Most gynecologic and radiation oncologists in the United States report
their results using the International Federation of Gynecology and Obstetrics (FIGO) staging
system. The initial staging system was proposed
by a subcommittee of the League of Nations in 1929 and was revised in 1937 and 1950. FIGO
accepted the League of Nations staging in 1950. The
current clinical staging system of FIGO has changed minimally since 1962. Ureteral
obstruction was found to have an adverse effect on survival, and
this was reflected in the 1971 FIGO modifications.14 The FIGO classification was again changed in 1974: with a better understanding
of the effect of an occult lesion on prognosis, frankly invasive
cancers were removed from stage IA. Stage IV was also subdivided into
cases with bladder or rectal invasion. Stage IVB was assigned to patients
with distant metastases. In 1974, the Society of Gynecologic Oncologists (SGO) proposed the definition
of microinvasion as stromal invasion 3 mm or less below the basement
membrane and absence of capillary lymphatic (C/L) space involvement.15 As will be noted, microinvasive carcinoma of the cervix has been poorly
defined in the past and is still a focus of persistent controversy. Most of the changes in FIGO staging in the past decade have occurred in
stage I disease. In 1985, FIGO defined stage IA as “preclinical
invasive carcinoma, diagnosed by microscopy only.” Stage IA was
subdivided into IA1 (minimal microscopic stromal invasion) and IA2 (tumor
with invasive component 5 mm or less taken from the base of the epithelium
and 7 mm or less horizontal spread).16 C/L space involvement did not exclude patients from being placed in stage
IA.16 In 1995, FIGO further reclassified stage I cervical cancer. Stage IA was
subdivided by depth of stromal invasion in an attempt to delineate the
different clinical behaviors and treatments for carcinoma with invasion
of less than 3 mm and less than 5 mm. C/L space involvement does
not alter the stage but should be recorded. Stage IB was subdivided into
lesions less than or greater than 4 cm.17,18Figure 3 shows a bulky barrel 6-cm cervical carcinoma currently staged as IB2. These
carcinomas are sometimes treated with external beam irradiation, one 72-hour
cesium, and extrafascial hysterectomy (Fig. 4). The complete 1995 FIGO staging is shown in Table 1. As in the 1985 FIGO staging system, extension to the corpus is disregarded. 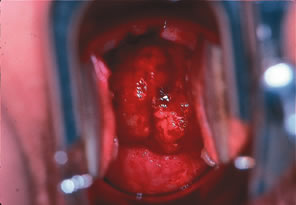 Fig. 3. Large bulky barrel IB2 carcinoma of the cervix at time of diagnosis. Fig. 3. Large bulky barrel IB2 carcinoma of the cervix at time of diagnosis.
|
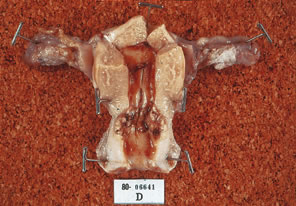 Fig. 4. Hysterectomy specimen of a 49-year-old treated with a IB2 cervical carcinoma
by radiation and completion extrafascial hysterectomy. Note residual
tumor in specimen at area of endocervix. Fig. 4. Hysterectomy specimen of a 49-year-old treated with a IB2 cervical carcinoma
by radiation and completion extrafascial hysterectomy. Note residual
tumor in specimen at area of endocervix.
|
Table 1. 1995 FIGO Staging for Carcinoma of the Cervix
Stage | Description |
0 | Carcinoma in situ, intraepithelial carcinoma |
I | Carcinoma is strictly confined to the cervix (extension to the corpus should
be disregarded). |
IA | Invasive cancer identified only microscopically. All gross lesions even
with superficial invasion are stage IB cancers. Invasion is limited to
measured stromal invasion with maximum depth of 5 mm and no wider than 7 mm. (The
depth of invasion should not be more than 5 mm taken from
the base of the epithelium, either surface or glandular, from which
it originates. Vascular space involvement, either venous or lymphatic, should
not after the staging.) |
IA1 | Measured invasion of stroma no greater than 3 mm in depth and no wider
than 7 mm |
IA2 | Measured invasion of stroma greater than 3 mm and no greater than 5 mm
and no wider than 7 mm |
IB | Clinical lesions confined to the cervix or preclinical lesions greater
than stage IA |
IB1 | Clinical lesions no greater than 4 cm |
IB2 | Clinical lesions greater than 4 cm |
II | The carcinoma extends beyond the cervix but has not extended to the pelvic
wall. The carcinoma involves the vagina but not as far as the lower
third. |
IIA | No obvious parametrial involvement |
IIB | Obvious parametrial involvement |
III | The carcinoma has extended to the pelvic wall. On rectal examination, there
is no cancer-free space between the tumor and the pelvic wall. The
tumor involves the lower third of the vagina. All cases with hydronephrosis
or a nonfunctioning kidney are included unless they are known
to be due to other causes. |
Stage IIIA | No extension to the pelvic wall |
Stage IIIB | Extension to the pelvic wall and/or hydronephrosis or nonfunctioning kidney |
Stage IV | The carcinoma has extended beyond the true pelvis or has clinically involved
the mucosa of the bladder or rectum. A bullous edema as such does
not permit a case to be allotted to stage IV. |
Stage IVA | Spread of the growth to adjacent organs |
Stage IVB | Spread to distant organs |
FIGO, International Federation of Gynecology and Obstetrics.
Stage IA carcinoma should include minimal microscopically evident stomal
invasion as well as small cancerous tumors of measurable size. Stage
IA should be divided into those lesions with minute foci of invasion
visible only microscopically as stage IA1 and macroscopically measurable
microcarcinoma as stage IA2 to gain further knowledge of the clinical
behavior of these lesions. The term “IB occult” should
be omitted.
The diagnosis of both stages IA1 and IA2 cases should be based on microscopic
examination of removed tissue, preferably a cone, which must include
the entire lesion. The lower limit of stage IA2 should be measurable
macroscopically (even if dots need to be placed on the slide prior
to measurement), and the upper limit of stage IA2 is given by measurement
of the two largest dimensions in any given section. The depth of
invasion should not be more than 5 mm taken from the base of the epithelium, either
surface or glandular, from which it originates. The second
dimension, the horizontal spread, must not exceed 7 mm. Vascular space
involvement, either venous or lymphatic, should not alter the staging
but should be specifically recorded, as it may affect treatment decisions
in the future.
Lesions of greater size should be classified as stage IB.
A patient with a growth fixed to the pelvic wall by a short and indurated
but not nodular parametrium should be assigned to stage IIB. It is
impossible, at clinical examination, to decide whether a smooth and indurated
parametrium is truly cancerous or only inflammatory. Therefore, the
case should be placed in stage III only if the parametrium is nodular
on the pelvic wall or if the growth itself extends to the pelvic
wall.
The presence of hydronephrosis or nonfunctioning kidney due to stenosis
of the ureter by cancer permits a case to be assigned to stage III even
if, according to the other findings, the case should be assigned to
stage I or II.
The presence of bullous edema, as such, should not permit a case to be
allocated to stage IV. Ridges and furrows in the bladder wall should be
interpreted as signs of submucous involvement of the bladder if they
remain fixed to the growth during palpation (i.e., examination from the
vagina or the rectum during cystoscopy). A finding of malignant cells
in cytologic washings from the urinary bladder requires further examination
and biopsy from the wall of the bladder.
A parallel TNM staging system has been proposed by the American Joint Committee
on Cancer (AJCC).19 The AJCC criteria for the various stages are defined and compared in Tables 2 and 3. All histologic types are included. Unfortunately, the TNM classification
is impractical for staging cervical carcinoma because lymphatic or
intra-abdominal spread cannot be reliably evaluated clinically. Cervical
cancer is one of the few female genital cancers that is not surgically
staged. Table 2. Comparison of Cervical Cancer Staging
AJCC Primary | FIGO Tumor (t) | Description |
TX | | Primary tumor cannot be assessed |
T0 | | No evidence of primary tumor |
Tis | 0 | Carcinoma in situ |
T1 | | Cervical carcinoma confined to uterus (extension to corpus should be disregarded) |
T1a | Ia | Preclinical invasive carcinoma, diagnosed by microscopy only |
T1a1 | Ia1 | Minimal microscopic stromal invasion |
T1a2 | Ia2 | Tumor with invasive component 5 mm or less in depth taken from the base
of the epithelium and 7 mm or less in horizontal spread |
T1b | Ib | Tumor larger than T1a2 |
T2 | II | Cervical carcinoma invades beyond uterus but not to pelvic wall or to the
lower third of vagina |
T2a | IIa | Without parametrial invasion |
T2b | IIb | Parametrial invasion |
T3 | III | Cervical carcinoma extends to the pelvic wall and/or involves lower third
of vagina or causes hydronephrosis or nonfunctioning kidney |
T3a | IIIa | Tumor involves lower third of the vagina, no extension to pelvic wall |
T3b | IIIb | Tumor extends to pelvic wall or causes hydronephrosis or nonfunctioning
kidney |
T4* | IVa | Tumor invades mucosa of bladder or rectum and/or extends beyond true pelvis |
Regional lymph nodes (n) |
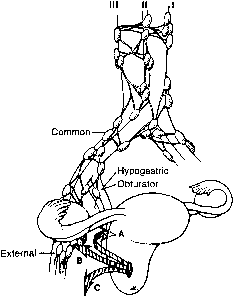
(Regional lymph nodes include paracervical, parametrial, hypogastric, obturator, common, internal and external iliac, presacral and sacral.) |
NX | | Regional lymph nodes cannot be assessed |
N0 | | No regional lymph node metastasis |
N1 | | Regional lymph node metastasis |
Distant metastasis (m) |
MX | | Presence of distant metastasis cannot be assessed |
M0 | | No distant metastasis |
M1 | | IVb Distant metastasis |
AJCC, American Joint Committee on Cancer; FIGO, International Federation
of Gynecology and Obstetrics.
*Presence of bullous edema is not sufficient evidence to classify a tumor
T4.
Table 3. AJCC Stage Grouping for Carcinoma of the Cervix
| Primary | Regional | Distant |
Stage | Tumor | Lymph Nodes | Metastases |
0 | Tis | N0 | M0 |
IA | T1a | N0 | M0 |
IB | T1b | N0 | M0 |
IIA | T2a | N0 | M0 |
IIB | T2b | N0 | M0 |
IIIA | T3a | N0 | M0 |
IIIB | T1 | N1 | M0 |
| T2 | N1 | M0s |
| T3a | N1 | M0 |
| T3b | Any N | M0 |
IVA | T4 | Any N | M0 |
IVB | Any T | Any N | M1 |
AJCC, American Joint Committee on Cancer.
Microinvasion The controversy regarding staging for squamous cell microinvasion has persisted
since the earlier definitions, which were primarily of European
origin.20–23 Microinvasive carcinoma cannot be defined by biopsy. A conization (or
hysterectomy) is needed with 12 or more sections of the cone to rule out
foci of deeper invasion. Microinvasive adenocarcinoma is a clinical
entity that is not well defined. No data are available to suggest the
efficacy of less-than-radical treatment for this entity. In 1983, van
Nagell and colleagues 24 described a group of patients who had radical hysterectomy and pelvic
lymph node dissections and added their series to the then-available data
from the literature. With stromal invasion of less than 3 mm, only 1 of 397 patients
had positive pelvic nodes; 8 of 98 patients with invasion
of 3.1 to 5 mm had positive nodes. Since that time, several studies
have questioned the 1985 FIGO staging because of the relatively high
incidence of lymph node metastases in the group of patients with invasion
of 3.1 to 5 mm.25–27 Burghardt and associates28 evaluated 486 patients with stage IA lesions, subclassified into 1985 FIGO
IA1 and IA2. They concluded that although IA1 lesions could be treated
conservatively, the treatment in stage IA2 lesions must be individualized. These
worldwide observations, prompted by the SGO definition, led
in part to the 1995 FIGO revisions for stage IA. The issue of C/L space involvement has yet to be resolved for FIGO stage
IA. Earlier studies, such as that of Roche and Norris,29 suggested that C/L involvement had little impact on node metastases when
patients had microinvasion. The group from M. D. Anderson, however, noted
in their review that of patients with less than 3 mm of invasion
and no C/L space involvement, 0.3% had pelvic node metastases; 2.6% of
those with C/L space involvement had pelvic node metastases.30 Lymph vascular space involvement appears to be a predictor of lymph node
metastases. Most gynecologic oncologists in this country continue to
use the 1974 SGO definition and will treat only patients with less than 3 mm
of invasion and no C/L invasion by conservative surgery.27,30 The evaluation of treatments based on the 1995 FIGO definition, with C/L
space taken into consideration, will be determined by future studies. The
importance of C/L space involvement and the question of whether
these data should be incorporated into the staging system of stage IA
needs to be settled.31 However, many Europeans see little need for even the 1995 changes, and
as recently noted by Burghardt and colleagues,32 the FIGO classification was never meant to be a treatment guideline. Clinical Staging Cervical carcinoma is predominantly a Third World problem. Thus, only certain
diagnostic studies are allowed by FIGO, and these tests are available
in most countries. A few caveats about clinical staging should
be remembered: - A biopsy, not a cervical cytologic smear, is necessary to establish the
diagnosis.
- The physical examination should include a survey of the skin, careful palpation
of lymph node-bearing areas, speculum examination, and a bimanual
rectovaginal examination.
- Only the procedures and studies allowed by FIGO can be used in clinical
staging.
- Once the stage is determined, it cannot be changed. For instance, a woman
who has clinical stage IB and has a metastatic para-aortic node detected
at the time of radical hysterectomy still has stage IB disease.
- Patients seen after treatment initiation should be listed as having unstaged
cervical carcinoma.
- If cancer remains after therapy has been completed or if invasive cancer
is documented within 6 months of treatment conclusion, the patient still
has the original stage disease, but it is classified as “persistent” in
most institutions.
Clinical staging is often inaccurate: 17% to 24% of patients with clinical
stage I and 30% to 50% of those with presumed stage II have more advanced
disease when surgical staging is done.33,34 Examination under anesthesia (EUA), particularly when performed by a gynecologic
oncologist and a radiation oncologist at the same time, may
increase the accuracy of clinical staging by 25%.35 EUA allows a more thorough visual inspection of the upper vagina and a
better bimanual examination of the parametria. Patients who have a large-volume
tumor and require cystoscopy or proctoscopy may benefit from
simultaneous EUA. EUA for staging is used in about 46% of patients who
have cervical cancer in the United States,36 but because the procedure is relatively expensive, and because of managed
care and cost containment, this figure will no doubt decrease in the
future. FIGO allows the following surgical procedures for staging: cervical biopsy
or conization, endocervical curettage, EUA, cystoscopy, and proctoscopy. Biopsy-proven
bladder or rectal involvement is uncommon in early-stage, small-volume
tumors. However, if the tumor is positioned anteriorly
or posteriorly, one of these two studies may be indicated. If proctoscopy
or cystoscopy (bullous edema) is abnormal, a biopsy must be
done and be positive to place the patient in a stage IVA category. The
Patterns of Care Study clearly noted a decrease in the use of cystoscopy (from 38% to 25%) and
proctoscopy (from 30% to 19%) in patients with
stage IB, IIA disease.36 This retrospective study did not evaluate tumor volume as a criterion
for performing or not performing these endoscopic procedures. Pretreatment blood or serum studies may lead the physician to perform more
sophisticated radiographic studies to exclude more advanced disease. An
elevated creatinine level suggests urinary tract involvement. Elevated
liver function tests suggest liver involvement, and an elevated
serum calcium level suggests bone involvement. Radiographic Studies, Routine FIGO allows a chest x-ray, intravenous pyelogram (IVP), barium enema, and
skeletal x-ray (not a bone scan) to be used for staging. Although metastatic
spread to the lung is rare in early-stage disease, it is present
in up to 5% of patients with more advanced disease.10 A chest x-ray should be obtained in all patients. If a solitary nodule
is found, histologic evaluation by needle aspiration, or preferably resection
by video-assisted thoracotomy or open biopsy (especially in tobacco
users), is needed. We have noted a few cervical cancer patients
with a second small solitary primary lung cancer who benefited from surgical
removal of the lung lesion. IVP studies are less expensive than
computed tomography (CT) scans and are abnormal in about 15% of patients.37 Almost one third of patients have an abnormal IVP with stage III disease. IVP
studies are not routinely indicated for women with small (less
than 2 cm) cervical lesions who are scheduled for radical hysterectomy. An
upper gastrointestinal (UGI) series should be ordered only for symptomatic
patients. The Patterns of Care Study noted that only 21% of
patients with stage IIB through IVB had a barium enema done.36 A barium enema should be ordered only in older women to help identify
other colon disease, such as diverticulosis or colon cancer. Radiologic
skeletal surveys are rarely ordered, and isotope bone scans should be
used for symptomatic patients. Most of the lesions in bone are lytic
and can be detected by chest x-ray and IVP. Radiographic Studies, Advanced Although the information from these procedures cannot be used to change
the FIGO stage, they are very useful in treatment planning. As noted
by Heller and associates,38 the lymphangiogram may be a more sensitive test to establish para-aortic
node metastases than the ultrasound or CT scan, particularly in women
who are at low risk for metastases to these nodes. The authors in this
Gynecologic Oncology Group (GOG) study suggested that a negative lymphangiogram
may be adequate to eliminate surgical staging in a select
group of patients with small-volume tumors. In this prospective study, all 320 patients
with negative studies had para-aortic node dissections. Although
the lymphangiogram may be more useful than CT in detecting
disease in nodes that are smaller, it is often painful and difficult
to perform in morbidly obese patients or those with pedal edema. CT scans are inaccurate for determining parametrial invasion. However, if
the nodes are larger than 1.5 cm, para-aortic node metastases can be
detected in as many as 91% of women.39 CT scans should be done with intravenous contrast to give optimal information (Fig. 5). However, CT scanning is seldom beneficial in evaluating patients with
small-volume disease.40 The specificity for detecting lymph node metastases is high, but the sensitivity
is low.38,41 CT is correct in evaluating 92% of lesions staged IIIB through IVB.42–44 Evaluation of enlarged lymph nodes should be complemented by CT-guided
fine-needle aspiration. The results of these tests cannot be incorporated
into FIGO staging. 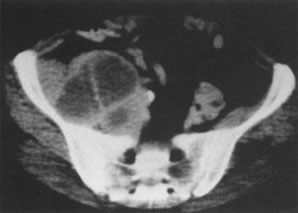 Fig. 5. Computed tomography with contrast in a 44-year-old woman. This necrotic
node deviating the ureter medially at the pelvic brim was not palpable
in this patient with a stage IB (1 cm) adenosquamous cell carcinoma
of the cervix. Fig. 5. Computed tomography with contrast in a 44-year-old woman. This necrotic
node deviating the ureter medially at the pelvic brim was not palpable
in this patient with a stage IB (1 cm) adenosquamous cell carcinoma
of the cervix.
|
The GOG study showed that ultrasound had a sensitivity of only 19% for
detecting metastases to para-aortic nodes.38 This indicates that the more expensive CT or magnetic resonance imaging (MRI) studies
may have more utility. Ultrasound can be used safely in
pregnancy and can be used to evaluate the upper urinary tract. Transrectal
ultrasound has been used to evaluate parametrial extension. The
sensitivity of transrectal ultrasound in this evaluation was only 78%, with
a specificity of 89% and diagnostic accuracy of 87%.45 One group has reported that transvaginal color Doppler ultrasound might
aid in the diagnosis of early-stage disease.46 This technique is expensive. Endosonography produces superior results
compared to CT in assessing local extension.47 These authors performed the procedure, which consisted of vesical, vaginal, and
rectal endosonography, under anesthesia. At our institution, the cost of imaging a cervical cancer patient with
abdominopelvic MRI is about $1,225 versus $1925 for a similar study by
CT. MRI should be considered in the evaluation of pregnant patients with
invasive cervical cancer. In the past few years, studies in the literature
have suggested that MRI is superior to CT in delineating the
primary tumor site, tumor dimensions, and the local extent of disease.48–50 Static images enhanced with gadolinium—diethylenetriamine—penta-acetic
acid (Gd-DIPA) may not improve staging accuracy but may be
useful in selected patients to confirm tumor necrosis or stage IVA disease.51,52 MRI may not be as useful as CT in detecting nodal metastases; however, in
centers with expertise, it may offer more useful information than
conventional CT or ultrasound. In a recent study, pretreatment MRI in
patients with cervical tumors larger than 2 cm decreased the number of
other diagnostic tests ordered, the number of invasive procedures, and
the number of unnecessary surgeries; thus, it was considered cost-effective
in this setting.53 Routine use of contrast-enhanced MRI is still controversial. MRI, like
CT, cannot be used for FIGO staging. Positron emission tomography (PET), a new technique, is based on the decay
of radionuclides and the emission of positively charged particles (positrons). These
positrons penetrate short distances and combine with
an electron, converting mass into energy. The emission of gamma rays
is scanned to reconstruct a three-dimensional representation of tissue
events.54 Different radionuclides can be used to evaluate tumor metabolism and organ
function. PET needs further evaluation, but it has the potential
for delineating primary disease more accurately. It may more precisely
localize nodal disease. Fine-needle aspiration should be used to confirm the suspect imaging studies. If
the results are positive, radiation treatment fields may merit
change (i.e., extended fields for positive para-aortic nodes).55 If results are negative, a decision must be made for surgical staging. Fine-needle
aspiration is of particular use in aspirating suspect lung
lesions, liver lesions, or suspected bone metastases. CT- or MRI-directed
aspiration of nodal masses that yields positive results does not
change FIGO staging. Current Serum Studies, Advanced The so-called serum tumor markers include squamous cell carcinoma antigen (SCCA), carcinoembryonic antigen (CEA), and CA-125. CEA levels have
not been found to correlate with clinical stage and are seldom used as
a marker for cervical cancer.56 CA-125 levels are elevated in some patients with advanced or recurrent
cervical adenocarcinoma.57,58 However, neither CA-125 nor CEA measurement is used in the routine management
of cervical cancer patients. The SCCA level has been found to be elevated in patients with large-volume
tumors, deep stromal invasion, and nodal metastases and correlates
with treatment response.57,59 Also, one recent study suggests that the combination of SCCA and CEA measurement
may help identify patients needing neoadjuvant chemotherapy
in advanced squamous cell carcinoma.60 Another recent study suggests that patients with elevated CA-125 and SCCA
levels may have more blood vessel involvement in stage IB, IIA disease, and
that such patients should be considered for treatments other
than radical surgery.61 Bolger and colleagues62 noted a higher incidence of positive nodes in 220 patients treated surgically
when the SCCA level was elevated. Elevated SCCA level had no independent
prognostic significance. The National Institutes of Health and the National Cancer Institute held
a Consensus Development Conference in Cervical Cancer in April 1996. One
of their conclusions was that the “optimal role for imaging
studies in defining the extent of disease and in planning radiation therapy
needs further investigation, as does the measurement of serum tumor
markers in patients with invasive cervical cancer.”63 Surgical Staging: Celiotomy Clinical staging for cervical cancer is inaccurate. Investigators in the 1970s
and 1980s began using surgical staging primarily to assess the
status of para-aortic nodes. Some patients with occult para-aortic node
metastases are potentially curable when extended-field irradiation
is added. The earlier studies of para-aortic node sampling, usually to
the level of the inferior mesenteric artery, noted a relatively high
incidence of node positivity, increasing as the clinical stage was more
advanced (Table 4).64–70 Thus, of patients with stage III disease who received only pelvic irradiation, about
one third would receive inadequate irradiation. This progressively
higher incidence of positive para-aortic nodes prompted such
organizations as the GOG to require para-aortic node sampling before
placing patients with advanced cervical cancer on phase 3 studies if
imaging modalities did not suggest “suspicious nodes.” Table 4. Incidence of Para-aortic Node Metastases by Stage in Patients
with Cervical Carcinoma, 1977-1981
| Stage | IIA | Stage | IIB | Stage | III |
Author | No. | % | No. | % | No. | % |
Ballon64 | 16 | 19 | 32 | 19 | 24 | 16.7 |
Bonanno65 | 23 | 4 | 73 | 12 | 52 | 31 |
Buchsbaum3 | 4 | 0 | 15 | 6.7 | 104 | 32.7 |
Chung34 | 15 | 6.7 | 17 | 17.6 | 14 | 42.9 |
Hughes66 | 35 | 8.5 | 45 | 24.4 | 96 | 23.9 |
Lagasse33 | 22 | 18.2 | 58 | 32.8 | 63 | 30.2 |
Nelson67 | 16 | 12.5 | 47 | 14.9 | 39 | 38 |
Sudarsanam68 | 21 | 14 | 22 | 18 | 19 | 26.3 |
Welander69 | 222 | 22.7 | 41 | 19.5 | 38 | 26.3 |
Wharton70 | 10 | 0 | 47 | 21.2 | 34 | 32.3 | Also, patients with large, bulky stage IB lesions are treated in some institutions
with only pelvic field irradiation. Two studies have noted
positive para-aortic nodes in these large stage IB lesions in 20%64 and 35%71 of patients. Podczaski and associates72 noted a 14% incidence of positive para-aortic nodes in stage IB cervical
cancer patients, but 21 of 35 total patients had bulky cervical disease. Serious bowel complications have been noted in cervical cancer patients
who have had operative evaluation through a transperitoneal approach
followed by radiation therapy. In a series of patients who had transperitoneal
evaluation of para-aortic nodes, Piver and Barlow73 reported that 21.7% died of radiation-induced bowel injuries. In a similar
group of patients who had transperitoneal para-aortic node sampling
followed by para-aortic node irradiation, Wharton and colleagues70 noted that in 27.6% of the patients, severe intestinal complications occurred
later; half the patients with intestinal complications died. In
a retrospective study, Berman and associates74 noted that of 33 patients operated on by a transperitoneal approach, 30% required
operative intervention for later small bowel obstruction. Weiser
and colleagues75 prospectively analyzed two surgical approaches to para-aortic nodes: transperitoneal
and extraperitoneal. No significant differences were found
between the two techniques in the sensitivity to detect nodal metastases
or the incidence of surgical complications. If the patients had
later irradiation, however, both bowel obstruction and other regional
enteric injuries were significantly more common in the transperitoneal
group. To avoid these complications, an extraperitoneal approach has
been advocated. EXTRAPERITONEAL APPROACH. Schellhas,76 using an upper midline incision, was one of the first to report an extraperitoneal
approach to para-aortic nodes. He noted few complications
using this small incision (7 to 8 cm), and pelvic portal irradiation
could be used without delay. One disadvantage is that it is difficult
using this incision to remove the left para-aortic nodes, particularly
in obese patients. We previously used and reported on an extraperitoneal
midline incision to remove pelvic and para-aortic nodes.77 Although no significant complications were noted in this small series, and
easy access to both sides of the aorta was obtained, we have abandoned
this as a staging procedure because of the delay in initiating radiation
therapy. A modification of the extraperitoneal inguinal incision was described in
the gynecologic literature by Berman and associates.74 This is actually a modified Gibson incision. The group from UCLA prefers
to make the skin incision on the left because the left para-aortic
lymphatic channels are lateral and posterior to the aorta. They argue
that it is technically more feasible to dissect the precaval nodes from
a left-sided incision if a single incision is to be used for extraperitoneal
para-aortic node sampling. We prefer to make the incision on
the right. To gain access to the node-bearing areas, an incision is initiated just
cephalad to the umbilicus and 3 cm medial to the right iliac crest (Fig. 6). The three fascial layers are incised (Fig. 7). The lateral anastomotic branches of the deep circumflex and musculophrenic
arteries and associated veins must be avoided. Exposure of the
extraperitoneal space is achieved by rolling the peritoneum medially and
cephalad (Fig. 8). The para-aortic area is exposed by blunt dissection and node sampling
is done, removing the precaval fat pad over the vena cava. With a Deaver
retractor on the medial and cephalad portion of the incision, para-aortic
nodes can be sampled to the level of the inferior mesenteric
artery, directly anterior and lateral to the aorta. 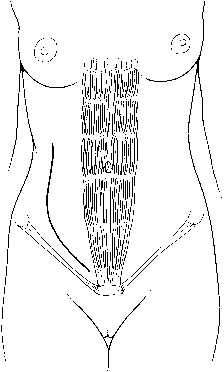 Fig. 6. The J-shaped incision. After being initiated superior to the umbilicus, the
skin incision is carried parallel to the inguinal ligament, just 2 cm
medial to the pubic tubercle.(Gallup DG: Extraperitoneal approach to paraaortic nodes. In Gallup DG, Talledo
OE [eds]: Surgical Atlas of Gynecologic Oncology, pp 107–124. Philadelphia, WB Saunders, 1994.) Fig. 6. The J-shaped incision. After being initiated superior to the umbilicus, the
skin incision is carried parallel to the inguinal ligament, just 2 cm
medial to the pubic tubercle.(Gallup DG: Extraperitoneal approach to paraaortic nodes. In Gallup DG, Talledo
OE [eds]: Surgical Atlas of Gynecologic Oncology, pp 107–124. Philadelphia, WB Saunders, 1994.)
|
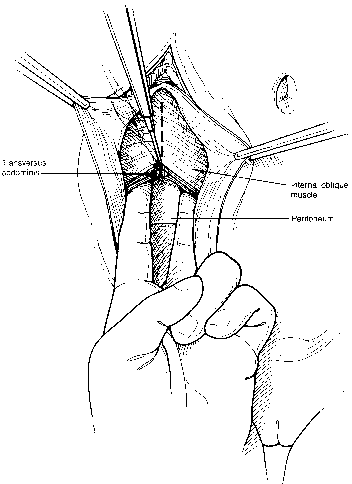 Fig. 7. The external oblique fascia is retracted with small clamps. The internal
oblique and transversus are then sectioned with cautery. The surgeon's
fingers push the peritoneum posteriorly while the incision is
made, thus protecting the underlying peritoneum.(Gallup DG: Extraperitoneal approach to paraaortic nodes. In Gallup DG, Talledo
OE [eds]: Surgical Atlas of Gynecologic Oncology, pp 107–124. Philadelphia, WB Saunders, 1994.) Fig. 7. The external oblique fascia is retracted with small clamps. The internal
oblique and transversus are then sectioned with cautery. The surgeon's
fingers push the peritoneum posteriorly while the incision is
made, thus protecting the underlying peritoneum.(Gallup DG: Extraperitoneal approach to paraaortic nodes. In Gallup DG, Talledo
OE [eds]: Surgical Atlas of Gynecologic Oncology, pp 107–124. Philadelphia, WB Saunders, 1994.)
|
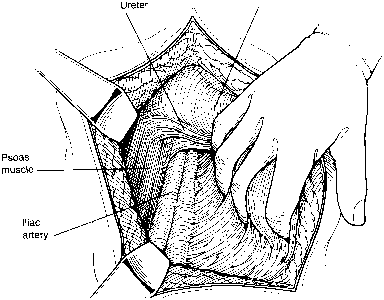 Fig. 8. The peritoneum is bluntly dissected by the surgeon's hand; this maneuver
is facilitated by sectioning and ligating the round ligament. The
psoas muscle and external iliac artery are palpated, and the peritoneum
is gently swept from lateral and caudad to medial and cephalad. The
ureter remains on the medial leaf of the retracted peritoneum.(Gallup DG: Extraperitoneal approach to paraaortic nodes. In Gallup DG, Talledo
OE [eds]: Surgical Atlas of Gynecologic Oncology, pp 107–124. Philadelphia, WB Saunders, 1994.) Fig. 8. The peritoneum is bluntly dissected by the surgeon's hand; this maneuver
is facilitated by sectioning and ligating the round ligament. The
psoas muscle and external iliac artery are palpated, and the peritoneum
is gently swept from lateral and caudad to medial and cephalad. The
ureter remains on the medial leaf of the retracted peritoneum.(Gallup DG: Extraperitoneal approach to paraaortic nodes. In Gallup DG, Talledo
OE [eds]: Surgical Atlas of Gynecologic Oncology, pp 107–124. Philadelphia, WB Saunders, 1994.)
|
In the left-sided approach, left para-aortic node dissection is more easily
accomplished. Berman and colleagues74 described lifting the peritoneum from the underlying vena cava to resect
the precaval fat pad. They caution that gentle traction must be used
when dissecting the precaval nodes to avoid injuring the inferior mesenteric
vessels. The inferior vena cava lies posterior to the aorta. We
have had technical difficulties in removing precaval nodes through
a left-sided approach. Also, avulsion of the inferior mesenteric artery
has been reported using the left-sided J-shaped incision.64 In some patients, the lower portion of the J-shaped incision may lie in
future radiation fields. Because of the potential delay in irradiation, we have subsequently used
a supraumbilical, transverse “sunrise” incision (Fig. 9).78,79 The incision is carried laterally in a downward fashion to the level of
the iliac crests. In the event of palpable, bulky disease in the lower
common iliac or pelvic nodes, the incision can be carried in a caudad
fashion to remove the nodes. (The advantage of pelvic node debulking
in cervical cancer patients who were later irradiated has been reported
by Downey and associates.80) The fascia is then incised in a transverse manner and the rectus muscles
are transected. The transversus muscle is incised, and the incision
in the transversus is carried caudad while the surgeon retracts the
peritoneum medially. The surgeon's hand is inserted deep into the
caudad portion of the incision (Fig. 10). In thin patients, the para-aortic nodes lateral and anterior to the
aorta can be removed by a right abdominal approach. However, if exposure
is limited, the peritoneum must be mobilized from the left side, as
previously described. 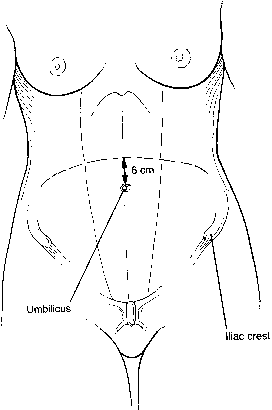 Fig. 9. In the center, the “sunrise” incision is about 6 cm above the
umbilicus. The bifurcation of the aorta can be assessed preoperatively
by computed tomography scanning with a radiopaque object at the level
of the umbilicus. Thus, the site of the incision can be varied.(Gallup DG: Extraperitoneal approach to paraaortic nodes. In Gallup DG, Talledo
OE [eds]: Surgical Atlas of Gynecologic Oncology, pp 107–124.Philadelphia, WB Saunders, 1994.) Fig. 9. In the center, the “sunrise” incision is about 6 cm above the
umbilicus. The bifurcation of the aorta can be assessed preoperatively
by computed tomography scanning with a radiopaque object at the level
of the umbilicus. Thus, the site of the incision can be varied.(Gallup DG: Extraperitoneal approach to paraaortic nodes. In Gallup DG, Talledo
OE [eds]: Surgical Atlas of Gynecologic Oncology, pp 107–124.Philadelphia, WB Saunders, 1994.)
|
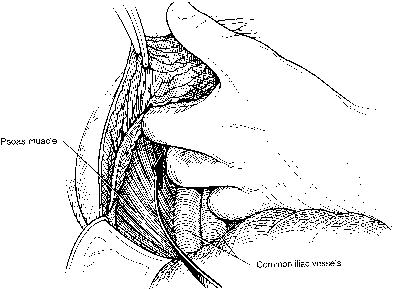 Fig. 10. Once the external iliac vessels are palpated, the peritoneum is bluntly
dissected from lateral and caudad to medial and cephalad, separating
it from the underlying common iliac vessels and the aorta and vena cava.(Gallup DG: Extraperitoneal approach to paraaortic nodes. In Gallup DG, Talledo
OE [eds]: Surgical Atlas of Gynecologic Oncology, pp 107–124. Philadelphia, WB Saunders, 1994.) Fig. 10. Once the external iliac vessels are palpated, the peritoneum is bluntly
dissected from lateral and caudad to medial and cephalad, separating
it from the underlying common iliac vessels and the aorta and vena cava.(Gallup DG: Extraperitoneal approach to paraaortic nodes. In Gallup DG, Talledo
OE [eds]: Surgical Atlas of Gynecologic Oncology, pp 107–124. Philadelphia, WB Saunders, 1994.)
|
Using this technique, patients with negative nodes had a mean number of 12.2 removed
from the para-aortic area.79 All but two patients underwent external beam irradiation within 2 weeks (Fig. 11). 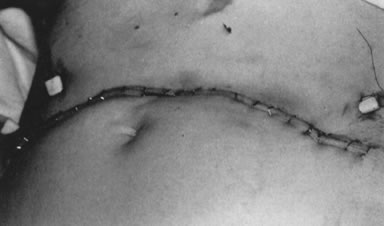 Fig. 11. “Sunrise” incision on first postoperative day in a patient
with stage IIIB carcinoma of the cervix. Note position of drain sites, far
removed from radiation fields. She began external beam irradiation
on postoperative day 7. Fig. 11. “Sunrise” incision on first postoperative day in a patient
with stage IIIB carcinoma of the cervix. Note position of drain sites, far
removed from radiation fields. She began external beam irradiation
on postoperative day 7.
|
Surgical staging may offer a survival advantage in a few select patients, primarily
because of occult distant metastases and local failures.81 Operative morbidity in patients who are good surgical candidates is low, but
intraoperative vascular injuries, use of blood transfusions, and
hematoma formation requiring re-exploration has been reported.38,82 A recent report noted the rare recurrence of skin metastases near a drain
site of a patient who had surgical staging for stage IIB cervical
cancer.83 LAPAROSCOPIC APPROACH. Experience with laparoscopic removal of nodes is accumulating. In one of
the early reports from this country, laparoscopy was followed by laparotomy
in patients with cervical cancer scheduled for radical hysterectomy
and node dissection, and in patients weighing less than 200 lb, 98% of
the nodes had been removed through the laparoscope.84 The mean yield for para-aortic nodes removed bilaterally was 6.3 nodes. A
subsequent report by Spirtos and colleagues85 revealed a mean bilateral para-aortic node count of 7.9. In this series
of 40 patients, 2 patients had to be explored to control bleeding. Recio
and associates86 reported the use of laparoscopic staging for large (more than 5 cm) stage
IB2 cervical cancers: a mean number of 7 para-aortic nodes were removed, all
patients were discharged from the hospital within 24 hours, and
all began irradiation within 1 week of surgery. Para-aortic lymph node resection through the laparoscope for cervical cancer
staging has been reported from other countries, with a similar mean
number of nodes removed.87,88 In a report from Taiwan, 43% of patients who had macroscopic invasion
of para-aortic nodes did not have suspicious nodes on CT scanning.88 One major concern is that laparoscopic para-aortic node sampling is a transperitoneal
technique. Although animal studies suggest that adhesions
occurring in pigs undergoing extraperitoneal lymphadenectomy are similar
to those undergoing transperitoneal laparoscopy, none of these animals
were irradiated.89 Another major concern is the occurrence of vena caval injuries requiring
laparotomy, even in the hands of experienced laparoscopists.90 The pressure needed for adequate visualization can result in gas embolism
in the presence of a large vena caval laceration. Further, scattered
reports suggest that laparoscopy can disseminate otherwise isolated
pelvic disease in cervical cancer patients. A report from the University
of Alabama noted squamous cell carcinoma in an umbilical trocar site.91 Pastner and Damien92 reported a similar occurrence, and Cohn and colleagues93 reported a patient with a stage IIB cervical adenocarcinoma who developed
intraperitoneal spread after laparoscopic lymphadenectomy. Clearly, para-aortic lymphadenectomies can be safely done by expert laparoscopists
in scattered areas of this country. The GOG is conducting
a study of open removal of pelvic and para-aortic nodes plus abdominal
hysterectomy versus laparoscopic node sampling plus laparoscopic-assisted
vaginal hysterectomy. This LAP-2 study has slow accrual, perhaps
because many gynecologic oncologists are not qualified to remove para-aortic
nodes through the laparoscope. Investigators reporting the use
of this procedure emphasize that it is an evolving technique with a learning
curve.84–88,90 SCALENE NODE SAMPLING. The issue of sampling left supraclavicular nodes has been raised, particularly
in patients with positive para-aortic nodes. In one study of 72 patients
with disease of various stages, no patient had metastatic
disease in the removed scalene fat pad.94 Ketcham and associates95 found that only 6 of 108 patients had scalene node metastases when the
nodes were nonpalpable. Even in patients with positive para-aortic nodes, others
have reported a similarly low rate of metastases.96,97 Before scalene node biopsy is done, a chest CT should be performed to
rule out metastases in patients with nonpalpable nodes. If the para-aortic
nodes are positive, scalene node biopsy could give information useful
for subsequent treatment planning. |