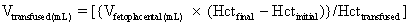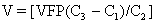In 1963, Liley9,10,11,12 initiated the in utero therapy of Rh hemolytic disease by developing the technique of fetal intraperitoneal transfusion (IPT). During an amniocentesis, Liley inadvertently placed his needle into the fetal abdomen. After returning from Africa, one of Liley's research associates described how IPT was commonly used there to transfuse children with sickle cell anemia. Liley concluded that this could be a feasible route for IUT if the fetal peritoneal cavity could be entered intentionally.13,14 After instilling radiopaque dye into the amniotic cavity by amniocentesis, Liley made use of the fetal ability to concentrate this contrast material in its lower gastrointestinal tract, thereby providing a radiographic target for needle placement. He then employed paper clips fastened with adhesive tape to mark the maternal skin surface. During the next step of the procedure, Liley inserted a 16-gauge Tuohy needle into the fetal peritoneal cavity and advanced an epidural catheter through it to aspirate 200 mL of fetal ascitic fluid and replace it with 100 mL of packed red blood cells. After three fetal deaths, Liley reported his first success in September 1963.11 Virtually simultaneous, though more aggressive attempts to access the fetal circulation directly included hysterotomy-facilitated catheter placement into chorionic plate vessels or into the fetal femoral artery, saphenous vein, or internal jugular vein.15,16,17,18
Before real-time ultrasound was available, IPT involved blindly injecting radiopaque dye into the amniotic cavity. The fetus would then swallow this contrast material, allowing for identification of its peritoneal cavity at fluoroscopy. Often a “chicken-wire” grid molded to the contour of the maternal abdomen was used to ensure accurate needle placement under fluoroscopic visualization.19,20 This technique was later modified to include the direct injection of radiopaque dye into the fetal abdomen.21 The use of static gray-scale ultrasound imaging for placement of the transfusion needle was first reported in 1975, significantly reducing fetal radiation exposure by eliminating the need for pretransfusion amniography.22,23 Real-time ultrasonography to guide the transfusion needle during IPT was first described in 1977.24,25,26 Only a short time later, fluoroscopy was no longer used. Saline, which produces small bubbles on real-time ultrasound when briskly shaken and injected under pressure, was used instead to observe the transfusion needle successfully entering the fetal abdominal cavity.24,25 Bowman and associates27 stressed that the diameter of the epidural catheter is important to the success of the IPT. To avoid side holes with small diameters causing hemolysis during red blood cell infusion, they recommended that the end of the epidural catheter be removed before being used for IPT. They estimated the appropriate transfusion volume for IPT using the following calculation:
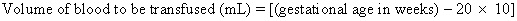
IPT remained the only technique for in utero transfusion therapy until 1981, when Rodeck and co-workers28 performed the first direct fetal intravascular transfusion (IVT) by placing a needle into chorionic plate vessels under fetoscopic visualization. One year later, Bang and colleagues29 successfully transfused a fetus by inserting a needle into the umbilical vein under ultrasound guidance. Seven different techniques for IUT have been reported since then (Table 1). The use of fetoscopy in guiding the IUT needle is described in several studies. This method failed to achieve great popularity.30,31,32,33 Both the direct fetal intravascular approach and fetal intravascular exchange transfusion were evaluated by investigators in the United States during the following 4 years.34,35,36,37 Proponents of exchange transfusion postulated that with this technique the fetus was less likely to become volume-overloaded while more fetal cells were removed from circulation. Advocates of direct IVT believed that the procedure time was shorter in comparison with fetal-exchange IVT. They also asserted that the placental vascular bed would absorb the increased circulatory volume without negatively affecting the fetus. The direct intravascular technique became more widely adopted as experience with both techniques grew. Ultrasound imaging resolution continues to improve, and simple direct IVT has become the procedure of choice at most centers in the United States; IPT alone is performed rarely unless direct access to the umbilical cord is not technically feasible. Transfusion of red blood cells directly into the fetal circulation seems especially important in the treatment of the hydropic fetus whose peritoneal absorption of red blood cells is not inhibited, but it is thought to require several weeks.38,39,40 Comparing neonatal outcome after IVT to that after IPT using historic controls, Harman and co-workers41 clearly demonstrated that IVT significantly improves the chance for survival in the hydropic fetus.
TABLE 1. Techniques for Intrauterine Transfusion
- Intraperitoneal
- Intravascular by cordocentesis
- Exchange transfusion
- Simple direct transfusion
- Exchange transfusion
- Intravascular by intrahepatic venous puncture
- Simple direct transfusion
- Simple direct transfusion
- Intracardiac by fetal cardiocentesis
- Simple direct transfusion
- Simple direct transfusion
- Combined approaches
- Exchange intravascular transfusion followed by intraperitoneal transfusion
- Simple direct intravascular transfusion followed by intraperitoneal transfusion
- Exchange intravascular transfusion followed by intraperitoneal transfusion
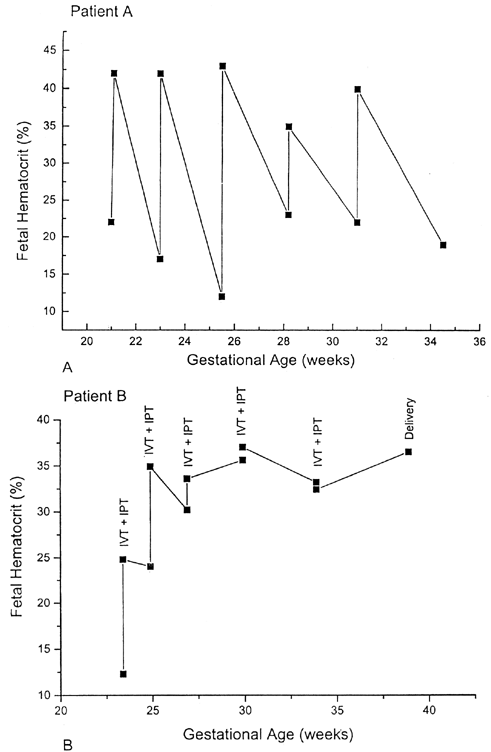
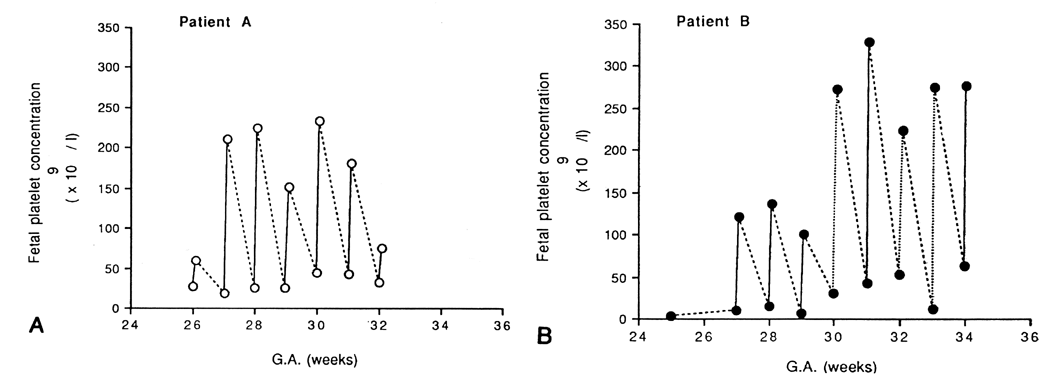
Because complications unique to IVT had been reported, the need for IVT in the severely anemic but nonhydropic fetus remained controversial.42,43,44 Moreover, with the use of direct IVT alone, fetal hematocrits between procedures varied widely. The combination of IVT and IPT therefore was tested at Baylor College of Medicine.45 First, packed red blood cells with a hematocrit of 80% or higher were infused by IVT to increase the fetal hematocrit to approximately 40%. Next, a standard IPT was performed to create a red blood cell reservoir in the fetus for later absorption between IUTs.This combined approach resulted in a more stable fetal hematocrit and longer intervals between procedures (Fig. 1).35,45 As a result of the combined IVT/IPT approach, the average daily decline of the fetal hematocrit was only 0.01% compared with 1.14% when IVT alone was used. Other centers have validated these findings and also use the combined IUT technique.46,47
As an alternate site for IUT, the intrahepatic umbilical vein has been used when access is impossible at the placental cord insertion.48,49,50 Nicolini and co-workers49,50 transfused 72 severely anemic fetuses intrahepatically with a 90% success rate. There were few complications, which consisted mainly of fetal bradycardia and intraperitoneal bleeding. Fetal bradycardia during IUT often is related to inadvertent umbilical arterial puncture and vasospasm.7,51 During intrahepatic transfusion, the incidence of fetal bradycardia was low because of the absence of the umbilical artery at the needle insertion site. Only occasional fetal intraperitoneal bleeding occurred in this series. The extravasated blood was absorbed from the peritoneal cavity in all cases, and there were no adverse fetal effects.49,50
Westgren and co-workers52 described direct fetal intracardiac transfusion (ICT) in patients with severe Rh hemolytic disease. Six patients with evidence of severe erythroblastosis fetalis underwent the procedure at 19- to 31 weeks' gestation. A total of 25 ICTs were completed, with a complication rate of 20%. According to these authors, ICT offers an alternative if direct IVT into the umbilical cord is impossible.52 Using ICT, Harman7 described the resuscitation of five fetuses from exsanguinating hemorrhage after umbilical cord puncture. After the initial ICT was successful, serial IVTs were performed subsequently with good outcome.