To some extent, sophisticated interpretation of FHR monitor strips is an art rather than an exact science. Because of this subjective and sometimes controversial nature of FHR interpretation, the National Institute of Child Health and Human Development convened a research planning workshop to develop standardized, unambiguous, quantitated definitions for interpretation of intrapartum FHR tracings. By so doing, the predictive value of intrapartum EFM can be assessed in prospective trials in a more reproducible manner. It is hoped that this initiative will ultimately yield data that facilitate FHR monitor strip interpretation in an evidence-based manner. Interested readers should consult the workshop's report to review the detailed, standardized definitions published in 1997.10
Currently, intrapartum EFM is best viewed as a screening test to confirm fetal well-being rather than a diagnostic test confirming fetal compromise. Clinical factors, stage of labor, evolution of a particular pattern, and the characteristics of the entire FHR monitor strip must influence any given interpretation. No single method of classification or interpretation, regardless of its complexity, can be expected to provide clear guidelines for the management of borderline or difficult-to-classify patterns. Finally, because the status of the fetus during the labor process is dynamic, the data produced by intrapartum EFM are continuous, and the FHR strips must be examined on an ongoing basis.
Analysis of intrapartum EFM strips must be approached in an organized, systematic manner to optimize the value of the information obtained, to avoid overwhelming the practitioner with the volume of data obtained, and to prevent the practitioner from overlooking important clinical events. In general, the following six characteristics should be included in any thorough analysis:
- The technical quality of the monitor strip, to ensure that artifact does
not make interpretation impossible (inadequate data must not be interpreted; instead, the
quality of the data obtained must be improved)
- Determination of whether external or internal monitoring methods are being
employed
- Evaluation of the FHR baseline rate
- Analysis of the FHR reactivity (external monitor) or beat-to-beat variability (internal
monitor)
- Inspection for the presence of FHR accelerations
- Consideration of periodic FHR decelerations associated with uterine contractions
During the analytic process, multiple abnormalities in a particular FHR tracing are more significant than isolated findings. Fetal scalp pH determination or evoked fetal response to scalp stimulation or vibroacoustic stimulation may be helpful in the accurate assessment of fetal status in the face of nonreassuring FHR data.
Uterine Contractions
The intrauterine baseline pressure is defined simply as the pressure extant between contractions. An approximation of this variable can be obtained only by means of an intrauterine pressure catheter monitoring system. The upper limits of normal baseline intrauterine pressure may be defined according to the stage of labor: for latent labor, less than 5 mm Hg; for the active phase, less than 12 mm Hg; and for the second stage of labor, less than 20 mm Hg. Baseline pressures greater than these levels may represent uterine hypertonicity and sometimes are associated with abruptio placentae, uterine hyperstimulation with oxytocin, fetal malposition (e.g., occiput posterior and occiput transverse), or cephalopelvic disproportion.11
Although no uniform definition exists, uterine tachysystole has been defined as six contractions in 10 minutes in consecutive 10-minute intervals. Uterine hyperstimulation has been defined as a similar contraction frequency or a series of contractions lasting more than 2 minutes in combination with potentially worrisome FHR changes (e.g., late decelerations or fetal bradycardia).12 If tachysystole occurs in association with an elevation of baseline intrauterine pressure, the risk of uterine hyperstimulation is increased significantly. Similarly, if oxytocin induction or augmentation becomes complicated by tachysystole, the oxytocin should be discontinued or the rate of infusion decreased, at least temporarily, to avoid uterine hyperstimulation.
The graphic representations of normal uterine contractions on intrapartum EFM strips tend to be bell-shaped (i.e., symmetric), with a frequency in active labor of approximately one contraction every 2 to 3 minutes, as measured from the beginning of one contraction to the beginning of the next. Experienced clinicians recognize, however, that some patients may have an irregular contraction pattern throughout labor with an entirely normal progress of labor. The potential issue of apparently inadequate uterine contraction frequency is of no concern as long as satisfactory labor progress is occurring.
Similarly, there is no simple relationship between the intensity of uterine contractions and the rate of cervical dilatation. External tocodynamometry provides no information regarding the strength or intensity of uterine contractions. Even with an intrauterine pressure catheter system, the deflection on the intrapartum EFM strips is not necessarily an accurate indicator of the quality of labor. Nonetheless, contractions that occur less frequently than every 5 minutes and have a peak amplitude of less than 25 mm Hg seldom are associated with a normal labor curve, particularly in the active phase of labor. Once again, however, a detailed analysis of the frequency and intensity of uterine contractions is necessary only when the rate of cervical change is abnormal.
Fetal Heart Rate Baseline Value
The FHR baseline value is defined as the approximate heart rate between uterine contractions. The normal range is 110 to 160 bpm. Baseline rates below this range (less than 110 bpm) are considered bradycardia, whereas fetal baseline tachycardia is defined by a FHR greater than 160 bpm for two contraction cycles or longer than 5 minutes. Deviations from this normal baseline range are not necessarily indicative of fetal compromise. For example, a baseline rate of 100 to 110 bpm, if not associated with other FHR abnormalities or a significant decrease from a previously higher baseline rate, may be associated with an entirely reassuring intrapartum EFM strip.
Severe FHR baseline bradycardia (less than 100 bpm) is more likely to be associated with fetal jeopardy, although fetal congenital heart block can produce an identical FHR tracing that is not associated with acute compromise. It is important to exclude fetal demise (with bedside ultrasound) when severe fetal baseline bradycardia is suspected. On confirmation of severe fetal bradycardia, rapid intrauterine resuscitation is indicated. These efforts may include maternal repositioning into either the right or left lateral recumbent position, maternal oxygen therapy, intravenous hydration, and oxytocin infusion discontinuation. If these measures prove unsuccessful in resolving the FHR bradycardia, preparations for emergent delivery may need to be undertaken.
Fetal tachycardia may not be as clear an indicator of potential fetal compromise as severe baseline bradycardia. Fetal baseline tachycardia is classified as mild from 160 to 180 bpm and as severe when greater than 180 bpm. The differential diagnosis for the etiology of fetal tachycardia includes fetal compromise, maternal fever, prematurity, fetal infection, fetal arrhythmia (more common with fetal heart rates greater than 200 bpm), maternal thyrotoxicosis, fetal anemia, and maternal drug ingestion (prescribed or illicit). After excluding these potential confounding etiologies, and if combined with other FHR abnormalities, the index of suspicion for fetal compromise should be increased. For example, the combination of fetal tachycardia with diminished variability and late decelerations would be nonreassuring, as would the combination of fetal tachycardia with severe variable decelerations. The measures for intrauterine resuscitation described above for fetal bradycardia can also be effective interventions for fetal tachycardia. In general, regardless of the type of nonreassuring FHR pattern, steps should be taken (and documented) to optimize fetal status.
Fetal Heart Rate Variability
The presence of normal FHR beat-to-beat variability is a reassuring sign of fetal well-being and is attributed to the interaction of an intact fetal sympathetic and parasympathetic nervous system. Fetal heart rate variability is defined as fluctuations in the baseline FHR of two cycles per minute or greater.10 These fluctuations are visually quantitated as the amplitude of the peak to trough (in bpm) as below:
Absent: amplitude range undetectable
Minimal: amplitude range more than undetectable but less than 6 bpm
Moderate: amplitude range 6 to 25 bpm
Marked: amplitude range more than 25 bpm
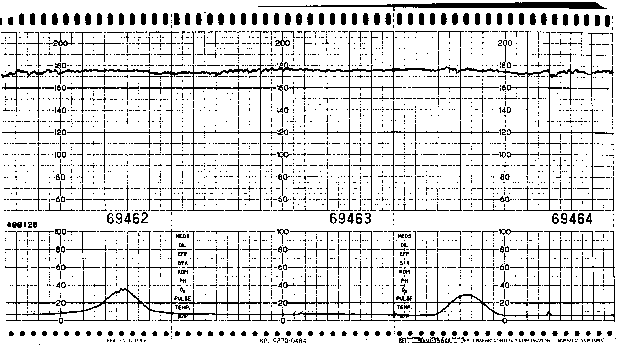
Accurate assessment of beat-to-beat variability requires direct EFM (i.e., analysis of instantaneous FHR data via scalp electrode) because external Doppler techniques yield a “processed” signal (via autocorrelation) that may exaggerate the magnitude of beat-to-beat variability present. Although satisfactory beat-to-beat variability is very reassuring, its absence is not necessarily associated with fetal compromise. Diminished variability (Fig. 1) may be associated with maternal narcotic use, administration of other drugs (e.g., diazepam, sodium thiopental, possibly magnesium sulfate), fetal neurologic dysfunction that predates labor, fetal baseline tachycardia (especially when associated with B-mimetic tocolytic therapy), or, most commonly, fetal sleep state.
|
Accordingly, the presence of diminished beat-to-beat variability in the absence of other FHR abnormalities, or decreased variability that is readily attributable to a known cause rarely indicates fetal compromise. In contrast, absent beat-to-beat variability coupled with late decelerations is nonreassuring and may be an indication for digital scalp stimulation (or vibroacoustic stimulation) to evoke FHR accelerations or fetal scalp sampling for pH determination to provide evidence of fetal well-being. Clearly, accurate assessment of the intrapartum fetal status requires an overview of the entire clinical scenario, not the simple categorization of isolated, perhaps even transient, FHR tracing characteristics.
Infrequently, the baseline beat-to-beat variability may be increased (greater than 25 bpm). This so-called saltatory pattern may be observed early in labors complicated by fetal malposition or disproportion and on occasion deteriorates into a pattern characterized by significant variable decelerations.13 The ultimate clinical significance of the saltatory pattern remains unclear, and operative intervention for this finding in isolation seems unwarranted.
Also infrequent is the sinusoidal FHR pattern. Characterized by the smooth, undulating oscillation of the baseline (mimicking a sine wave), short-term beat-to-beat variability is absent in this pattern. The amplitude of the long-term baseline variability ranges from 5 to 15 bpm, and the frequency of the cycles generally is three to five cycles per minute. This FHR pattern must be present for at least 10 minutes to avoid overdiagnosing this rare pattern, which classically is associated with severe fetal anemia (e.g., secondary to isoimmunization).14 The sinusoidal FHR pattern has also been reported in association with abruptio placentae and maternal administration of alphaprodine.8 When persistent, a true sinusoidal pattern may signify significant fetal compromise, and appropriate diagnostic or therapeutic maneuvers should be initiated. More commonly, however, a sinusoidal-like pattern is not of clinical significance.
Fetal Heart Rate Accelerations: Spontaneous and Evoked
Periodic intrapartum FHR accelerations (more than 14 bpm above baseline lasting more than 14 seconds and less than 2 minutes)10 may be spontaneous, associated with uterine contractions, or related to fetal movements. As in the setting of antenatal surveillance (e.g., nonstress test), intrapartum FHR accelerations provide evidence of intact fetal central nervous system function and, thus, fetal well-being. The presence of spontaneous FHR accelerations clearly is a reassuring sign. The converse, however, is not true; the absence of such accelerations is not necessarily worrisome, and other FHR tracing characteristics should be used to evaluate fetal status.
Intrapartum FHR accelerations also can be evoked with either scalp stimulation of vibroacoustic stimulation. Clark and associates15 reported that the presence of FHR accelerations (15 bpm for at least 15 seconds) after digital or instrumental fetal scalp stimulation uniformly was associated with a fetal scalp pH greater than 7.19. On a practical basis, the fetal scalp stimulation test is useful for evaluation of the fetus showing a nonreassuring FHR pattern, particularly when the cervix is not sufficiently dilated to permit fetal scalp sampling for pH determination. Smith and associates16 observed that similar FHR accelerations evoked by transabdominal vibroacoustic stimulation in the setting of nonreassuring FHR data were associated with a fetal scalp pH of greater than 7.25. The presence of evoked FHR accelerations is an excellent predictor of fetal well-being, although scattered reports have described isolated instances of fetal acidemia in fetuses with a reactive response to intrapartum vibroacoustic stimulation.17 It merits repeating that when confronted with nonreassuring FHR data, the entire intrapartum clinical setting needs to be assessed.
Fetal Heart Rate Decelerations
The evaluation of FHR decelerations arguably represents the most challenging aspect of FHR data analysis. Despite the myriad of classification systems proposed, we prefer to categorize these decelerations (typically, but not always, decreases of at least 10 to 15 bpm from the baseline value) simply as early, variable, or late in character. Most authentic disagreements about the analysis of FHR decelerations stem from differing assessments of the FHR baseline value. One unifying generalization typifies the discussion: The deeper the deceleration, the longer it lasts, the less beat-to-beat variability present, and the greater the late component, the greater the chance that fetal compromise is imminent or already present. The types of FHR decelerations are described in what most authorities agree is the order of increasing potential concern.
Early decelerations display an onset, nadir, and recovery that is synchronous with the onset, peak, and end of the uterine contraction. In short, early decelerations mirror the uterine contraction pattern. Generally ascribed to fetal head compression (with resultant vagal stimulation), early FHR decelerations are the least common variety of intrapartum FHR deceleration observed and usually are of no clinical significance.
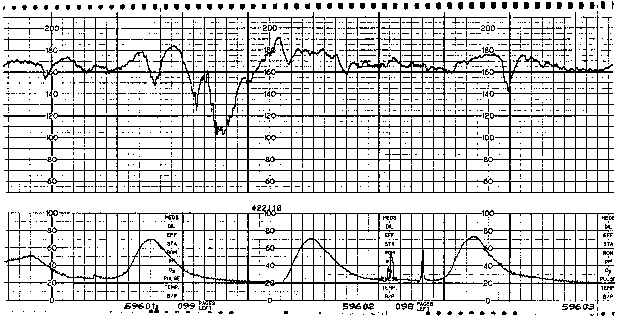
Variable FHR decelerations are variable in shape as well as in timing with respect to the uterine contraction pattern. Characterized by an abrupt decline (defined as onset of deceleration to beginning of nadir less than 30 seconds)10 from the FHR baseline value and an equally abrupt recovery, variable decelerations display a variety of waveforms, such as V, U, W, or combinations (Fig. 2). The decline in FHR below baseline is more than 14 bpm, lasting more than 14 seconds and less than 2 minutes from onset to return to baseline.10 Although they may occur throughout the contraction cycle, this variety of deceleration occurs most frequently in the vicinity of the peak of a given contraction. A severe variant of the variable deceleration is defined by a decline of the FHR to less than 60 bpm (or a decrease of the FHR by at least 60 bpm from the baseline value) lasting 60 seconds or longer.
|
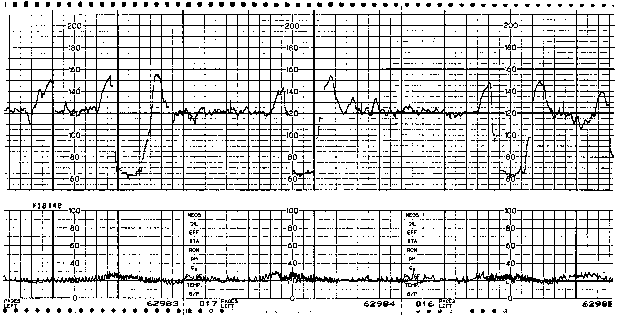
The etiology of variable decelerations is likely related to umbilical venous and arterial occlusion. Initially, with occlusion of the thin-walled umbilical vein, venous return to the fetal right atrium is reduced, producing a reflex tachycardia. This pattern often is observed as a shoulder on the FHR monitor strip immediately before the abrupt variable FHR deceleration. Shoulders that precede and follow variable decelerations are indicative of intact fetal central nervous system function and, thus, relative fetal well-being (Fig. 3). When cord occlusion becomes complete (including the umbilical artery), the low-resistance placental circulation is no longer in series. Instead, the elevated peripheral resistance that is caused by umbilical artery occlusion predictably leads to fetal hypertension, with subsequent baroreceptor stimulation. The baroreceptor and resultant vagal responses ultimately produce the parasympathetically mediated FHR deceleration. With relief of the umbilical cord occlusion, the sequence is reversed.
|
In isolation, variable FHR decelerations are not indicative of fetal compromise. If unremitting umbilical cord occlusion results in persistent, deep, variable FHR decelerations, however, the potential exists for the development of a fetal metabolic acidemia that cannot be managed satisfactorily by the fetal buffer system. Appropriate therapeutic interventions, including change of maternal position or amnioinfusion,8 may resolve the variable FHR deceleration pattern. The practice of amnioinfusion for the prevention or relief of umbilical cord compression, with resultant variable decelerations, is an accepted method of intrapartum management.8 Amnioinfusion has been reported to reduce the incidence of neonatal acidemia, to reduce the thickness of meconium, and to reduce the rate of operative intervention for nonreassuring FHR strips.18 Contraindications include persistent late FHR decelerations, fetal scalp pH less than 7.20, and significant vaginal bleeding; relative contraindications may include multiple gestation and malpresentation.
Amnioinfusion with normal saline (room temperature) can be accomplished via either bolus or continuous infusion using an infusion pump or gravity (intravenous bag 3 to 4 feet above the catheter). A bolus infusion of up to 800 mL may be administered, initially at a rate of 10 to 20 mL per minute until FHR decelerations resolve, and then up to an additional 250 mL to reach a maximum of 800 mL total. For a continuous infusion, a rate of 10 mL per minute is administered by infusion pump for 60 minutes, followed by an infusion at 3 mL per minute. Indications for discontinuation of the amnioinfusion include observation of a significant increase in the baseline intrauterine pressure, uterine hyperstimulation, or FHR bradycardia.
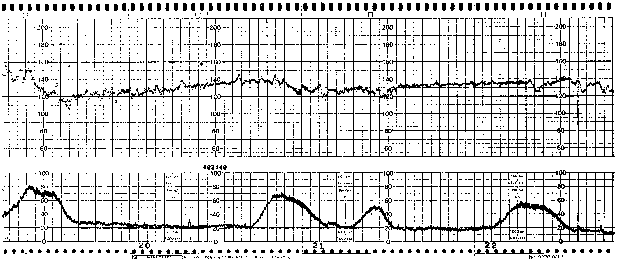
Late FHR decelerations are characterized by a decline after the onset of the uterine contraction, a nadir after the peak of the contraction, and a slow recovery to the FHR baseline value after the end of the uterine contraction (Fig. 4). Late declarations have been described as being of two distinct etiologies.8 The first, caused by reflex, is mediated by the fetal central nervous system. The second, direct myocardial depression, is seen more often in a setting of metabolic acidemia. Classically ascribed to relative uteroplacental insufficiency, occasional or intermittent late decelerations are not at all uncommon during most labors. The presence of persistent late decelerations, however, must be carefully evaluated because they may be secondary to transient fetal hypoxemia in response to decreased placental perfusion associated with uterine contractions.
The potential for fetal compromise in the presence of persistent late FHR decelerations mandates appropriate diagnostic and therapeutic interventions. Therapeutic maneuvers include maternal repositioning in the lateral recumbent position, satisfactory intravenous hydration, maternal oxygen therapy, and relief of uterine hyperstimulation, if present. The clinical index of suspicion for potential fetal compromise should be heightened if late FHR decelerations are accompanied by diminished beat-to-beat variability or baseline fetal tachycardia. If palliative measures are unsuccessful and late FHR decelerations persist, an attempt to evoke a FHR acceleration via either digital examination or vibroacoustic stimulation, or a fetal pH determination via scalp sampling may be considered. Although isolated late FHR decelerations are not an indication for immediate cesarean section, persistent late FHR decelerations remote from the time of anticipated vaginal delivery often ultimately prompt operative abdominal delivery.
Finally, a prolonged deceleration is a decrease in the FHR from baseline of more than 14 bpm lasting more than 2 minutes and less than 10 minutes from onset to return to original baseline.10 A prolonged FHR deceleration lasting more than 10 minutes is defined as a baseline FHR change.
A combination of various types of FHR decelerations may occur. For instance, FHR decelerations may show an abrupt decline from the baseline value, with a nadir after the peak of the contraction followed by a slow recovery to the baseline level. These variable decelerations with a late component may appear in labors that are complicated by intermittent umbilical cord occlusion, fetal malposition, or cephalopelvic disproportion. Persistence of this pattern of FHR deceleration, with progressively longer recovery times to the baseline level, may be associated with an increased potential for fetal compromise. Clearly, it would be an error to consider only shallow, smooth decelerations with a slow return to the baseline value as late. An important principle of accurate fetal assessment is an appreciation of the timing and depth of any FHR deceleration, regardless of the type of FHR pattern that may have preceded it. The evaluation and treatment of variable FHR decelerations with a late component logically proceed as described above for classic late FHR decelerations.
The FHR decelerations observed with maternal pushing during the second stage of labor are not typically indicative of potential fetal compromise and usually are not an indication for immediate operative delivery. Despite their depth, which can be significant, these decelerations tend not to persist. Unless complicated by a protracted second stage of labor, development of a late component, loss of previously normal beat-to-beat variability, or a prolonged deceleration, these FHR decelerations likely reflect fetal head compression, with resultant vagal stimulation, rather than evidence of uteroplacental dysfunction. Under ambiguous clinical circumstances, fetal scalp sampling for pH determination may be helpful to assess fetal status more accurately.
Fetal Scalp Sampling
The potential applications of intrapartum fetal scalp sampling for pH determination have been discussed throughout this chapter. It is our impression that since the publication of previous editions of this chapter, the practice of scalp pH determination has become much less common on many labor and delivery units. In other centers, however, scalp pH determination remains a frequently used diagnostic tool. Regardless, scalp blood pH determination may, at times, be extremely helpful in the clinical decision-making process. This fetal assessment tool, however, is undeniably invasive and at least moderately uncomfortable for the patient. Therefore, perhaps even more than with most interventions, the clinician must provide the patient and her family with a thorough discussion of the rationale for its use and its potential risks and benefits.
Originally introduced by Saling and Schneider in 1962,19 fetal scalp sampling for pH determination preceded the development of continuous EFM for intrapartum fetal assessment. In current practice, fetal scalp sampling for pH survey typically is reserved for the determination of fetal status when FHR data are ambiguous or nonreassuring. The technique of scalp sampling requires a cooperative patient in either the lateral decubitus or lithotomy position with uterine displacement. The amniotic membranes must be ruptured and the cervix dilated at least 2 to 3 cm to allow the vaginoscopic cone to be placed firmly against the fetal scalp. The area to be sampled is swabbed clean, and silicone gel may be placed at the planned puncture site to facilitate beading of the blood. A 2-mm blade on a long handle is used to make a sharp stab incision. The free-flowing blood is collected in preheparinized capillary tubes. After collection of the blood, one end of the tube is plugged, and pressure is maintained with a swab on the incision site to facilitate hemostasis. Contraindications to fetal scalp sampling include maternal infectious processes (e.g., positive HIV status, hepatitis, active genital herpes simplex virus, positive group B streptococcus status) and maternal coagulation disorders (e.g., hemophilia, possibly idiopathic thrombocytopenic purpura).
Most authorities interpret a scalp pH of greater than 7.25 as reassuring. Values between 7.20 and 7.25 are considered borderline, and the test may be repeated in approximately 15 minutes, after a general re-evaluation of the entire FHR pattern and labor progress. Fetal scalp pH values below 7.20 are nonreassuring and are an indication to proceed with delivery by the most expeditious route. If significant doubt exists about the integrity of the specimen, or if the FHR pattern is patently inconsistent with an abnormal scalp pH value, an immediate repeat determination may be performed as preparations are being made to effect delivery.
Continuous fetal pulse oximetry is an emerging technology for intrapartum fetal assessment. With transcervical placement of an infrared sensor against the fetal cheek, it is possible to monitor fetal oxygen saturation continuously and thus, potentially, identify the truly compromised fetus more accurately. A recent prospective, randomized, multicenter controlled trial of continuous intrapartum fetal pulse oximetry20 showed a greater than 50% reduction in the number of cesarean sections performed for nonreassuring fetal status in the study group. Interestingly, the overall cesarean birth rate was unchanged because of an increase in cesarean birth for dystocia in the study group. Although this promising assessment tool may improve clinicians' ability to identify acidemic and depressed fetuses, further study is needed before there is widespread application of this technology.
Fetal scalp sampling for pH determination, fetal scalp stimulation, and vibroacoustic stimulation should not be considered in a vacuum. These data provide valuable clinical markers of fetal status, but comprehensive intrapartum fetal assessment requires an appraisal of the entire maternal-fetal clinical circumstance, including the stage of labor, the presence or absence of meconium-stained fluid, and the trends evident in the FHR data. Individual patient care is optimized when management decisions reflect the fact that no single parameter (clinical, electronic, or biochemical) is an infallible guide. The accomplished clinician will gather, analyze, and synthesize data that affect all of the pertinent intrapartum forces to maximize the efficiency and outcome of clinical decision making.