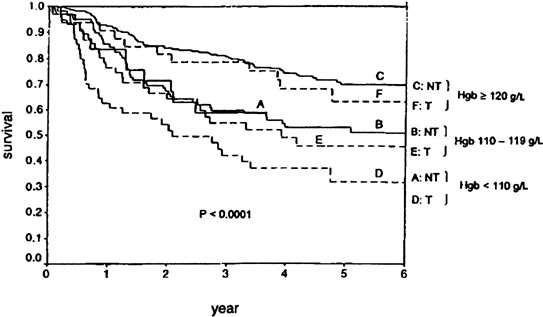
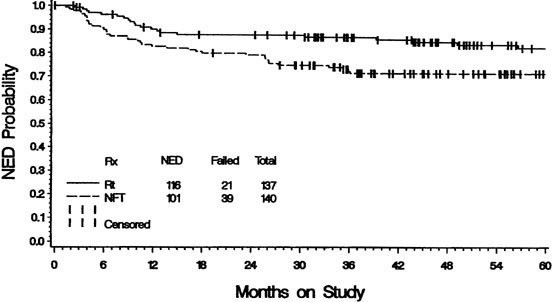
Radiation therapy for intact carcinoma of the cervix usually involves a
combination of EBRT and brachytherapy (usually ICRT). The goal of treatment
is to balance EBRT and brachytherapy in a way that maximizes the
likelihood of local-regional tumor control while minimizing the risk
of treatment complications. For many patients with local-regionally advanced
disease, these treatments must also be carefully integrated with
chemotherapy to achieve the greatest likelihood of cure. The primary goals of EBRT are to sterilize regional disease and to shrink
central tumor to facilitate subsequent brachytherapy. The degree of
tissue penetration achieved by a radiation beam is related to the energy
of the x-rays delivered from the radiation source. One of the earliest
sources used for EBRT was 60Co, which produced a relatively deeply penetrating beam. However, linear
accelerators now provide higher energy photon beams (15 to 25 MV) that
are better suited for EBRT because they permit more homogeneous delivery
of radiation to deep tissues with relative sparing of superficial
tissues. In ICRT for cervical cancer, radioactive sources placed in the uterine
cavity are used to deliver a very high dose to the cervix and uterus with
relative sparing of surrounding tissues, such as the bladder, rectum, small
bowel, and superficial soft tissues. Because the radiation dose
is proportional to the square of the distance from a source of radiation, tissues
close to a source will receive a much higher dose than
those farther away. The therapeutic advantage of ICRT always depends
on the ratio of the distance between the sources and the tumor and the
distance between the sources and critical normal tissues. Radium was
first used to treat uterine malignancies shortly after its discovery at
the turn of the century and was convenient because of its very long
half-life (Table 4). However, because the daughter product of radium—radon gas—can
pose a radiation protection problem, 137Cs, which provides similar energy, has now replaced radium in many practices. TABLE 4. Radioisotopes Commonly Used in Treatment of Cervical Cancer
| | | | Photon Energies (MeV) | |
Name | Symbol | Half-Life | Range | Average | Comments |
Cobalt |
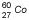
| 5.26 years |
1.17, 1.33 |
|
Commonly used for EBRT in the past; occasionally used in HDR or LDR brachytherapy |
Cesium |
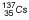
| 30.0 years |
0.662 |
|
Most commonly used source for LDR ICRT |
Radium |
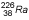
| 1600 years |
0.047–2.45 |
0.83* | Most commonly used source for LDR ICRT before 1980s; potential for leakage
of radon gas led to gradual replacement |
Iridium |
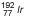
| 74.2 days |
0.136–1.06 |
0.38 | Most commonly used source for gynecologic interstitial brachytherapy and
for HDR ICRT |
EBRT, external-beam radiation therapy; ICRT, intracavitary radiation therapy; LDR, low-dose–rate; HDR, high-dose–rate
* Average γ energy with 0.5-mm platinum filtration encasing source
Khan FM: The Physics of Radiation Therapy. Baltimore, MD: Williams & Wilkins, 1994:
Low-dose–rate ICRT with cesium or radium provides an additional
therapeutic advantage by permitting recovery of sublethal injury to normal
tissues during the course of irradiation. In more recent years, computer
technology has made it possible to deliver ICRT at high dose rates (more
than 100 cGy/min) using high-activity sources, usually 192Ir. These approaches are discussed in further detail in the Dose Rate section. Radiation Therapy Planning EBRT is used to shrink bulky endocervical tumors to bring them within the
range of the high-dose portion of the ICRT dose distribution (Fig. 1), to shrink exophytic tumors that distort anatomy, and prevent optimal
brachytherapy, and to sterilize disease (paracentral and nodal) that
lies beyond the reach of the ICRT system. 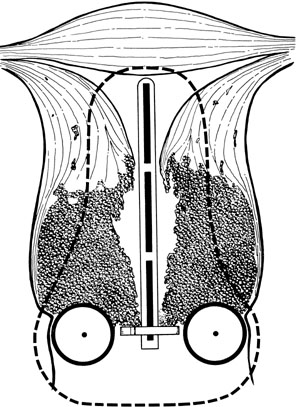 Fig. 1. Invasion of the myometrium of the uterine isthmus, producing a bulky barrel-shaped
lesion. The dotted line represents the volume that would typically
receive a minimum of 30 Gy from a 48-hour intracavitary treatment. Tumor
cells in the periphery of the tumor may receive a dose inadequate
to achieve central tumor control. An initial course of external-beam
radiation therapy (EBRT) may shrink central disease and bring it
within the volume treated to high dose with the intracavitary system.(Fletcher GH: Textbook of Radiotherapy, p. 479. Philadelphia, Lea & Febiger, 1966.) Fig. 1. Invasion of the myometrium of the uterine isthmus, producing a bulky barrel-shaped
lesion. The dotted line represents the volume that would typically
receive a minimum of 30 Gy from a 48-hour intracavitary treatment. Tumor
cells in the periphery of the tumor may receive a dose inadequate
to achieve central tumor control. An initial course of external-beam
radiation therapy (EBRT) may shrink central disease and bring it
within the volume treated to high dose with the intracavitary system.(Fletcher GH: Textbook of Radiotherapy, p. 479. Philadelphia, Lea & Febiger, 1966.)
|
Patients with bulky central disease usually begin treatment with a course
of EBRT. Some practitioners (e.g., Perez and colleagues28) prefer to maximize the brachytherapy component of treatment and deliver
the first ICRT treatment as soon as EBRT has shrunk the tumor sufficiently
to permit good placement of an intracavitary system (with bulky
tumors, this may still require 40 Gy or more of EBRT).28 Subsequent irradiation of the pelvic lymph nodes is done using anterior
and posterior fields with a block that shields central structures that
received the greatest dose from ICRT. Other radiation oncologists opt
to treat most patients with large central lesions with an initial EBRT
dose of 40 to 45 Gy to the whole pelvis. The first approach (brachytherapy as soon as possible) delivers a greater
proportion of the central dose with brachytherapy and may reduce the
volume of bladder and rectum treated to a high dose; however, with this
approach, more reliance is placed on the extremely complex match between
the three-dimensional dose distribution from the ICRT system and
the edge of the central block in the external fields. Initial treatment
of the whole pelvis to a total dose of 40 to 45 Gy provides a homogeneous
distribution to the entire region at risk for microscopic disease
and may produce somewhat more shrinkage of central disease before
ICRT. In fact, both approaches have been in use for several decades and, when
optimally employed, appear to yield excellent tumor control rates
with acceptable complication rates. However, it is probably best not
to exceed a radiation dose of 40 to 45 Gy to the whole pelvis because
higher doses have been associated with higher complication rates without
improvement in pelvic disease control.29 Patients who have only microinvasive disease are most often treated with
simple hysterectomy. However, medical problems or morbid obesity may
complicate surgery, and in such cases, ICRT alone yields good results. Some
patients with very small stage IB disease (less than 1 cm) may
also be treated with ICRT alone, particularly if there are relative contraindications
to a course of EBRT.30 Patients who have relatively small lesions and a narrow vagina should
be treated with ICRT first to optimize the brachytherapy portion of treatment
before EBRT causes additional narrowing of the vaginal apex. A
total dose (EBRT plus ICRT) of 50 to 55 Gy appears to be sufficient to
sterilize microscopic disease in the pelvic nodes in most patients. Nodes
known to contain gross disease and heavily involved parametria should
be treated with additional EBRT with a small boost field to a total
dose of 60 to 65 Gy. External Beam Radiation Therapy Technique For most patients with cancer of the cervix, relatively high beam energies (15 to 25 MV) are
preferred because they spare superficial tissues
that are not usually at risk for disease. When lower energy beams (e.g., 60Co or 6 to 10 MV) are used, multiple-field techniques (usually anterior, posterior, and
lateral fields) should be used to reduce the dose to
subcutaneous tissues (Fig. 2). Treatment technique and custom blocking must be carefully tailored to
encompass the patient’s known disease and potential sites of
microscopic disease. Today, treatment is usually planned using CT or MRI
scans that may help the radiation oncologist define a target volume. However, during
treatment planning, it is important to take into account
that the position of the cervix can move by as much as 3 to 4 cm
with changes in bladder filling. 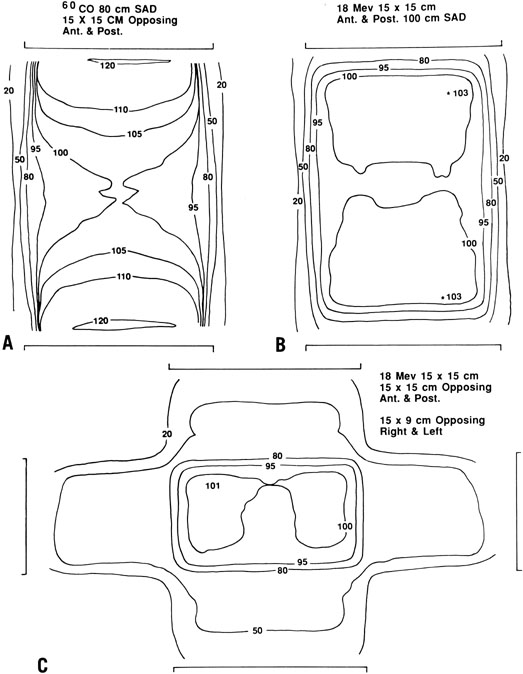 Fig. 2. Comparative dose distributions from external-beam radiation therapy (EBRT) in
a patient with an anterior-posterior diameter of 22 cm. A. Anterior and posterior opposed beams using 60Co. Note the relatively high dose that must be delivered to superficial
tissues in order to achieve the desired dose of 200 cGy to the deep pelvis. B. An 18-MeV beam gives a more favorable ratio between the dose to deep
tissues and the dose to superficial tissues. C. In some cases, further sparing of superficial tissues may be achieved
by delivering a portion of the dose with lateral fields (also with an 18-MeV
beam). Fig. 2. Comparative dose distributions from external-beam radiation therapy (EBRT) in
a patient with an anterior-posterior diameter of 22 cm. A. Anterior and posterior opposed beams using 60Co. Note the relatively high dose that must be delivered to superficial
tissues in order to achieve the desired dose of 200 cGy to the deep pelvis. B. An 18-MeV beam gives a more favorable ratio between the dose to deep
tissues and the dose to superficial tissues. C. In some cases, further sparing of superficial tissues may be achieved
by delivering a portion of the dose with lateral fields (also with an 18-MeV
beam).
|
To treat the whole pelvis, treatment fields typically extend from the L4-5 interspace
superiorly to the midpubis or to a line 4 cm below the
lowest vaginal disease (Fig. 3). Lateral borders are placed at least 1 cm lateral to the pelvic margins. The
lateral margins and shielding should be more generous if massive
obesity reduces the reproducibility of the treatment setup. 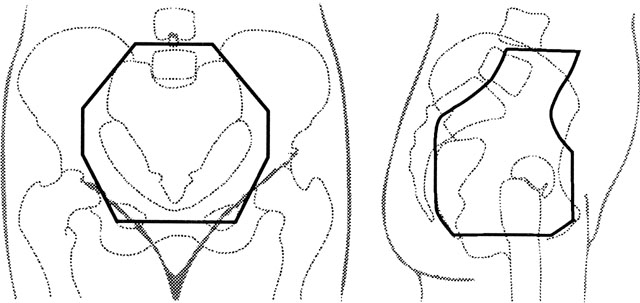 Fig. 3. An example of anterior (left) and lateral (right) fields with custom blocking that may be used in a four-field pelvic irradiation
technique for treatment of carcinoma of the cervix. In this
patient, the external iliac and common iliac lymph nodes are visible
after injection of radiopaque dye for a lymphangiogram. The anterior half
of S1–S3 is included in the lateral fields to give adequate
coverage of presacral nodes, and care must be taken not to shield tumor
with custom blocks. Fig. 3. An example of anterior (left) and lateral (right) fields with custom blocking that may be used in a four-field pelvic irradiation
technique for treatment of carcinoma of the cervix. In this
patient, the external iliac and common iliac lymph nodes are visible
after injection of radiopaque dye for a lymphangiogram. The anterior half
of S1–S3 is included in the lateral fields to give adequate
coverage of presacral nodes, and care must be taken not to shield tumor
with custom blocks.
|
If a four-field technique is used, the anterior border of the field usually
includes the anterior tip of the pubis; to cover the tumor, presacral
nodes, and uterosacral ligaments, the posterior border usually includes
S3 (see Fig. 3). Custom blocks may be used to shield anterior small bowel, soft tissue, and, in
some cases, low rectum on the lateral fields. However, care
must be taken not to shield potential sites of disease. Because the inguinal
nodes drain the lower third of the vagina, they should be included
in the treatment volume whenever the distal vagina is involved with
tumor. The risk of major complications is increased when the dose of whole-pelvic
radiation exceeds 40 Gy at 2 Gy per fraction or 45 Gy at 1.8 Gy per
fraction.29 There is no clear evidence that fraction sizes of less than 2 Gy significantly
decrease the rate of late complications, although daily fractions
of 1.8 Gy may reduce the severity of acute radiation effects when
large treatment fields or concurrent chemotherapy is used. Although 40 to 45 Gy
is usually sufficient to control microscopic tumor in the pelvis, additional
treatment must be given to control gross disease. ICRT
is used to deliver a high dose to cancer in the cervix; enlarged pelvic
nodes or lateral parametrial tumor may lie beyond the high-dose range
of ICRT but may be given additional treatment with small external-beam
fields. Whole-pelvis EBRT fields typically are designed with an upper border placed
either at the L4-5 interspace or at L5-S1. However, the fields may
be extended superiorly if the para-aortic nodes are known to be involved
or are believed to be at high risk for involvement. Because cervical
cancer typically follows an orderly progression along the lymph node
chain, the upper border is selected by balancing an estimate of the
risk of disease at a given level against the expected morbidity from
large-volume EBRT. In general, the upper border is placed at least 4 to 6 cm
above known disease. This is probably sufficient if the patient
has had a lymph node dissection with negative nodes above the level of
involvement. Bulky or multiple nodes probably warrant greater extension
of the fields, particularly if the patient has not had surgical evaluation
of the nodes. Although the survival rate of patients with aortic
node metastases is significantly less than that of patients with similar-stage
disease who do not have this finding, about 20% to 40% of
patients with aortic node metastases are curable with radiation
alone, depending on the extent of pelvic disease (Table 5). TABLE 5. Survival of Patients with Biopsy-Proven Para-Aortic Node Involvement
After Extended-Field Radiation Therapy
Study | Number of Patients | % of Patients with FIGO Stage I or II Tumors | 5-Year Survival Rate (%) |
Piver et al:23 |
31 |
35 |
10 |
Berman et al:93 |
98 |
65 |
25* |
Podczaki et al:94 |
33 |
70 |
31 |
Ballon et al:95 |
18 |
78 |
23 |
Kim et al:96 |
43 |
79 |
24 |
Brookland et al:97 |
15 |
80 |
40* |
Komaki et al:98 |
15 |
93 |
40 |
Cunningham et al:99 |
24 |
100 |
48 |
Rubin et al:100 |
14 |
100 |
50 |
* Three-year survival rate
In the late 1980s, two randomized trials evaluated the role of prophylactic
extended-field irradiation in patients with locally advanced cervical
cancer.31,32 Patients
who had known clinical evidence of aortic node involvement were ineligible
for these trials. The Radiation Therapy Oncology Group (RTOG) 79-2032
compared pelvic irradiation with extended-field (upper border L1-L2) radiation
therapy in patients with stage I or II disease. No routine surgical or
radiographic evaluation of the aortic lymph nodes was performed. Analysis
of this trial demonstrated a significantly better survival rate for patients
who received prophylactic aortic irradiation in addition to pelvic irradiation
and ICRT. A second trial, performed by the European Organization for Research
and Treatment of Cancer (EORTC),31 involved
a similar randomization but had more extensive clinical staging of the
abdomen (patients were required to have negative para-aortic nodes as
determined by lymphangiography) and included patients with more advanced
disease (bulky stage II or stage III). The EORTC trial failed to demonstrate
a significant improvement with extended fields. The locally advanced tumors
included in this trial and the corresponding high local failure rate may
have reduced the potential benefit of large-field irradiation. The relative
benefit of prophylactic extended-field irradiation may also be reduced
when more accurate evaluations of regional metastases are available. Today,
prophylactic aortic irradiation is performed infrequently because a later
RTOG trial 6 found an even greater advantage
from pelvic irradiation and concurrent chemotherapy.
Although EBRT plays an important role in the treatment of cervical cancer, the
high central dose delivered with ICRT is also an extremely important
component of curative treatment of cervical cancer. Patients should
be treated with EBRT alone only in the rare case of massive, poorly
responsive disease in which the uterine canal cannot be probed, even
with ultrasound guidance. In our experience, this represents fewer than 1% of
cases. In other cases, locally advanced disease is better
treated with a combination of EBRT to the whole pelvis, ICRT, and
carefully planned parametrial or nodal boosts.29 Brachytherapy Technique INTRACAVITARY RADIATION THERAPY. Intracavitary Applicator Systems. A number of different intracavitary applicator systems have been developed
to treat cervical cancer. Nearly all include an intrauterine tube
and some form of vaginal applicator to accommodate radiation sources; the
length of the intrauterine tube (tandem) and the design of the vaginal
applicators vary between systems.
The Fletcher-Suit-Delclos applicator used at The University of Texas
MD Anderson Cancer Center33,34
consists of a rigid metal tandem with an adjustable flange that can be
set to correspond to the length of the uterine cavity and two cylindrical
colpostats that are positioned in the vaginal fornices (Fig.
4). The applicator is loaded with radium or cesium in the patient’s
room after the accuracy of placement has been verified radiographically
and the patient has recovered from anesthesia. Remote afterloading units
are now available with Fletcher-Suit-Delclos–type applicators.
These systems reduce the radiation exposure to personnel to a negligible
level.
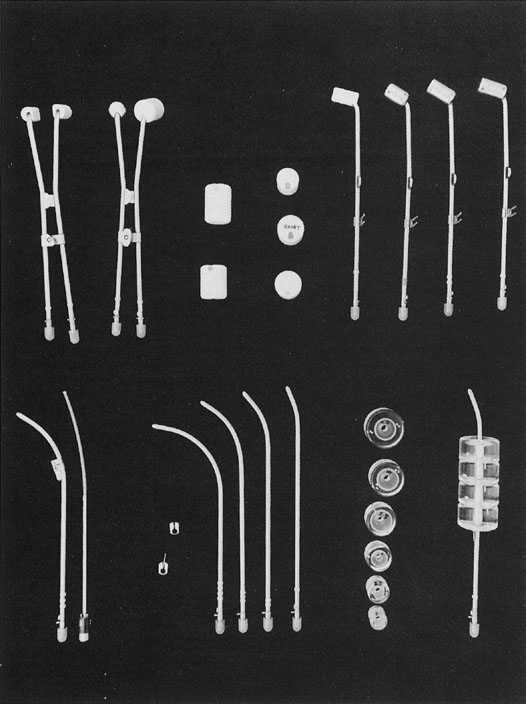 Fig. 4. Fletcher-Suit-Delclos applicator modified for use with Nucleotron Selectron
remote afterloading system. Several types of afterloading colpostats (at
the top, from left to right) are used in different clinical situations: mini
colpostats for treatment of patients with a narrow vagina; regular (2-cm
diameter) colpostats and plastic jackets used to increase
the size to medium (2.5-cm diameter) and large (3-cm diameter); and 15-degree
and 30-degree angle colpostats (lateral view) designed to
accommodate various positions of the cervical portio. Colpostats are
designed to have the vaginal sources approximately perpendicular to the
vaginal axis in order to minimize the dose to the bladder and rectum. Uterine
afterloading tandems of several different curvatures and metal
flanges with and without a “keel” (to stabilize the
tandem during packing) are shown in this figure (below). Vaginal cylinders (available
in a variety of diameters) are used for the irradiation
of a selected part of the vagina or the entire vagina. Fig. 4. Fletcher-Suit-Delclos applicator modified for use with Nucleotron Selectron
remote afterloading system. Several types of afterloading colpostats (at
the top, from left to right) are used in different clinical situations: mini
colpostats for treatment of patients with a narrow vagina; regular (2-cm
diameter) colpostats and plastic jackets used to increase
the size to medium (2.5-cm diameter) and large (3-cm diameter); and 15-degree
and 30-degree angle colpostats (lateral view) designed to
accommodate various positions of the cervical portio. Colpostats are
designed to have the vaginal sources approximately perpendicular to the
vaginal axis in order to minimize the dose to the bladder and rectum. Uterine
afterloading tandems of several different curvatures and metal
flanges with and without a “keel” (to stabilize the
tandem during packing) are shown in this figure (below). Vaginal cylinders (available
in a variety of diameters) are used for the irradiation
of a selected part of the vagina or the entire vagina.
|
Recommendations for loading of Fletcher-Suit-Delclos applicators should
not be generalized to other types of applicators because differences
in the dose distribution around the colpostats can result in substantial
differences in the doses delivered to the mucosa of the upper vagina, base
of the bladder, and anterior rectal wall. Placement of Intracavitary Systems. Delclos and associates34 described the following conditions for successful ICRT: - The geometry of the radium sources must prevent underdosed regions on and
around the cervix.
- An adequate dose must be delivered to the para-cervical areas.
- Mucosal tolerance must be respected.
The physics of ICRT depend on the inverse square law. Figure 5 illustrates the influence of the inverse square law on the depth dose
from colpostats and uterine tandems. The radiation dose to a point in
the pelvis depends on the amount of cesium in each source, the distance
from each source to the reference point, and the length of time the
radioactive sources remain in place. Optimal placement of the uterine
tandem and vaginal ovoids produces a pear-shaped distribution laterally, delivering
a high dose to the cervix and paracervical tissues and a
reduced dose to the rectum and bladder (Fig. 6). 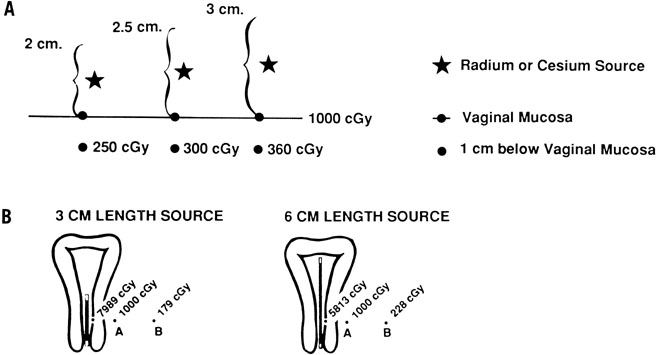 Fig. 5. Intracavitary applicators for treatment of carcinoma of the cervix take
advantage of the inverse square law. The dose delivered by a radioactive
source varies inversely as the square of the distance from the source
to that point. A. The influence of colpostat diameter on the ratio between vaginal surface
dose and the dose 1 cm below the vaginal surface. With a 2-cm colpostat, the
radium (or cesium) source is 1 cm from the vaginal surface
and 2 cm from the deep submucosal point. Consequently, the dose at a depth
of 1 cm is (1/2)2 × 1000 cGy = 250 cGy. A 2.5-cm ovoid will deliver (1.25/2.25)2 × 1000 cGy = 300 cGy, and a 3-cm ovoid will deliver (1.5/2.5)2 × 1000 cGy = 360 cGy to a point 1 cm below the vaginal surface. For
this reason, the larger diameter colpostats should be used
whenever possible to optimize the ratio between the dose delivered to
the vaginal surface and that delivered to deep paracolpal tissues. B. The dose at point A is kept constant at 1000 cGy. As the length of the
source increases (keeping the overall activity of radium or cesium constant), the
dose to the mucosa decreases and the dose at point B increases. Because
of this, the linear source used in a uterine tandem should
be as long as the patient’s vaginal anatomy will permit. Fig. 5. Intracavitary applicators for treatment of carcinoma of the cervix take
advantage of the inverse square law. The dose delivered by a radioactive
source varies inversely as the square of the distance from the source
to that point. A. The influence of colpostat diameter on the ratio between vaginal surface
dose and the dose 1 cm below the vaginal surface. With a 2-cm colpostat, the
radium (or cesium) source is 1 cm from the vaginal surface
and 2 cm from the deep submucosal point. Consequently, the dose at a depth
of 1 cm is (1/2)2 × 1000 cGy = 250 cGy. A 2.5-cm ovoid will deliver (1.25/2.25)2 × 1000 cGy = 300 cGy, and a 3-cm ovoid will deliver (1.5/2.5)2 × 1000 cGy = 360 cGy to a point 1 cm below the vaginal surface. For
this reason, the larger diameter colpostats should be used
whenever possible to optimize the ratio between the dose delivered to
the vaginal surface and that delivered to deep paracolpal tissues. B. The dose at point A is kept constant at 1000 cGy. As the length of the
source increases (keeping the overall activity of radium or cesium constant), the
dose to the mucosa decreases and the dose at point B increases. Because
of this, the linear source used in a uterine tandem should
be as long as the patient’s vaginal anatomy will permit.
|
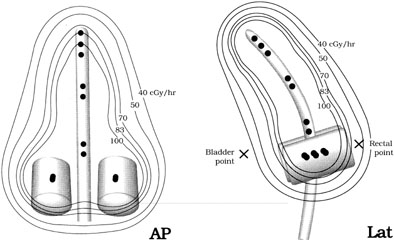 Fig. 6. Dose distributions from a typical intracavitary system using a Fletcher-Suit-Delclos
applicator modified for use with a Nucleotron Selectron
remote afterloading system. Black dots (•) represent 5-mg Ra-equivalent
cesium sources. This placement of the sources generates a dose
distribution nearly identical to that produced when a 15-mg and two 10-mg
linear radium sources are placed in the tandem and two 15-mg sources
are placed in two small (2-cm diameter) colpostats. Fig. 6. Dose distributions from a typical intracavitary system using a Fletcher-Suit-Delclos
applicator modified for use with a Nucleotron Selectron
remote afterloading system. Black dots (•) represent 5-mg Ra-equivalent
cesium sources. This placement of the sources generates a dose
distribution nearly identical to that produced when a 15-mg and two 10-mg
linear radium sources are placed in the tandem and two 15-mg sources
are placed in two small (2-cm diameter) colpostats.
|
The manipulations necessary to optimize placement of the intracavitary
system in different anatomic situations can only be learned with experience. To
maximize the chance of local control in the treatment of bulky
cervical tumors, treatment is prescribed to deliver the highest dose
to the tumor that will not produce an unacceptable risk of major complications. Because
there may be a narrow margin between tumor control
and normal tissue complications, the radiation oncologist must make every
effort to optimize the intracavitary system to obtain a favorable
ratio between the dose to the tumor and the dose to adjacent normal tissues. To
maintain an acceptable dose rate to bladder and rectum, the
axis of the tandem is usually placed in a midposition in the pelvis and
is loaded with approximately 5 to 6 mgRaEq of 137Cs per centimeter. A relatively long line of intrauterine sources delivers
a higher dose to the endocervix and to paracervical tissues; if anatomy
permits, 6 to 7 cm is usually loaded with active sources. The vaginal
ovoids are placed up against the cervix, centered on the cervical
portio; on a lateral radiograph, this usually means that the colpostats
are bisected by the tandem. The vagina is packed to displace the rectum
and bladder away from the intravaginal sources and to keep the system
in place. Colpostats of different sizes are selected as accommodated
by the patient’s vaginal anatomy and are usually loaded with 10 to 20 mgRaEq
of 137Cs according to the size of the colpostats; typical loadings usually produce
a dose rate of 80 to 100 cGy per hour at the lateral surface of
the vagina. Timing.
Therapy usually is accomplished in two equal applications given approximately
2 weeks apart. For patients who have received initial EBRT, the first
intracavitary placement should be administered as soon as possible after
completion of EBRT (usually 1 to 5 days). In most cases, the entire course
of treatment should be completed in less than 8 weeks. Excessive protraction
of the overall treatment time will compromise its effectiveness.35,36,37
Treatment Prescription and Dose Specification. Methods of ICRT treatment prescription and dose specification have generally
been developed empirically and differ significantly, making it difficult
to compare experiences between institutions. There is no inherently
correct way to specify the inhomogeneous radiation dose distribution
delivered with an intracavitary system. The most common method of
paracentral specification has been to describe the dose at a single
reference point, usually one located 2 cm lateral to and 2 cm superior
to the external cervical os in the plane of the intrauterine tandem (point
A). Point A is a reference point developed as part of the Manchester
system, which also incorporated a set of fairly rigid rules guiding
application geometry and radioactive source loadings. Point A bears
no consistent relation to tumor volume or target volume and has been
localized in different ways by different investigators. In 1985, the International
Commission on Radiation Units and Measurements38 recommended that reference points like point A not be used because “such
points are located in a region where the dose gradient is high
and any inaccuracy in the determination of distance results in large
uncertainties in the absorbed doses evaluated at these points.” Instead, it
was recommended that doses be specified in terms of (1) total
reference air Kerma (TRAK), which is expressed in milligray at 1 meter
and gives information similar to that described by the “concept
of mg-hr”; (2) description of the reference volume, that
is, the tissue volume encompassed by a reference isodose surface; and (3) the
dose calculated to specific reference points associated with
normal tissues within the treatment volume (bladder, rectum, and vagina). Despite these recommendations, point A is still the most common method
used to specify intracavitary implants in the treatment of cervical cancer. However, dose
specification for the purpose of treatment comparison
should never be confused with dose prescription. Treatment should
always be prescribed after careful consideration of information about
the quality of the application, size of the applicator system, position
of the system in the pelvis, exposure to normal tissue structures, initial
tumor extent, and residual tumor, as well as the dose to tumor
and normal tissue reference points. Treatment should also take into account the dose to lateral and more distant
pelvic structures. With ICRT, the dose to lateral structures is
maximized when a relatively long tandem and large colpostats are used
because a broader distribution of sources and greater displacement of
the vagina permit more activity to be used without exceeding the maximum
tolerable dose to bladder or rectum. However, care must be taken not
to exceed a tolerable dose to the whole pelvis. The total mg-hrs of
radium (the product of the total activity of 226Ra sources and the duration of treatment) has been used to limit the integral
dose to pelvic structures; clinicians found that exposures of more
than 6500 mg-hr after 40 Gy of EBRT tended to cause excessive pelvic
fibrosis and bowel damage even when the doses to bladder and rectum
were within acceptable limits.39 Although mgRaEq hours are often specified in a similar fashion when 127Cs is used to treat cervical cancers, care must be taken to correct for
differences in the filtration of material used to encapsulate radium
and cesium sources; to avoid confusion, TRAK dose is a preferred measure. A
variety of reference points also have been used to estimate the
dose to lateral pelvic structures. Point B (in the Manchester system) is
usually located 3 cm lateral to point A, but its location in the pelvis
varies considerably according to the position of the intracavitary
system. Some clinicians also record the dose at a pelvic reference point
P located just inside the lateral pelvic wall at the approximate
level of the obturator lymph nodes.40 In the Fletcher system, bony landmarks are used to estimate the locations
of the iliac lymph nodes (Fig. 7). The external iliac nodes generally lie in a plane that intersects the
tip of the pubic symphysis and the middle of S2. The common iliac nodes
lie along a plane between the anterior aspect of L4 and the bisector
of the line between the pubis and S2. Most clinicians also calculate
the dose of radiation at reference points on the lateral vaginal wall, posterior
bladder wall (usually on the posterior border of a contrast-filled
Foley bulb), and anterior rectal wall as viewed on orthogonal
radiographs of the pelvis.38 In most cases, the doses to reference points on the vaginal surface are
kept within a range of 110 to 130 Gy; the doses to the bladder and rectal
reference points are usually less than 75 Gy and 70 Gy, respectively. However, CT-based
three-dimensional analyses of the doses delivered
to normal structures from ICRT demonstrate that standard reference
points routinely underestimate the maximum dose delivered to these structures.41,42 The artifact caused by standard intracavitary systems and other logistical
concerns have made it difficult to perform routine three-dimensional
analysis of treatment doses, but this is expected to be an active
area of research in the next decade. 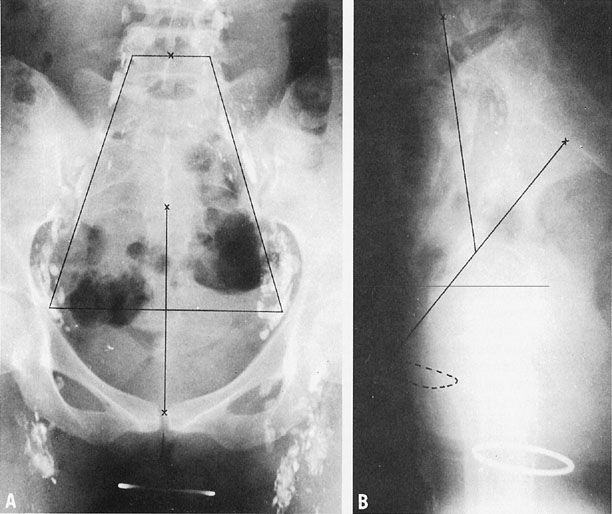 Fig. 7. Anteroposterior (A) and lateral cross-tablele (B) orthogonal radiographs of a patient with a normal findings on a lower-extremity
lymphangiogram. The basic reference line is from the top of
the symphysis to the junction of S1 and S2 (B). A line is drawn from the midpoint of the basic reference line to the
middle of the anterior aspect of L4. A. A trapezoid is defined by points 6 cm from the midline and 2 cm from the
midline at L4.(Durrance FY, Fletcher GH: Computer calculation of dose contribution to
regional lymphatics from gynecological radium insertions. Radiology 91:140, 1968.) Fig. 7. Anteroposterior (A) and lateral cross-tablele (B) orthogonal radiographs of a patient with a normal findings on a lower-extremity
lymphangiogram. The basic reference line is from the top of
the symphysis to the junction of S1 and S2 (B). A line is drawn from the midpoint of the basic reference line to the
middle of the anterior aspect of L4. A. A trapezoid is defined by points 6 cm from the midline and 2 cm from the
midline at L4.(Durrance FY, Fletcher GH: Computer calculation of dose contribution to
regional lymphatics from gynecological radium insertions. Radiology 91:140, 1968.)
|
With typical sources loaded in a Fletcher-Suit-Delclos intracavitary system, the
dose-rate at point A is usually 45 to 50 cGy per hour. Typically
patients who have received 40 to 45 Gy to the pelvis are treated
with two 48-hour applications, yielding a total dose to point A (with
EBRT and ICRT) of 85 to 90 Gy for locally advanced tumors or 80 to 85 Gy
for small IB1 tumors. However, these doses may vary depending on the
clinical situation and other factors discussed above. Dose Rate. Until relatively recently, most ICRT was delivered at a low dose rate of 40 to 60 cGy
per hour. This was necessitated by practical considerations: the
use of relatively low activity sources limited exposure of personnel
to ionizing radiation during loading and unloading of the applicators
and during nursing visits. However, laboratory and clinical studies
also demonstrated that radiation therapy delivered at these low
dose rates has an important radiologic advantage.43 The protracted continuous exposure has an effect similar to that of fractionated
EBRT. The repair of sublethal injury in normal tissue cells
exceeds that of tumor cells, maximizing the therapeutic ratio of ICRT.
The development of computer-driven remote afterloading devices has made
it possible to deliver brachytherapy with high activity sources with negligible
exposure to personnel. Using these devices, radiation is delivered by
advancing a single high-activity source along a track from the machine
through the brachytherapy applicator. The length of time the source rests
in various positions determines the distribution of radiation dose. This
form of high-dose–rate brachytherapy is now used for a number of
applications, including postoperative treatment of the vaginal apex, and
in some practices, treatment of intact cervical cancers. However, because
the dose-rate effect is lost, treatment must be divided into multiple
fractions to achieve an acceptable ratio between the rates of tumor control
and late radiation complications. Clinicians still disagree about the
number of fractions needed to achieve optimal results; the number of high-dose–rate
treatments used to treat cervical cancers in different centers ranges
from 3 to 15.44,45, 46
Over the past 10 to 20 years, there has been a gradual increase in the
use of high-dose–rate ICRT to treat intact carcinoma of the cervix
in the United States. In a random survey of radiation therapy practices
in the United States, 9% of patients treated between 1992 and 1994 were
treated with this method.45 High-dose–rate ICRT is the dominant method of brachytherapy treatment
in a number of other countries, including Japan and Germany. Despite
the possible radiobiologic concerns, high-dose–rate ICRT
has a number of advantages. Treatment does not require hospitalization. Although
more applications are needed than with low-dose–rate
treatment, the ability to give all treatment on an outpatient basis is
frequently more convenient for patients and their physicians. Personnel
exposure to ionizing radiation is virtually eliminated, and shielded
inpatient hospital rooms are not required. For physicians who treat
relatively few cases, capital costs may be less because the treatment
can be delivered with the same high-dose–rate unit that is used
for other purposes, eliminating the need to purchase cesium (which can
be used only for cases of cervical or inoperable endometrial cancer). It
has also been argued that the retraction methods used for high-dose–rate
therapy, fixation of the applicator position during planning
and treatment, and computerized optimization of source dwell positions
may produce a more favorable ratio between the dose to tumor and
normal tissues. Advocates believe that these factors overcome the radiobiologic
disadvantages of treatment with large fractionated doses
of radiation. However, we still prefer the use of low-dose–rate ICRT when possible, particularly
for patients who have large tumors and poorly distensible
vaginas. For such patients, the maximum dose to normal tissues
may be as high as or higher than the dose to tumor. In such circumstances, low-dose–rate
ICRT would be expected to have an important
advantage over high-dose–rate therapy. Unfortunately, no well-designed
randomized study has compared the two approaches; the large number
of patients that would be required, the expense of such a trial, and
the unwillingness of most practitioners to maintain the equipment
and sources needed to practice both low-dose–rate and high-dose–rate
therapy make it unlikely that such a study will ever be done. Although
retrospective studies of patients treated for cervical cancer
suggest that good survival rates can be achieved with high-dose–rate
treatment, poor follow-up, small numbers of patients, and
selection biases compromise many of these studies. The largest United
States experience reported to date still included fewer than 200 patients
with all stages of disease; in that study, patients with stage III
disease who were treated with high-dose-rate ICRT had a significantly
poorer outcome than those in a historic comparison group treated with
low-dose–rate therapy, but within the limits of statistical power, patients
with earlier disease appeared to have similar outcomes with
the two approaches.47 INTERSTITIAL BRACHYTHERAPY.
Although ICRT is the most important method of brachytherapy used for
treatment of cervical cancer, some tumors, particularly those that involve
the distal vagina, may be difficult to treat adequately with ICRT alone.
In a small percentage of these cases, distal vaginal lesions may require
treatment with a supplemental dose of radiation delivered using intralesional
192Ir interstitial implants. Some clinicians have advocated
broader use of interstitial therapy as an alternative to ICRT for treatment
of locally advanced cervical cancers.48,49
With this approach, needles are inserted transperineally into the cervix
and paracervical tissues; a Lucite template is used to guide and maintain
parallel positioning of the needles. With this technique, the dose to
paracervical tissues is usually somewhat higher than that delivered with
ICRT, but the central dose to the cervix is usually somewhat lower. Thus
far, most published series of interstitial brachytherapy as an alternative
to ICRT have included fewer than 75 patients, and results have not been
demonstrably better than those achieved with ICRT in patients with similar-stage
disease.50,51 However,
this approach can be very useful for occasional recurrent cancers or intact
cervical cancers that cannot be treated with ICRT.
|