A cordocentesis is performed by the advancement of a needle in the sonographic plane to the targeted puncture site. The technique has two variations: freehand and needle-guided.
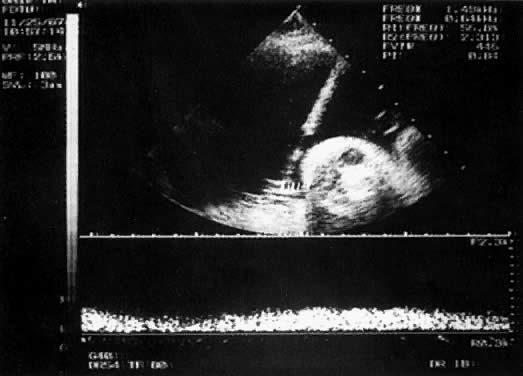
With the freehand technique, the transducer and needle are managed either by one person or by a team of two. If one is working alone, a second person is required to withdraw the sample or to inject through the needle. Because the needle direction can be readjusted laterally, many prefer the freehand to the needle-guided technique. With the needle-guided technique, the needle's travel is limited to one plane (Fig. 1). The needle tract is displayed on the ultrasound screen, permitting precise alignment of the tract with the vessel (Fig. 2).
|
The needles used for cordocentesis are between 20 and 25 gauge. A 20 gauge is used most often for freehand procedures because its stiffness facilitates redirection; however, the risk of bleeding and bradycardia10 can be minimized by the use of a smaller needle. Further, studies of midtrimester amniocentesis suggest that the larger the gauge of the needle, the greater the risk of membrane rupture. Generally, smaller gauge needles are used for needle-guided procedures. A 22-gauge needle is satisfactory for both diagnostic and therapeutic procedures. Choice of needle length depends on the thickness of the maternal abdominal wall, amniotic fluid volume, and sampling site. A 5-inch needle generally is adequate. If necessary, a therapeutic amniocentesis can be performed to facilitate cordocentesis.
Cordocentesis is done on an outpatient basis in a location with close proximity to the delivery suite. A relaxed, informal setting helps to alleviate the mother's anxiety, which tends to improve her cooperation. After reviewing the benefits, risks, and options, an informed written consent is obtained. If the fetus is viable, a nonstress test or ultrasound assessment is performed before the procedure to document the presence or absence of fetal well-being. The mother is placed in a recumbent position with a lateral tilt, and a suitable target site is chosen. Neither local anesthetic nor maternal sedation is needed for diagnostic procedures, but may be employed for therapeutic procedures of any length. We have used 5 to 10 mg diazepam intravenously with good results. Prophylactic antibiotics are unnecessary.
Ideally, the placental umbilical cord origin is chosen for sampling because it is fixed. Alternatively, a midsegment (free loop) may be used. The umbilical vein is preferred because of the lower associated risk of a reactive fetal bradycardia.11 Maternal repositioning may be necessary to improve access to the cord. Hepatic vein12 or cardiac sampling13 are alternatives if cordocentesis is not feasible. Although the transplacental approach may not be avoidable when the placenta is anterior, it increases the risk of fetal-maternal hemorrhage and possible sensitization.14 We specifically seek to avoid the placenta when evaluating a fetus for hemolytic anemia by seeking a free loop to puncture.
The maternal abdomen is prepared aseptically. We use acetone to remove the acoustic coupling gel (unsterile), followed by alcohol and povidone-iodine. The sterile-gloved operator prepares all necessary equipment on a sterile tray and drapes the mother. If a linear transducer or needle-guided technique is being used, an assistant also dons sterile gloves and places the transducer in a sterile sheath. Sterile acoustic coupling gel is applied to the abdomen, and the sampling site is relocated. The needle is inserted and advanced until it abuts the cord. With a controlled thrust, the vessel is punctured. If a free loop is chosen, it is imaged longitudinally and the needle advanced gradually until it pins the cord against either the uterine wall or a fetal body part. It is then punctured with a thrust. The stylet is removed; blood should be seen to fill the hub. At the placental origin, the vessel punctured is identified either by injection of a small amount of saline or blood (flush method) or by measurement of the vessel pressure. Streaming of fluid toward the fetus indicates that the vein has been punctured. With a midsegment puncture, the flush method can prove misleading. When necessary, the fetal heart rate can be monitored by placement of a Doppler gate in the umbilical artery.
If fetal movement is a concern (particularly if the placenta is posterior or whenever a free loop is targeted), immediately upon puncture, 0.3 mg/kg pancuronium (to a maximum of 0.6 mg) is given either intravascularly or intramuscularly into the fetal buttock or thigh with a 25-gauge needle. Neuromuscular blockade persists for 1 to 2 hours. Blood samples are withdrawn with 1-mL syringes because those with a larger barrel create too much negative pressure and can collapse the vein. The sample volume varies with the indication. We have routinely withdrawn 5 to 6 mL after 20-weeks' gestation without apparent sequelae. Heparinized syringes are unnecessary, except for those used for blood gas analysis. The samples are immediately transferred to a container with the appropriate anticoagulant or preservative.
Before the procedure, the operator should determine which studies are desired and should rank them in importance. The samples are taken in rank order in case the needle slips out of the lumen or a complication occurs, causing the procedure to end prematurely. We send a complete blood count at each procedure, using the elevated mean corpuscular volume as an indication that the sample is fetal in origin. We also routinely perform a Kleihauer-Betke stain of the blood smear if the placental cord origin is sampled. Other tests of purity include anti-i agglutination tests, human chorionic gonadotropin determination, and the measurement of factor V and VIII activity,15 but they rarely are necessary.
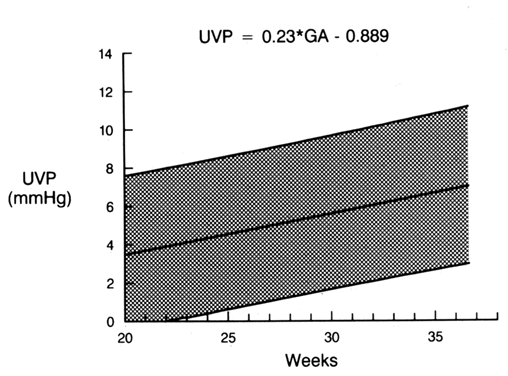
Umbilical venous pressure is measured by attachment of a pressure transducer to the needle. The value obtained should be corrected for amniotic fluid pressure (Fig. 3). This measurement is very useful in evaluating nonimmune hydrops. An elevated value suggests a cardiac etiology. After the needle is removed, the uterine and umbilical puncture sites are observed for bleeding and the duration of any loss is recorded. The fetal heart is viewed to determine whether a bradycardia is present. If the fetus is viable, external fetal heart monitoring is performed for a minimum of 1 hour. If pancuronium has been given, the tracing usually is nonreactive with a mild tachycardia. Before being discharged, the mother is counseled to call if she has contractions, leakage of fluid, bleeding, fever, or uterine tenderness. She is told that infection may present in an indolent fashion, mimicking a viral syndrome with myalgias and a low-grade fever. Activity is not restricted. Arrangements are made for the patient to be contacted with test results. If the patient is Rh-negative, the fetus should be typed and RhoGAM administered when appropriate.
The importance of a team approach cannot be stressed enough. Expert personnel are needed to support the mother through the procedure and to assist the operator (Fig. 4). The laboratories must be familiar with the analysis of small volumes of blood and must recognize the need for efficient and rapid service. Should there be a complication, the team must be familiar with resuscitative procedures and be prepared to move rapidly to perform a cesarean delivery. Likewise, the delivery suite staff should be aware that a procedure is taking place and be ready to provide anesthesia and nursing personnel as necessary.

 OD450 measurements to predict fetuses at risk for anemia. At a given gestational
age, however, there is wide variation in fetal hematocrit and
optical density. It has been estimated that in 2.1%
OD450 measurements to predict fetuses at risk for anemia. At a given gestational
age, however, there is wide variation in fetal hematocrit and
optical density. It has been estimated that in 2.1%