and
hydronephrotic kidneys (K) are all seen in the same transverse
image.
|
Assessment of Changes in Amniotic Fluid Volume Amniotic fluid is a physiologic extension of the fetus. Abnormalities of
amniotic fluid volume are nonspecific findings that warrant further
investigation. Polyhydramnios is defined as an amniotic fluid volume in
excess of 2000 mL. The clinical diagnosis is made by the findings of
a large-for-date uterine size, increased fluid, and a normal-size
fetus. The excess fluid is seen as large anechoic areas
on ultrasound. A single pocket of fluid that is 8 cm or larger suggests
polyhydramnios; this occurs in approximately 1% of pregnancies.60 An excess of fluid may be found in maternal diabetes, erythroblastosis, or
fetal malformation.59 In severe erythroblastosis, fetal hydrops may be seen at the time of ultrasound
study. In one third of pregnancies complicated by polyhydramnios, no cause is
determined.61 Fetal malformations can complicate up to 40% of cases when twins, diabetes, and
blood group incompatibility are excluded. Oligohydramnios can be associated with fetal renal abnormalities, IUGR, postdate
fetuses, or membrane rupture. The clinical diagnosis is made
when the fundal height is small and ultrasound examination shows little
or no amniotic fluid. When amniotic fluid is decreased, the fetal small
parts are crowded and the fetus is in contact with the uterine wall
in most scanning planes. The amniotic fluid index, a sum of the largest vertical pocket of amniotic
fluid in each of four quadrants of the uterus, is a useful semiquantitative
measurement of fluid volume.62 Severe oligohydramnios is present when the largest vertical pocket of amniotic
fluid measures less than 1 cm. Major fetal anomalies were observed
in 13% of patients with severe oligohydramnios. In the structurally
normal fetus with intact membranes, severe oligohydramnios may
be an indication for delivery.63 Assessment of Fetal Well-Being The biophysical profile is a test used in conjunction with antepartum fetal
heart rate testing to evaluate fetal well-being in high-risk
pregnancies.64 The test quantifies fetal breathing and body movements, fetal tone, and
amniotic fluid volume. It also incorporates the nonstress test as a
measure of fetal reactivity. Each of these five parameters are assigned
a score of 2 if normal and 0 if abnormal (Table 3). The fetus is observed until all parameters are seen or until 30 minutes
has elapsed. Table 3. Biophysical Profile Scoring
| Variable |
Score 2 |
Score 0 |
| Fetal breathing movements |
At least 30 s of sustained fetal breathing movements
in 30 min of observation |
Less than 30 s of fetal breathing movements min 30
min |
| Fetal movements |
Three or more gross body movements min 30 min |
Two or fewer gross body movements min 30 min |
| Fetal tone |
At least one limb motion from flexion to extension
and rapid return to flexion |
Partial or full limb extension, with no flexion; absence
of fetal movement |
| Fetal reactivity |
Two or more fetal heart accelerations of 15 beats/min
for 15 s in 40-min observation period |
Fewer than two fetal heart rate accelerations in 40
min |
| Amniotic fluid volume |
A pocket of fluid that measures at least 2 cm in two
perpendicular planes or AFI of 5 |
Largest pocket of fluid measures less than 2 cm in
two perpendicular planes or AFI less than 5 |
(Manning FA, Baskett TF, Morrison I et al: Fetal biophysical profile
scoring: A prospective study in 1184 high-risk patients. Am
J Obstet Gynecol 140:289, 1981.)
This observation period helps to distinguish the normal fetus with a physiologic
periodic steep cycle from the chronically asphyxiated fetus
with central nervous system depression. Fetal breathing movements occur
approximately 30% of the time and, when seen, demonstrate that
a complex fetal central nervous system function is intact.64 Fetal breathing movements are seen in the normal fetus after 24 weeks' gestation.65 A normal biophysical profile (score of 8 to 10) is associated
with a corrected perinatal mortality rate of 1 to 2 per 1000.66 A test score of 6 indicates the need to repeat testing within 24 hours. Scores
of 4 or less are highly predictive of intrauterine fetal jeopardy
and, in a term fetus, are an indication for delivery. Low scores
are associated with fetal distress in labor as well as low Apgar scores. Vintzileos
and colleagues67 observed a good correlation between a low biophysical profile and fetal
acidosis. In the extremely preterm fetus, a low score may not necessarily indicate
the need for immediate delivery. The clinician must weigh the risk of
fetal distress or demise with the risks of prematurity. In this clinical
situation, stabilizing any adverse maternal condition (such
as elevated blood glucose in a diabetic patient or high blood pressure
in a hypertensive patient) and repeating the biophysical test a
short time later may be indicated. Assessment of the Risk of Preterm Labor There has been considerable interest in ultrasonographic evaluation of
the cervix as a marker for assessing the risk of preterm birth (Fig. 17). Iams and colleagues68 published a large multicenter study confirming that patients with a short
cervix, as determined by endovaginal ultrasonography, are at increased
risk for preterm birth. It is clear that the length of the cervix
decreases with advancing gestational age and that there is no cervical
length at which the risk of preterm birth is zero. A cervical length
of less than 25 to 30 mm indicates an increased risk of preterm birth, both
in high-risk and in low-risk populations. A single
endovaginal cervical length measurement at 24 weeks can be used to assess
risk status.69 Cervical length may also provide a more objective and clinically effective
means of assessing the cervix in patients with cervical cerclage. 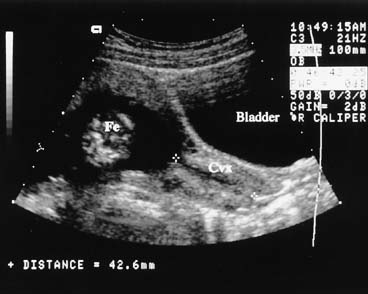 Fig. 17. If the maternal bladder is filled, the cervix (Cvx) can be seen
clearly and measured. Cervical length of less than 30 mm is associated
with an increased risk of preterm delivery. Fig. 17. If the maternal bladder is filled, the cervix (Cvx) can be seen
clearly and measured. Cervical length of less than 30 mm is associated
with an increased risk of preterm delivery.
|
Guiding lnvasive Procedures Ultrasound can be used to localize a favorable pocket of amniotic fluid
before performing amniocentesis.70 The needle can be inserted under direct visualization using the real-time
scanner.71 A needle guide is available to aid the obstetrician with needle placement, but
this can limit the operator's ability to move the needle
in three dimensions. It is preferable to insert the needle in a coplanar
fashion (i.e., in the same longitudinal plane as the ultrasound transducer), so
that the entire length of the needle track can be seen. Transplanar placement (i.e., perpendicular to the ultrasound plane) limits ultrasound view of
the needle. In chorionic villus sampling, the catheter is inserted into the placental
bed under ultrasound guidance.72 Similarly, simultaneous real-time ultrasound guidance is used in
percutaneous umbilical blood sampling or cordocentesis.73 Three-Dimensional Ultrasound 3D ultrasound offers a significant new method of imaging which may revolutionize
obstetric ultrasound. Initially, 3D images were obtained by
computer processing of multiple two-dimensional (2D) images. Presently, real-time 3D images can be obtained (Fig. 18).74 The 3D ultrasound image consists of a volume of data that can subsequently
be manipulated to display multiple longitudinal, transverse, and
coronal images. The sonologist can obtain images which may not be readily
available by conventional 2D sonography, and the images may not be
as operator dependent as with 2D sonography. A saggital view of the fetal
brain to look at the corpus callosum, for example, not readily obtained
on 2D study, can be obtained readily during volume manipulation
of a 3D data set. It is clear that if image quality is poor in 2D, then
it may also be suboptimal in 3D. 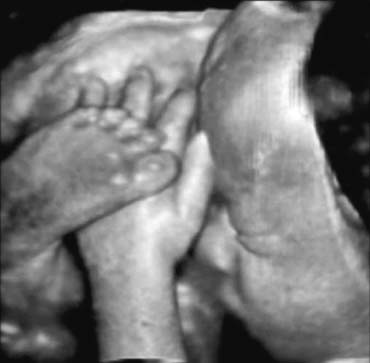 Fig. 18. 3D image of a fetus in the late first trimester. Courtesy of GE Medical
Systems. Fig. 18. 3D image of a fetus in the late first trimester. Courtesy of GE Medical
Systems.
|
There is an increasing body of research which indicates that 3D images
may improve the accuracy of anomaly detection of the fetal face, ears, and
distal extremities when compared to 2D images (Fig. 19).75,76 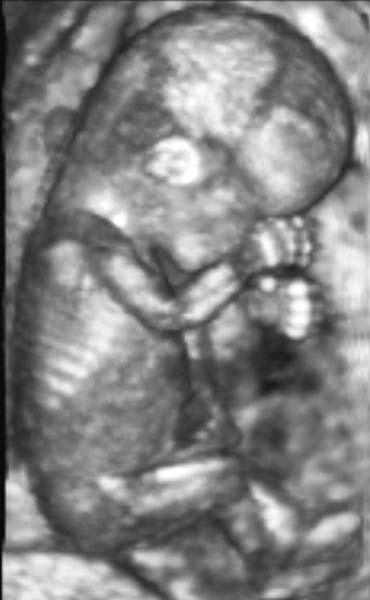 Fig. 19. 3D image of fetus with high detail of extremities. Courtesy of GE Medical
Systems. Fig. 19. 3D image of fetus with high detail of extremities. Courtesy of GE Medical
Systems.
|
|