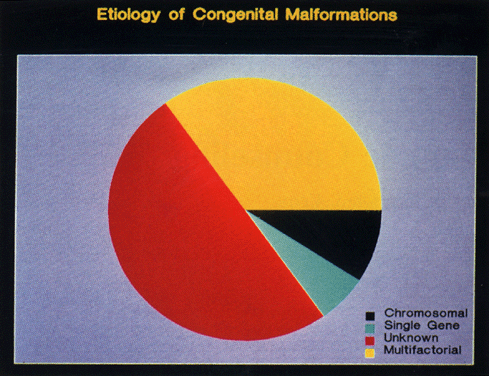
Every physician should be able to take an appropriate history and decide
whether referral for detailed genetic counseling is necessary. In this
section, the types of information that should be ascertained during
the evaluation are presented, with an emphasis on the specific areas
of genetic counseling relevant to the patient presenting for obstetric
or gynecologic care. Common indications for counseling are listed in Table 4. TABLE 4. Common Indications for Genetic Counseling
Pregnancy in a woman age 35 or older, or whose partner is age 45 years
or older
Chromosomal abnormalities in one of the parents
Genetic or congenital abnormality, or mental retardation, in a previous
offspring or other close family member
Diagnosis of heterozygosity in one member (autosomal- dominant or X-linked
disorders) or both members (autosomal-recessive disorders) of a couple
Exposure to a potential teratogen
Multiple spontaneous abortions, unexplained or anomalous stillbirths, or
neonatal deaths
Family history of site-specific cancer
The purpose of genetic counseling is to provide couples with facts about
the genetic disorder of concern to them, help them decide on the best
course of action, and assist them in adjusting psychologically to their
situation. The reproductive choices that are subsequently made should
be the couple's, not the counselor's. The desirability of
undertaking a pregnancy, use of alternative reproductive options (e.g., donor egg, sperm, or embryo), prenatal diagnosis, pregnancy termination, or
sterilization must all be discussed in an informative and, optimally, nondirective
fashion. Nondirective counseling implies that the counselor
will try to remain impartial and objective in providing information. Counselors
must try to avoid even nonverbal messages (facial expressions, ignoring
certain statements of the patient) that indicate
their own biases. Of course, being nondirective does not require the absence
of empathy, understanding, or even suggestion of a course of action
as long as the suggestion is intended to help the couple discover
their optimal choices. Difficult as it may be, physicians and others
undertaking genetic counseling should avoid imposing their own preferences
on patients. Before beginning genetic counseling, an accurate and specific diagnosis
of the condition for which the couple is seeking counseling is necessary. If
the woman is 40 years old and concerned about her chance for a
child with a chromosomal abnormality, counseling can be straightforward. On
the other hand, if a couple has a “retarded” relative
and no additional information is available, very little in the way
of helpful counseling can be offered. Therefore, it is sometimes necessary to postpone counseling until appropriate
medical records can be obtained. Such records should be perused
to determine how the diagnosis was made, and how other potential diagnoses
were excluded. For example, most neural tube defects are of polygenic/multifactorial
inheritance, with a recurrence risk of 2% for parents
with one affected child. However, in some cases an encephalocele
occurs as a component of Meckel's syndrome, an autosomal-recessive
disorder with a 25% recurrence risk. Neural tube defects may also be
secondary to trisomy 13 and thus carry a lower recurrence risk. If secondary
to ingestion of valproic acid, or if present with other anomalies
consistent with amniotic band syndrome, the recurrence risk for a
neural tube defect may be no greater than that of the general population. Physicians
must consider all possibilities before discussing recurrence
risks and potential approaches to prenatal diagnosis. A second requirement for counseling is that the counselor have knowledge
both of the disorder in question and of whether genetic heterogeneity
exists (i.e., whether transmission patterns may differ among families). For example, retinitis
pigmentosa can be transmitted in an autosomal dominant, autosomal
recessive, or X-linked fashion. Counselors should also be familiar
with the concepts of variable expression and nonpenetrance of single
gene disorders and be prepared, if appropriate, to examine crucial
family members. Genetic Counseling of the Prenatal or Preconception Couple A screening questionnaire can be useful (Fig. 2), but it must be reviewed with the patient to be sure she has understood
the questions. One should inquire about the health of the couple, their
first-degree relatives (sibs, parents, offspring), second-degree
relatives (uncles, aunts, nephews, nieces, grandparents), and third-degree
relatives (first cousins). Consanguinity of the couple (relationship
by descent from a common ancestor) may not be volunteered and must
be sought. Abnormal reproductive outcomes (spontaneous abortions, fetal
deaths, and anomalous liveborn infants) should be explored in detail. One
should determine the patient's and spouse's current exposure
to alcohol, drugs, infections, or other potentially toxic agents, as
well as to toxins (e.g., radiotherapy or chemotherapy) in the past.  Fig. 2. Prenatal genetic screening questionnaire. Fig. 2. Prenatal genetic screening questionnaire.
|
Parental ages should be determined, for the most common indication for
counseling and antenatal diagnosis is advanced maternal age. Advanced
paternal age (50 years or older) confers an increased risk for a child
with a new dominant mutation, such as achondroplasia or Marfan's
syndrome, but probably does not increase the risk for a chromosomal abnormality. Specific conditions for which the fetus is at increased risk should be
identified and carrier or prenatal testing offered if available. Risks
of prenatal diagnosis, accuracy, and limitations of the various modalities (e.g., amniocentesis, chorionic villus sampling [CVS], ultrasound) should
be clearly stated. Ethnic origin must be discussed because certain genetic disorders occur
at high frequencies in particular ethnic groups. For some of these conditions, carrier (heterozygote) testing of the potential parents is available (Table 5). If both parents prove to be heterozygous, prenatal diagnosis should
be offered. For example, Ashkenazi Jews are at increased risk for offspring
with Tay-Sachs disease and Canavan disease. The potential parents
should be screened to determine whether they are carriers of either
of these genes and, if so, whether they desire prenatal diagnosis. Prenatal
testing for sickle cell disease, β-thalassemia, and α-thalassemia—common
disorders in the black, Mediterranean, and Asian
populations, respectively—is also available. In the near future, screening
for cystic fibrosis (CF) heterozygotes may be recommended
in some white populations. Currently, however, the CF mutation can at
best be detected in 90% of known heterozygotes even with the use of
multiple probes for the most common mutations. In some ethnic groups (e.g., Latinos, blacks, Asians) the detection efficiency is significantly lower. Therefore, although
carrier testing is appropriate for relatives of
persons with CF, as well as for partners of CF patients who are planning
a pregnancy, general population screening has not yet been accepted. TABLE 5. Ethnic Origin and Heterozygote Screening
Ethnic Group | Genetic Disorders | Screening Test |
Ashkenazi Jews | Tay-Sachs disease | Serum or leucocyte |
| | hexosaminidase |
| Canavan disease | DNA analysis |
Blacks | Sickle cell anemia, | Hemoglobin |
| β-thalassemia | electrophoresis |
Mediterraneans | β-Thalassemia | MCV of <80, hemoglobin |
(Greeks, Italians) | | electrophoresis |
Southeast Asians | α-Thalassemia | MCV of <80, hemoglobin |
and Chinese | | electrophoresis |
Northern European | Cystic fibrosis | DNA analysis (not yet |
Whites | | generally accepted for |
| | population screening) |
MCV = mean corpuscular volume
Finally, all couples should be informed of the general population risk
for a child with a serious anomaly (5%).1 Although prenatal testing is possible for certain disorders, no test can
exclude the possibility of an abnormal child. Counseling the Consanguineous Couple A small number of couples will consult their physician because of concerns
about consanguinity. (Consanguinity is defined as relationship by
descent from a common ancestor). If one of the members of the couple is
the product of a consanguineous relationship or has a close relative
who is, there is no concern so long as the individual in question is
phenotypically normal and does not have an autosomal recessive or other
genetic disorder. A more pressing issue exists if the members of the couple are themselves
closely related. Offspring of first-cousin matings have a two-fold increase
in risk compared to the general population for perinatal and childhood
death, malformations, and mental retardation.16 These figures assume that the couple is phenotypically normal and that
there is no family history of an autosomal-recessive disorder. If a familial
disorder for which carrier testing is possible does exist, or
if ethnic background warrants (see Table 5), heterozygote testing should be performed. If both members are heterozygous
for a recessive trait, the risk to each offspring for the disease
is 25%. If an autosomal recessive disease exists in the family but
testing is unavailable, the couple should be informed that the likelihood
that they share a gene for a recessive familial disorder inherited
from a common ancestor is 1 in 16. If they do, the chance that each child
will inherit that gene from both parents is 1 in 4. The risk is greater
if relatives such as parents or sibs of the couple have the gene
or the disorder in question. Because of the complexity of these issues, couples
who are first cousins or more closely related should be referred
to a geneticist for counseling. Parenthetically, a child who is
the result of a closer mating (e.g., brother-sister, father-daughter, uncle-niece) has a much higher risk of
abnormality.16 Fortunately, as the genetic distance between relatives increases, the likelihood
of carrying identical mutant genes decreases sharply. Consistent
with this is the empiric finding that, as a group, second- and third-cousin
matings are not at increased risk for abnormal offspring. However, if
a genetic disorder does exist in a given family, couples should
be given specific counseling as to their risk for that disorder. Counseling the Couple with a Mentally Retarded Child Counseling should begin with a complete history of the pregnancy and the
affected child. The mother should be questioned regarding intrapartum
illnesses, infection, use of prescription and illicit drugs, and alcohol
ingestion. Details of the delivery, condition of the neonate at birth, presence
of other anomalies in the retarded child, reports of cytogenetic
studies including fragile-X testing, metabolic studies, patterns
of early childhood development and hospitalizations, and the possibility
of early abuse or neglect should be reviewed. If no etiology for
the retardation can be determined, parents can be offered empiric recurrence
risks (Table 6).17,18,19 TABLE 6. Risk for Retardation in Sibs of Retarded Probands
| Proband IQ | Proband IQ |
| 50–69(%) | <50(%) |
Bundey et al18 | 19.7 | 14.5 |
Costeff and Weller17 | 21.6 | 9.5 |
Herbst and Baird19 | 5.4 | 4.2 | Estimates of risk vary among studies, depending in part on completeness
of ascertainment of the population. For example, underascertainment is
likely in a registry study because investigators do not examine sibs; reliance
is placed on routine reporting to the registry. Recurrence
risks are increased for families with more than one retarded child, for
brothers of male index cases, and for sibs of index cases without other
neurologic abnormalities. Unfortunately, in the absence of a specific diagnosis, prenatal diagnosis
is impossible. In this situation parents should be explicitly informed
that amniotic fluid or CVS studies cannot exclude a recurrence. Genetic Counseling in Spontaneous Abortions The couple experiencing multiple spontaneous abortions will be identified
in the course of the routine genetic history cited above. A detailed
medical and family history should be taken, with an emphasis on information
relating to pregnancy losses (e.g., gestational age, complications of pregnancy, maternal health, teratogen
exposure, genotype or phenotype of abortuses, if known). Both genetic
and nongenetic causes should be considered. The first step in counseling couples experiencing fetal loss is education. Many
couples are mistakenly convinced that fetal loss has occurred
as a result of a preventable factor(s) for which they or their physician
are responsible. One should explicitly inform patients that at least 15% of
clinically recognized pregnancies result in fetal loss, and
that loss rates increase with increasing maternal age. Approximately 50% of
abortuses less than 13 weeks' gestational age, and 20% of abortuses
at ages 13 to 26 weeks have chromosomal abnormalities7; prevention of abortion in such situations is impossible and, indeed, undesirable. Empiric risk figures for future pregnancies should be stated. If a couple
has at least one liveborn and one or more pregnancy losses at less
than 13 weeks' gestation, the likelihood of another loss is 25% to 30%.20 This risk increases only slightly with increasing numbers of spontaneous
abortions. If a couple has no liveborns or if their abortus is known
to have been chromosomally normal, the risk rises to a maximum of 40% to 45%.21,22 No evidence supports the old concept that the recurrence risk is only
increased in the face of three spontaneous abortions, or that in such cases the recurrence risk is 80% to 90%. Although relatively infrequent causes of loss overall, structural chromosomal
rearrangements nonetheless play an important role in repetitive abortions. In 2% to 5% of couples experiencing repetitive abortions, either
the female or the male will show a balanced translocation or inversion. If
a couple's pregnancies include not only abortions, but
also fetal deaths or anomalous infants, the likelihood of a parental
rearrangement is higher than if the history includes abortions only. Evaluation
for repetitive abortions is indicated after two or three losses
in the first 4 months of pregnancy. However, the occurrence of an
unexplained fetal death or anomalous liveborn infant necessitates evaluation
irrespective of the number of prior abortions. Optimally, chromosomal
studies should be performed on the abnormal fetus or infant; however, if
this has not been done, parental chromosomal complements should
be determined. If a parental translocation or inversion is detected, referral
to a geneticist is appropriate. Discussions with persons found
to carry chromosomal rearrangements should include counseling regarding
prenatal chromosomal studies (CVS, amniocentesis) in future pregnancies. Other genetic causes of spontaneous abortion, namely mendelian (single
gene) and multifactorial disorders, are doubtless involved in some pregnancy
losses; however, the magnitude of their contribution is unknown. Luteal
phase deficiency, uterine and cervical abnormalities, and maternal
disease and infection are potential nongenetic causes of fetal loss
and should be evaluated if genetic studies are normal. Exposure to
an environmental toxin is only rarely the cause of a spontaneous abortion. The
role of immunologic factors in reproductive loss is currently
under investigation. Genetic Counseling After the Birth of an Abnormal Child CONFIRMATION OF DIAGNOSIS. If possible, the child should be examined by an experienced physician
or geneticist to confirm the diagnosis. If a chromosomal abnormality is
suspected, cytogenetic studies are necessary, even if the clinical findings
seem obvious. A complete family history, as described earlier, should
be elicited. Examination of first-degree relatives may be helpful
if an autosomal dominant disorder is suspected. In cases of an unexplained
or anomalous fetal or neonatal death, the physician should attempt
to obtain photographs as well as autopsy, cytogenetic, biochemical, and
radiographic reports. GENETIC COUNSELING. Initially, the parents may experience denial, shock, bewilderment, grief, fear, or
anger. Counseling at this point is best directed toward explaining
the nature of the infant's disorder and the prognosis, providing
answers to questions, and offering emotional support. This information
should ideally be given by the primary physician, in consultation
with the pediatrician and medical geneticist. The couple may insist
that nothing is wrong with the child, that she looks just like baby
pictures of another (unaffected) member of the family. The counselor(s) should
accept emotional outbursts with sympathy and empathy, while
maintaining professional objectivity. Some couples may benefit from
the support of relatives, social workers, clergy, or formal psychological
counseling. The initial stage of counseling is usually not the appropriate
time for extensive discussion regarding recurrence risks for
future offspring or the possible availability of prenatal testing. The
parents should be told, however, that such information is available. After the initial crisis has passed, more detailed genetic counseling is
required. Timing of subsequent genetic counseling will vary, depending
on the psychological readiness of the couple. Many couples will search
for exogenous, discrete factors that might have caused the abnormal
condition, in the subconscious desire to identify, understand, and control
this factor and thus prevent a similar outcome in future pregnancies. In
this process, there often occurs a tendency to blame the spouse
or oneself. Rarely is the “blame” realistic, as would
be the case, for example, for a disorder known to be associated with a
teratogen or for an inherited autosomal dominant trait. Most couples
can and should be reassured that nothing could have prevented the abnormal
pregnancy. They should be encouraged to view the abnormality as the
result of an unavoidable “accident” of which they are the
victims, not the perpetrators. Appreciation of various psychological defense mechanisms helps one understand
the difficulty some intelligent couples have in comprehending and
recalling genetic information. For this reason, more than one counseling
session may be necessary. A letter to the couple reviewing the factual
information provided during the counseling process is usually helpful. Occasionally
it may be appropriate to hold a genetic counseling
session which includes other members of the family. Antenatal Diagnosis of Fetal Anomalies With the increasing sensitivity of ultrasound diagnosis and the rise in
the number of couples being screened and undergoing prenatal diagnosis, antepartum
detection of anomalies is becoming more common. Once an
abnormality is detected, it is important that the couple understand the
immediate and long-term significance of the fetal defect. Couples often
find consultation with appropriate pediatric specialists (e.g., cardiologists, neurologists, geneticists) useful. Before the stage of
pregnancy at which the fetus would be viable, the option of pregnancy
termination should be discussed (see later discussion). Some couples will
choose to continue pregnancies with known fetal abnormalities, and
they need continued support. The likelihood that a couple will choose
to continue a pregnancy with a chromosomal abnormality is related to
the specific diagnosis and gestational age at the time the diagnosis is
made. Almost all couples with chromosomal abnormalities diagnosed by
CVS choose to terminate an abnormal pregnancy, regardless of prognosis. In
contrast, when a chromosomal abnormality is diagnosed by amniocentesis, pregnancies
with more optimistic prognoses (i.e., sex-chromosome abnormalities) are less likely to be terminated.23 Couples continuing pregnancies with known fetal abnormalities grieve for
the loss of their fantasized normal child and begin to accept and plan
for the birth of a handicapped child. When the diagnosis or prognosis
is uncertain, the remainder of the pregnancy may be especially difficult
for the parents. Pregnancy Termination for Genetic Indications When faced with a prenatal diagnosis of a severe fetal abnormality, many
couples elect pregnancy termination. Particularly after quickening, psychological
sequelae from late-pregnancy termination for genetic indications
can be as severe as those following a stillbirth.24,25 Depression and guilt may be more severe in patients terminating for a
genetic abnormality because the pregnancy is wanted (as opposed to an
elective termination) and the patient has made the active decision to
discontinue the pregnancy (as opposed to fetal demise or stillbirth).24 Grief reactions are common immediately after termination.24,25,26 Even after 6 months, some patients or their partners have not resolved
their grief; in these cases, psychiatric intervention for prolonged grief
reactions may be indicated.26 Although programs providing counseling and follow-up usually exist for
patients with pregnancy loss, stillbirth, or neonatal death, few are available
specifically for patients undergoing pregnancy termination for
genetic indications. Steps should be taken to facilitate grieving in
these patients. The couple may wish to view, hold, name, or bury the
fetus; mementos such as photographs, footprints, and infant clothing can
be offered. Even if the couple say that they do not want such keepsakes, arrangements
can be made to keep them available in case the parents
change their minds. Dilation and evacuation is the most common method of second-trimester pregnancy
termination performed in the United States.27 This method can provide adequate specimens for confirmation in some cases, particularly
if a definite diagnosis has already been made (e.g., chromosomal abnormality, mendelian disorder). In other cases, when an
autopsy is desired, or when the parents want to see and hold the fetus, induction
of labor with prostaglandins is more appropriate. Genetic Counseling and Screening of Gamete Donors The common practice of selecting donors because they have previously had
normal children does little to ensure genetically normal offspring. Even
two persons heterozygous for the same autosomal-recessive trait have
a 75% likelihood that a given child will be phenotypically normal. Gamete
donors should be genetically screened in a manner similar to that
for partners planning a normal pregnancy. Thus, one elicits the health
status of the donor and the first-, second-, and third-degree relatives; spontaneous
abortions, fetal deaths, and anomalous liveborn infants
are noted, as are age and exposure to drugs or toxins. As discussed elsewhere,27,28 absolute genetic grounds for excluding a donor include: - Presence of a disorder resulting from a single mutant gene (mendelian disorder, such
as Marfan's syndrome or retinitis pigmentosa)
- Presence of certain mendelian disorders in a relative, if it is not possible
to exclude the donor as a heterozygote (e.g., Huntington's disease in the donor's parent, Werdnig-Hoffmann
disease in a sib)
- Presence of a chromosomal abnormality in the donor (e.g., balanced translocation or inversion)
- Previous trisomic offspring
- Presence of a serious polygenic/multifactorial disorder (e.g., spina bifida, cardiac anomaly) in the donor or a first-degree relative
- Donor blood group that is incompatible with that of the recipient (e.g., Rh-positive donor and Rh-negative recipient).
These are the same findings that, if detected in couples attempting nonassisted
pregnancy, would warrant genetic counseling. Potential donors
whose ethnic origin is appropriate should additionally be screened for
Tay-Sachs disease, Canavan disease, α-thalassemia, β-thalassemia, sickle
cell disease, or cystic fibrosis. Even if the donor is heterozygous
for one of these disorders, he or she need not be rejected
as a donor in every instance; however, one must be certain that the other
parent is not heterozygous for the same disorder and that the couple
agrees to a heterozygous donor. Screening for many other disorders
is possible, but probably not cost-effective. The desirability of performing
cytogenetic studies to identify donors with balanced translocations, inversions, or
low-frequency aneuploidy is controversial. Such
studies may not be warranted unless the potential donor or a close relative
has a suspicious reproductive history, such as multiple abortions, an
unexplained fetal death, or an anomalous liveborn. In addition to these absolute indications for donor exclusion, various
relative indications could be suggested: - Serious polygenic disorders (e.g., cleft lip) in a donor's second- or third-degree relative
- Less serious polygenic disorders in the donor (e.g., mild hypertension)
- Advanced donor age (greater than 40 years for a male donor or 34 years
for a female donor)
- Prior exposure to mutagens, such as radiation therapy or chemotherapy
- Unexplained stillborn or anomalous liveborn offspring born to a donor or
a near relative, if one does not wish to perform cytogenetic studies
on the donor.
Other reasons for exclusion might be proposed. For example, persons with
human leukocyte antigen (HLA) DR3 are at increased risk for Addison's
disease. Offspring of donors with HLA-DR3 have a 50% likelihood
of inheriting the parental DR3 allele. However, given the number of such
HLA-disease associations, if we were to exclude donors for such reasons, the
supply of acceptable donors might quickly be exhausted. Thus, the
goal of screening should not be to select a genetically “perfect” donor—an impossibility—but rather to eliminate
donors whose likelihood of producing genetically abnormal offspring
is considerably increased. Recipient couples should be counseled that
despite appropriate screening of donors, it is impossible to guarantee
normal offspring. Late-Onset Disease Some genetic disorders do not manifest until adult life, sometimes after
reproduction has occurred. One example is a woman who has a mutated
breast cancer gene (BRCA1 or BRCA2) and is at increased risk for breast and ovarian cancer. Because the mutation
is inherited in autosomal dominant fashion, each child has a 50% chance
of inheriting it.29 Presymptomatic testing and prenatal diagnosis are now possible. Persons
who request such testing are best referred to centers that have experience
in the issues of accuracy of the available tests and their predictive
value. Careful consideration should be given to the potential benefits
and liabilities for the individual patient in knowing she is a
carrier of the gene in question. Medical, psychological, and economic
consequences must be explored, as well as issues of confidentiality.30,31 |