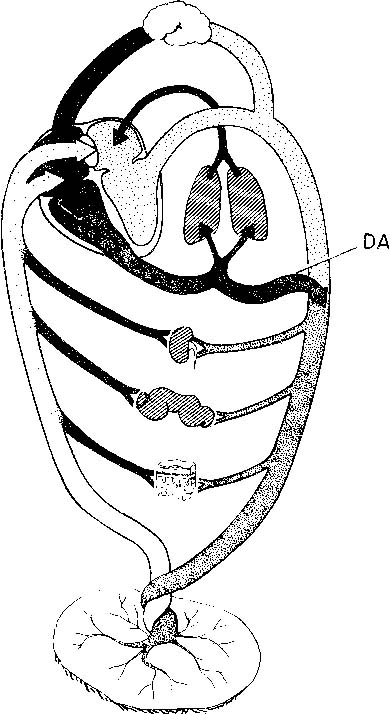
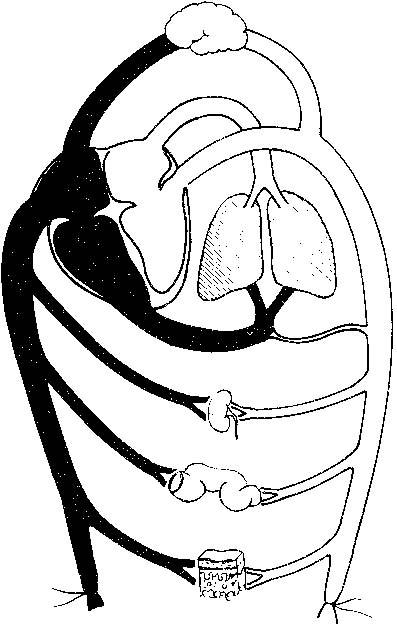
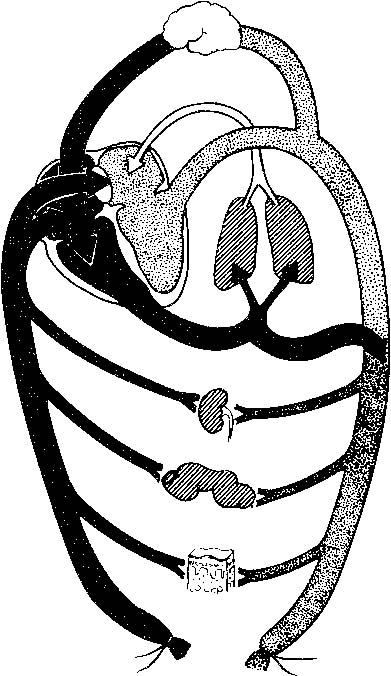
Anticipation, adequate preparation, accurate evaluation, and prompt initiation
of support are critical for a successful neonatal resuscitation. Communication
between the obstetric and the pediatric services can
augment this process of anticipation and adequate preparation immensely. As
an adjunct, a prenatal consult with the family regarding short- and
long-term management should be accomplished, as time permits. Parents
should share their beliefs and be involved in the decision-making
regarding the extent of resuscitation. This is especially critical in
cases of extremely premature infants or infants with a potentially lethal
congenital abnormality diagnosed prenatally. To ensure adequate preparation, ongoing
communication between the obstetric and the pediatric
services is essential. Important perinatal information includes details
of maternal medical condition(s) and subsequent treatment as well
as specific indicators of the fetal condition such as heart rate monitoring, lung
maturity, and antenatal ultrasound findings. Perinatal depression can arise as a result of antepartum, intrapartum, and/or
postpartum problems during which the fetus becomes hypoxic, hypercarbic, and
subsequently acidotic. The result of perinatal depression, regardless
of the inciting event, is that the infant fails to initiate
respirations or the respirations are insufficient to provide adequate
gas exchange. Table 1 lists many of the numerous conditions that place the newly born infant
at risk. TABLE 1. Risk Factors Associated With the Need for Neonatal Resuscitation
Autopartum (Maternal) | Intrapartum | Postpartum |
Diabetes mellitus | Multiple gestation | Apnea |
Hypertension | Nonvertex presentation | Bradycardia |
Substance abuse | Postdates | Respiratory distress |
Vascular disease | Macrosomia/microsomia | Hypoperfusion |
Vasoactive medications | Maternal hypotension | Anemia |
Smoking | Placental abruptio or previa | Congenital anomaly |
Poor maternal weight gain | Cord accidents | Infection |
Myasthenia gravis | Maternal infection | Prematurity |
Sexually transmitted disease | Operative delivery | Birth trauma |
Prior fetal or neonatal demise | General anesthesia | |
No prenatal care | Poly/oligohydramnios | |
Chronic disease states | Fetal anomaly | |
Anemia | Isoimmunization | |
Hemorrhage | Prematurity | |
Maternal age > 35 yr | Prolonged labor | |
Maternal age < 15 yr | Meconium stained amniotic fluid | |
| | Abnormal fetal heart rate pattern | |
| | Maternal sedation | |
Faix RG: Neonatal resuscitation. In Donn SM, Faix RG (eds): Neonatal Emergencies. Mt. Cisco, NY, Futura Publishing, 1991
Equipment Table 2 outlines the equipment necessary for most neonatal resuscitations. As
many as 10% of newly born infant will require active resuscitation. The
necessary equipment should therefore be readily available in
the delivery room for every birth. All equipment should be checked at
regular intervals as well as immediately prior to delivery. The expiration
dates of the medications should be monitored periodically as well. The
personnel responsible for the newly born infant should be familiar
with the organization of the equipment to promptly initiate resuscitation
and provide support. TABLE 2. Neonatal Resuscitation Equipment and Supplies
Suction Equipment
Bulb syringe
Mechanical suction
No. 5, 8, 10 French suction catheters
8 French feeding tube and 20-mL syringes
Meconium aspirator
Wall suction
Bag and Mask Equipment
Infant resuscitation bag with pressure-release valve or pressure manometer; the
bag must deliver 100% oxygen
Face masks in premature and term infant sizes (No. 1, 2, 3) with cushioned
rims
Oral airways
Oxygen source with intact flow meter and tubing
Intubation Equipment
Laryngoscopes with straight blades No. 0—premature
No. 1—term
Extra bulbs and batteries for laryngoscope
Endotracheal tubes 2.5 mm—Infants < 1 kg
3 mm—Infants 1–2.5 kg
3.5 mm—Infants > 2.5 kg
Stylet
Scissors
Gloves
Medications
Epinephrine 1:10,000, 3- or 10-mL ampules
Naloxone hydrochloride 0.4 mg/mL in 1-mL ampules or 1 mg/mL in 2-mL ampules
Volume expanders Whole blood
Fresh frozen plasma
Albumen (5%)/saline solution
Sodium bicarbonate 4.2% (5 mEq/10 mL)
Dextrose 10%, 250 mL
Sterile water 30 mL
Normal saline solution 30 mL
Other Equipment and Supplies
Radiant warmer
Stethoscope
Blood pressure monitor with appropriate cuffs
Adhesive tape
Syringes
Needles
Alcohol sponges
Umbilical catheterization tray
Umbilical tape
Umbilical catheters—3.5, 5 French
3-way stopcocks
5 French feeding tube
Cardiotachometer with electrocardiogram oscilloscope
Pressure transducer and monitor
Pulse oximeter
Modified from American Heart Association/American Academy of Pediatrics: Textbook
of Neonatal Resuscitation. Dallas, 1991
Personnel Many of the risk factors associated with the need for neonatal resuscitation
are known. However, a fetus or newly born infant may experience
unanticipated problems and require intervention to make a smooth transition
to extrauterine life. Thus, an individual trained in neonatal resuscitation
should be present at every delivery. Ideally, at least one
person should solely be responsible for the newly born infant. Such personnel
will vary according to local circumstances. For uncomplicated
resuscitations, it is possible for one person to provide this support. However, if
a high-risk delivery is anticipated in which significant
resuscitative intervention may be necessary, at least one additional
skilled person should be present. In addition, each infant of a multiple
gestation should have separate personnel. It is also imperative that
personnel attending deliveries maintain their resuscitative skills and
familiarize themselves with any new guidelines as put forth by the
American Academy of Pediatrics and the American Heart Association. In
institutions in which resuscitations are uncommon, periodic mock codes
may be helpful. Steps to Resuscitation Figure 4 presents an overview of resuscitation of the newly born infant. The first
step focuses on the prevention of cold stress. Cold stress induces
peripheral vasoconstriction which if persists leads to increasing acidosis. In
turn acidosis is a stimulus for pulmonary vasoconstriction thus
setting the stage for persistent pulmonary hypertension of the newborn
to develop. Cold stress occurs as the newly born infant leaves the
warm liquid environment of the amniotic sac and uterus. Heat loss can
be minimized by several maneuvers. The delivery room should be warm
and free of drafts. The infant should be placed underneath a preheated, radiant
warmer. The infant should be dried off thoroughly with warm
blankets and wet blankets removed immediately. The head is proportionately
larger than the body in newly born infants and is therefore a major
source of heat loss. Therefore, the head should be covered with a hat. Hyperthermia
should be avoided as well because it has been associated
with perinatal respiratory depression. The ultimate goal is a neutral
thermal environment. In this state, the infant maintains a normal
core temperature, yet oxygen consumption is minimal. 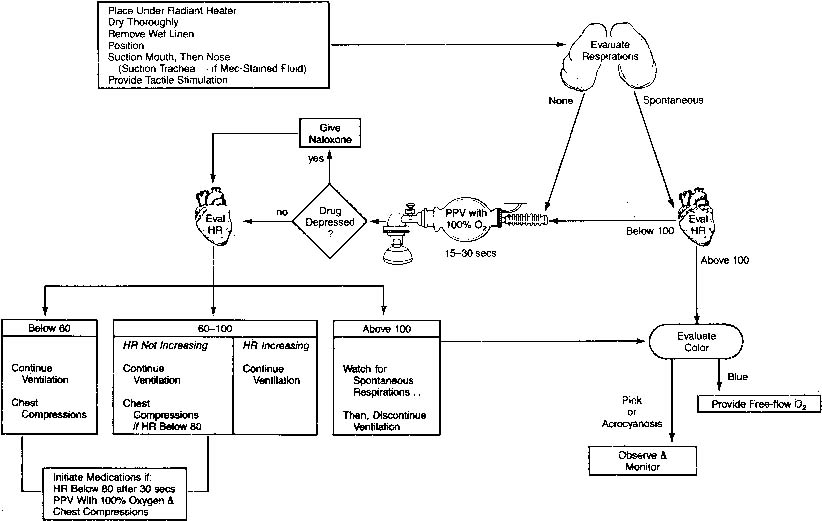 Fig. 4. Overview of neonatal resuscitation in the delivery room. HR, heart rate; PPV, positive
pressure ventilation.(American Heart Association/American Academy of Pediatrics: Textbook of
Neonatal Resuscitation. Dallas, American Heart Association/American Academy
of Pediatrics, 1991) Fig. 4. Overview of neonatal resuscitation in the delivery room. HR, heart rate; PPV, positive
pressure ventilation.(American Heart Association/American Academy of Pediatrics: Textbook of
Neonatal Resuscitation. Dallas, American Heart Association/American Academy
of Pediatrics, 1991)
|
The next step focuses on clearing the airway. This is accomplished by using
either a bulb syringe or a suction catheter. If time permits, the
infant’s nares and mouth should be suctioned after delivery of
the shoulders. If suctioning after delivery is needed, the mouth should
be suctioned first because suctioning of the nose may lead to gagging
and aspiration of oral secretions. In addition, infants are obligate
nose breathers. When the mouth is suctioned, care should be taken to
avoid vigorous suctioning of the posterior pharynx because this may cause
laryngeal spasm and stimulate a vagal response with resultant apnea
or bradycardia, thus delaying the onset of spontaneous breathing. Most newly born infants require only these two steps to initiate adequate
respirations and adapt successfully to extrauterine life. If respirations
are ineffective, tactile stimuli such as gentle rubbing of the
back or flicking of the heels may be added. Oxygen is indicated when cyanosis
is present. Blow by oxygen at a flow of 5 L/min can be administered
via a face mask and flow-inflating bag, an oxygen mask, or a hand
cupped around oxygen tubing. The maximal inhaled concentration of oxygen
is delivered by holding the oxygen source as close to the face as
possible. Although there is some evidence to suggest that 100% oxygen
may not be necessary, current guidelines recommend starting at
that level and weaning as the infant responds (see controversies section). Thus, the
goal of supplemental oxygen should be normoxia. This entire
process of drying, suctioning, and stimulation should take less
than 30 seconds. If an adequate response is not established, the subsequent
steps of active resuscitation should be performed. The ABCs (airway, breathing, circulation) that apply to older children
and adults also apply to the neonate. If adequate respirations are not
established despite drying, suctioning, and stimulation, additional resuscitative
measures should be performed immediately. In fact, if there
is any evidence of perinatal depression, vigorous resuscitation should
be initiated earlier to counteract any hypoxemia or acidemia that
may be present. Four percent of the newly born population requires bag
mask ventilation with supplemental oxygen. The establishment of adequate
ventilation must be emphasized because only a very small percentage
will need chest compressions and medications.16 Indications for positive pressure ventilation administered either via
bag and mask or via endotracheal tube include (1) an insufficient respiratory
pattern manifested by gasping and/or apnea, (2) a heart rate that
remains below 100 beats/min (bpm) for 30 seconds, and (3) persistent
central cyanosis despite administration of 100% oxygen.17 The airway is smaller and located more anteriorly than in older children
and adults. Moreover, because the neonate’s head is proportionately
larger than the body, the neck has a tendency to be flexed. The
ideal position is described as the neutral or “sniffing” position. Both
underextension (flexion) and hyperextension can obstruct
flow through the airway as well as obstruct visualization of the vocal
cords (Fig. 5). A blanket roll placed beneath the shoulder blades may be helpful; however, one
must be careful not to hyperextend the neck. 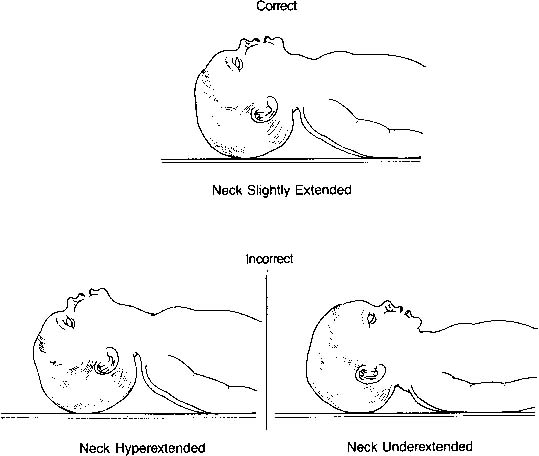 Fig. 5. Correct and incorrect positions for endotracheal intubation.(American Heart Association/American Academy of Pediatrics: Textbook of
Neonatal Resuscitation. Dallas, American Heart Association/American Academy
of Pediatrics, 1991) Fig. 5. Correct and incorrect positions for endotracheal intubation.(American Heart Association/American Academy of Pediatrics: Textbook of
Neonatal Resuscitation. Dallas, American Heart Association/American Academy
of Pediatrics, 1991)
|
Two types of bags are available for neonatal resuscitation. A self-inflating (Ambu) bag
is more convenient to use, especially for less experienced
resuscitators. However, there are some limitations. An oxygen reservoir
must be in place to deliver high concentrations of oxygen, otherwise
mixing with room air occurs, thus limiting oxygen delivery to only 40%. In
addition, the valve assembly in most of these bags
allows oxygen flow only when the bag is compressed or squeezed. Thus, this
type of bag cannot be used for blow by oxygen delivery. A flow-inflating (anesthesia) bag
requires more experienced resuscitators. It requires
continual flow for inflation and is susceptible to mechanical
failure because multiple adjustments need to be considered. The flow of
gas into the gas inlet as well as out through the flow-control valve
must be adjusted. Lastly, a tight seal must be present between the mask
and the infant’s face. At the other end of the spectrum, air
leak syndromes such as pneumomediastinum, pneumothorax, or worse yet, pneumopericardium
may occur because of the high pressures that can be
delivered with this type of bag. The advantages, however, include the
ability to provide a greater range of peak inspiratory pressures and
more reliable control of oxygen concentration. During bag mask ventilation, a tight seal is critical to administer an
adequate amount of positive pressure. First, the right mask size should
be chosen. It should cover the nose and mouth, but the eyes should remain
uncovered. Face masks with an air-filled cushion help to enforce
a tight seal without inflicting facial or ocular injury to the infant. Again, positioning
of the infant is key. If the infant is not in the
neutral position, flow of oxygen and positive pressure through the airway
may be obstructed. In addition, it is helpful to hold the chin to
the edge of the mask because this ensures a tight seal. This maneuver
also elevates the angle of the jaw, which effectively brings the tongue
forward and opens the airway. Positive pressure ventilation should be administered at a rate of 30 to 60 bpm, whether
via bag and mask or via an endotracheal tube. As noted
previously, the first few breaths may require longer inflation times
and a peak inflating pressure of 30 to 40 cm H2O for removal of fetal lung fluid and for alveolar expansion. Subsequent
breaths will likely require less positive pressure. Therefore, the appropriate
inflating pressure is that which is required to expand the
chest and improve gas exchange. This is clinically demonstrated by chest
rise, which should approximate 1/4 to 1/2 cm. If the infant initiates
respirations, positive pressure should gradually be reduced and can
be discontinued when a regular respiratory pattern is established. However, if
chest movement does not occur despite correct infant positioning, an
adequate seal, and an unobstructed airway or if significant peak
inflating pressures are required, intubation should be performed. Prolonged
ventilation often with high inflating pressures will be necessary
if parenchymal pulmonary disease and/or persistent pulmonary hypertension
of the newborn, regardless of the etiology, is present. Visualization of the vocal cords and therefore intubation can be difficult
in the newly born infant. Positioning of the infant is critical to
optimize adequate visualization of this small and anteriorly located
airway. Placing the infant in the neutral position cannot be overemphasized. As
with the face masks, appropriately sized equipment must be used. Laryngoscope
blades are available in two sizes—size 0 for
preterm infants and size 1 for term infants. Table 3 shows the appropriate endotracheal tube size and depth of insertion based
on gestational age and weight. A rough rule of thumb to determine
the distance in centimeters to which the tip should be inserted is to
add the number 6 to the infant’s weight in kilograms. In addition, the
endotracheal tubes for neonates have a dark black line at the
tip. Insertion of the endotracheal tube until this line just passes through
the vocal cords should position the endotracheal tube above the
carina. If a stylet is used, it is important to ensure that the stylet
does not extend beyond the tip of the endotracheal tube because this
may cause airway injury. The laryngoscope blade should be inserted into
the vallecula or onto the epiglottis. The vocal cords should come into
view as the laryngoscope blade is elevated superiorly. Cricoid pressure
may be helpful because the airway is anteriorly located in the neonate. Correct
placement should be checked initially by clinical means
but should be confirmed by chest radiograph as soon as possible. Again, the
chest should rise and fall with ventilation and the movement should
be symmetric. As a correlate, breath sounds should be audible and
symmetric throughout each lung field. It is important to listen not only
anteriorly but also in both axillae. Breath sounds should not be audible
over the stomach, and the stomach should not inflate. Condensation
should be seen within the tube on exhalation. Some also advocate the
use of an end-tidal carbon dioxide detector as a secondary confirmation
of endotracheal intubation. Although false-negative results can occur, false-positive
results are very unlikely.18 If there is any question whether the endotracheal tube is placed appropriately, direct
visualization with a laryngoscope should be performed. The
endotracheal tube should be secured as soon as correct placement
is determined. Chest radiography should confirm that the endotracheal
tube is located between the clavicles and the carina. TABLE 3. Suggested Tracheal Tube Size and Depth of Insertion According
to Weight and Gestational Age
Weight (gm) | Gestational Age (wk) | Tube Size (mm [ID]) | Depth of Insertion From Upper Lip (cm) |
<1000 | <28 | 2.5 | 6.5–7 |
1000–2000 | 28–34 | 3.0 | 7–8 |
2000–3000 | 34–38 | 3.5 | 8–9 |
>3000 | >38 | 3.5–4.0 | >9 |
ID, inner diameter.
Modified from American Heart Association/American Academy of Pediatrics: Textbook
of Neonatal Resuscitation. Dallas, 1994
If the infant’s heart rate is absent or if after 30 seconds of effective
ventilation the heart rate remains below 60 bpm, cardiac resuscitation
should be initiated. Heart rate can be assessed by listening
with a stethoscope over the precordium or feeling for pulsations at the
base of the umbilical cord. External cardiac massage can be provided
by two methods, as depicted in Figure 6. Compressions should be performed on the lower one third of the sternum
immediately above the xiphoid process and should be deep enough to generate
a palpable pulse. In most instances, this depth is one third of
the anterior-posterior diameter of the infant’s chest. The two
thumb-encircling hands technique is preferable because this results
in better peak systolic and coronary perfusion pressure. However, because
the hands surround the thorax with this maneuver, care must be taken
to avoid limiting thoracic expansion during ventilation. As an adjunct, the
most recent American Academy of Pediatrics/American Heart Association
guidelines no longer recommend simultaneous ventilation and chest
compressions. Instead a ratio of 3 compressions to 1 ventilation
should be performed such that 120 events/min occur—90 compressions/min
and 30 breaths/min.17 The infant should be reassessed 30 seconds after initiation of positive
pressure ventilation and external cardiac massage. If the heart rate
remains below 60 bpm, chest compressions should be resumed and resuscitative
medications should be considered. 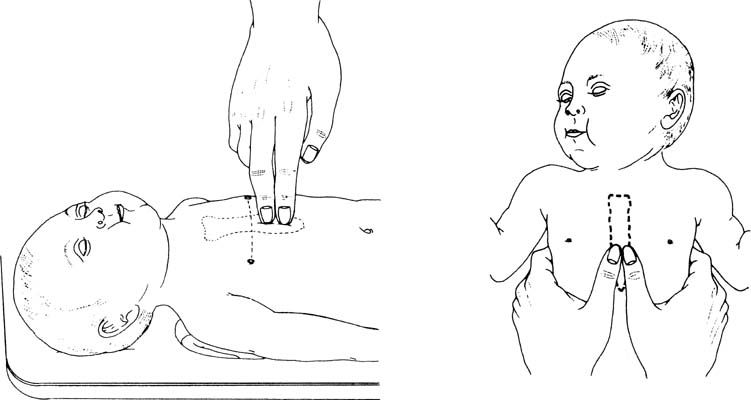 Fig. 6. Chest compression techniques for neonatal resuscitation.(American Heart Association/American Academy of Pediatrics: Textbook of
Neonatal Resuscitation, Dallas, American Heart Association/American Academy
of Pediatrics, 1991) Fig. 6. Chest compression techniques for neonatal resuscitation.(American Heart Association/American Academy of Pediatrics: Textbook of
Neonatal Resuscitation, Dallas, American Heart Association/American Academy
of Pediatrics, 1991)
|
Vascular Access Placement of a peripheral venous catheter can be difficult and time-consuming
in a well neonate. This difficulty is compounded if hypoxia or
acidosis is present. Thus, in a depressed newly born infant, the umbilical
vein presents a rapidly accessible route for the delivery of medications
or volume expanders. The catheter should be inserted to a depth
at which blood flows freely on aspiration, approximately 4 to 6 cm. This
method ensures placement of the catheter below the liver and avoids
potential damage from intrahepatic infusions. If, for some reason, the
umbilical vein cannot be cannulated and peripheral access is unsuccessful, intraosseous
access can be used as an alternative route for medications
or volume expansion.19 Medications and Volume Expansion Bradycardia is often the result of inadequate lung inflation or severe
hypoxia. Thus, adequate ventilation with supplemental oxygen is the most
important step in correcting bradycardia. Drugs are rarely indicated
in resuscitation of the newly born infant. Only if the heart rate remains at or below 60 bpm, despite a minimum of 30 seconds of adequate ventilation and chest
compressions, should medications be administered. Table 4 lists the medications that may be required for neonatal resuscitation
as well as their dosages and route(s) of administration.
TABLE 4. Medications for Neonatal Resuscitation
Click
to View Larger Table
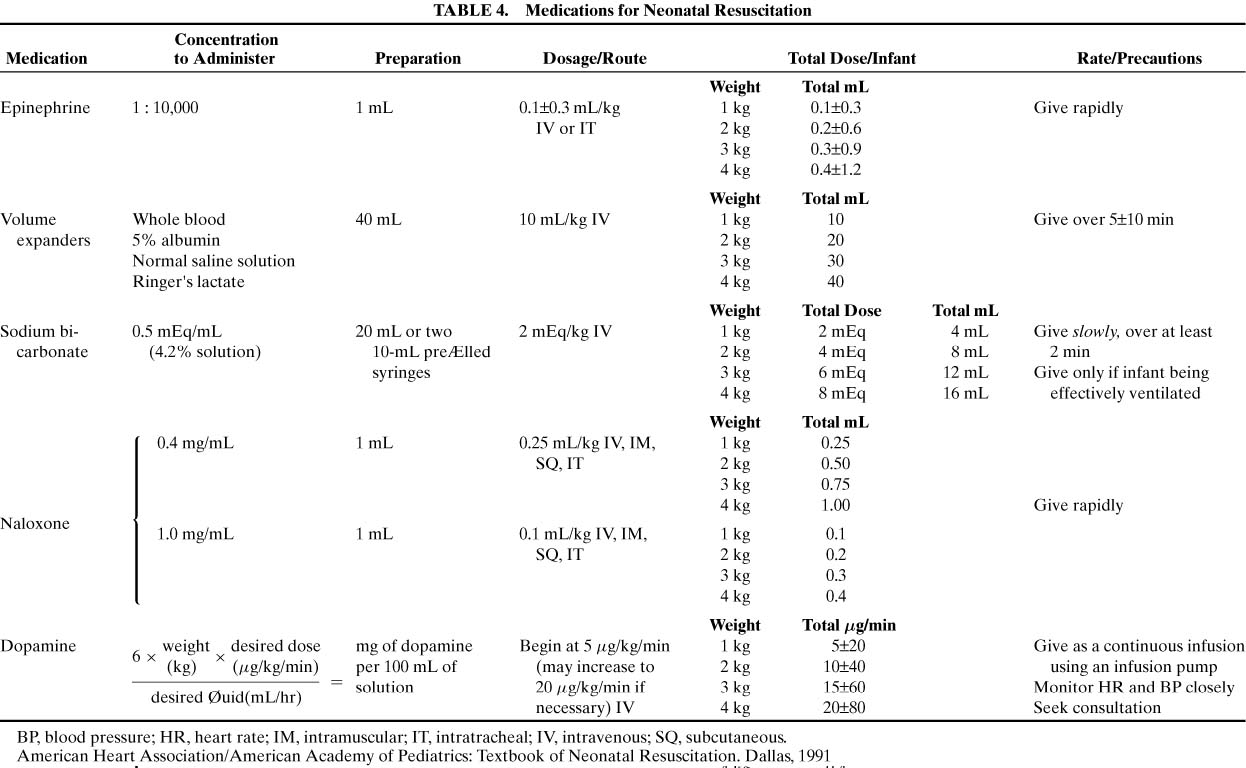
The primary drug used in delivery room resuscitations is epinephrine. Use
of epinephrine is indicated if there is asystole or if the heart rate
remains below 60 bpm after 30 seconds of assisted ventilation and chest
compressions. Epinephrine should be administered in a 1:10,000 dilution
at a dose of 0.1 to 0.3 mL/kg. Although it can be given via an
endotracheal tube, the intravenous route is preferable. If the infant’s
response is suboptimal, epinephrine doses can be repeated every 3 to 5 minutes. Hypovolemia owing to blood loss can prevent adequate response to resuscitation. Blood
loss can occur with placental abruption, placenta previa, cord
accidents, internal hemorrhage, twin-to-twin transfusion syndrome, or
fetal-maternal hemorrhage. Clinical signs of hypovolemia include
weak pulses, poor capillary filling, and low blood pressure but may
not be present until 20% to 25% of the infant’s
blood volume is depleted. Normal saline or Ringer’s lactate solution
should be used at a starting dose of 10mL/kg given over 5 to 10 minutes. Type
O-negative blood may be used instead if large-volume blood
loss needs to be replaced. The dose may be repeated depending on the
clinical response. Sodium bicarbonate should be administered only after metabolic acidosis
is documented despite establishment of adequate ventilation and circulation.20 Moreover, if ventilation is inadequate, respiratory acidosis will compound
the metabolic acidosis as bicarbonate is converted to carbon dioxide. In
a prolonged resuscitation, however, metabolic acidosis is likely
to result owing to ongoing hypoxemia, peripheral vasoconstriction, and
the accumulation of lactic acid. Correction of acidosis may increase
pulmonary blood flow and may enhance the effect of epinephrine. A dose
of 1 to 2 mEq/kg of a 0.5 mEq/mL solution should be infused slowly
over a period of at least 2 minutes. Narcotic drugs used to relieve labor pain are able to cross the placenta. Naloxone, a
narcotic antagonist that does not depress respiratory activity, may
be indicated in a newly born infant whose respiratory depression
is due to maternal narcotic administration within 4 hours prior
to delivery. It is contraindicated if there has been chronic maternal
narcotic use because it may precipitate acute withdrawal symptoms in
the neonate, including seizures. Although there are multiple routes of
administration for naloxone, intramuscular delivery can only be performed
after ventilation and circulation have been established. Repeat
doses of naloxone may be necessary because the duration of action is shorter
than that of the narcotics used for labor analgesia. |