In the case of endometrial carcinoma, the current consensus among experts
in the field of periodic health examinations is not to recommend screening
for endometrial cancer and its precursors because there is no
scientific evidence to support such examinations in menopausal and postmenopausal
women.31 The arguments against screening for endometrial carcinoma are the following: Although endometrial carcinoma is common, morbidity rates are low, comparatively
less important in number than breast carcinoma, colon carcinoma, lung
carcinoma, leukemia, lymphoma, brain carcinoma, pancreas carcinoma, and
ovary carcinoma.
Based on the incidence of endometrial carcinoma in asymptomatic
women, it would take about 1000 procedures to detect a single case of
either a carcinoma or its precursor, AH.32,33
The techniques available for diagnosing endometrial disease in asymptomatic
women suffer from pitfalls in interpretation or instrumentation. One
is the difficulty in interpreting relatively inexpensive cytologic
material34; the other is that office biopsy aspiration techniques are relatively
expensive and uncomfortable to painful, and tissue insufficient for diagnosis
rates may be 25%.
No controlled randomized trials have been done to evaluate the effectiveness
of screening in endometrial carcinoma. Even in high-risk menopausal
women, screening would detect only 50% of all cases of endometrial
carcinoma.35
Most patients with disease eventually become symptomatic (i.e., presenting
with abnormal uterine bleeding, yet have early clinical stage disease
at the time of surgical diagnosis and treatment). This contention
is supported by the excellent 5-year survival rates of patients with stage
I endometrial carcinoma (i.e., 80% to 91%).36 The clinicopathologic and epidemiologic data suggest that about 80% of
endometrial carcinomas are slow growing with a favorable course,30 and earlier treatment of asymptomatic carcinomas would be no more effective
than treatment given when symptoms appear.
Elderly people are difficult to enroll into screening programs, and the
dropout rate is relatively high. This is particularly true if painful
techniques are used for endometrial evaluation.
The incidence of endometrial carcinoma and its precursors is low in women
aged younger than 50 years and in women receiving combination-type
HRT (estrogen/progestins).28
Screening for endometrial carcinoma or its precursor, AH, in asymptomatic, postmenopausal
women is presently not recommended because of the low
incidence of endometrial carcinoma in this group of women, estimated
to be 1.7 cases per 1000 women per year, and the low prevalence, in
the order of 1 per 1000 women.32 In the Postmenopausal Estrogen/Progestin Intervention (PEPI) trial, no
patients developed endometrial carcinoma while on daily estrogen-only
replacement therapy, 0.625 mg, during a follow-up of 36 months versus 1% of
women who developed endometrial carcinoma on placebo.37 The mean transit time of AH to endometrial carcinoma has been estimated
to be 4.56 to 5.5 years.7 Who Should Be Screened Women receiving unopposed estrogens need endometrial sampling once every 2 years (relative
risk increases only after 2 years of estrogen use), particularly
if endometrial hyperstimulation has been documented previously
and has not been treated by short-term administration of progestins. Also, if
the informed, high-risk individual requests an endometrial
evaluation before or during HRT or at any time during her periodic
health examinations, she should not be deprived of an office-based investigative
procedure to rule out endometrial pathology. An endometrial
evaluation also should be performed in women at high risk for endometrial
carcinoma, such as women with history of Lynch II syndrome.38 The term diagnosis, as opposed to screening, refers to the application of a test to women presenting with symptoms (most
commonly abnormal uterine spotting or bleeding) that presumably
are related to endometrial carcinoma or its precursors. A study addressed
the optimal evaluation strategy for patients with a first period of
postmenopausal bleeding at various risks for endometrial carcinoma and
AH.39 Among four options—office endometrial biopsy, dilation and curettage (D&C), hysterectomy, and observation alone (unless bleeding
recurred)—office biopsy with the Vabra technique was the most
cost-effective initial means, costing less than $41,000 U.S. per year
of life saved for patients with a 10% risk of having endometrial
carcinoma or AH. For patients at 5% risk, the cost of endometrial
biopsy increased, however, to $66,000 U.S. per year of additional
life saved for 60-year-old patients. Neither D&C nor hysterectomy
was as cost-effective as office biopsy as an initial diagnostic
evaluation procedure in patients with any risk for carcinoma/AH and abnormal
uterine bleeding. Based on this decision-analytic model, the patient’s
age and the risk for endometrial carcinoma/AH seem to be
important determinants for the use of a given endometrial evaluation
technique. Screening and Diagnostic Techniques At present, seven methods exist for assessing the endometrium: cervical/vaginal
cytology, endometrial cytology, endometrial biopsy, transvaginal
ultrasonography (TVS), magnetic resonance imaging, hysteroscopy, and
D&C. CERVICAL/VAGINAL CYTOLOGY. The main drawbacks of this method are that it detects mainly advanced endometrial
carcinoma and has a high false-negative rate (80%) in
postmenopausal, asymptomatic endometrial carcinoma patients. In one
study, the odds ratio of endometrial carcinoma in symptomatic postmenopausal
women was three times greater in the presence of histiocytes with
phagocytosis of acute inflammatory and red blood cells compared with
controls.40 Histiocytes alone failed to predict either endometrial carcinoma or hyperplasia. Endometrial
cells on cervical smears carried a fourfold odds
ratio for EH. Vaginal cytology may detect recurrent cancer in women
treated for endometrial carcinoma. Because the risk of recurrence of endometrial
carcinoma (11% to 17%) and adjunct radiotherapy
complications (70%) are greatest during the first 3 postoperative
years and because most patients with recurrence are symptomatic
and only few survive their recurrent disease, annual follow-up examination
that includes vaginal cytology is sufficient.36 It is generally accepted that the best yield is obtained with tests that
directly sample the endometrial lining.34 Numerous endometrial cell samplers are available commercially. Most of
them obtain cellular samples either by brushing or by aspirating the
superficial endometrial mucosa (Table 4). All endometrial cell samplers have been used under experimental conditions; the
results in detection rates do not represent detection rates
at large. Nevertheless, if cytologic atypia is the only feature to look
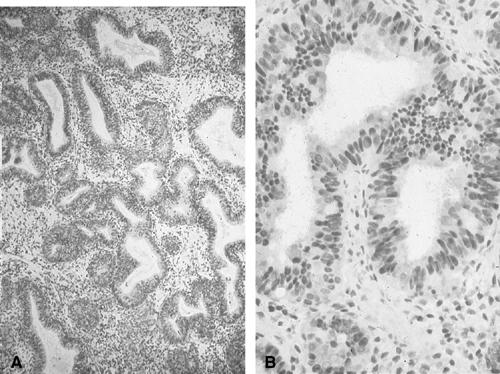
for, endometrial cytology may be highly accurate in distinguishing
carcinoma from normal or hyperplasia without cytologic atypia (Fig. 3). In one study, endometrial cytology using plastic brushes yielded 79% sensitivity, 95.4% specificity, and 80.5% negative
predictive value.41 If the smear contains normal endometrial cells, the patient may have either
a normal or a hyperplastic uterine lining. Often, hyperplasia without
cytologic atypia is indistinguishable from normal proliferative
endometrium. Because this form of hyperplasia is not a carcinoma precursor, however, the
patients with symptoms such as uterine bleeding can
be treated conservatively. Most cytologic laboratories lack expertise
for distinguishing cytologic atypia related to neoplasia from atypia
associated with degeneration or repair. As a result, false-positive rates
may be too high to justify the routine use of cytology for endometrial
disease. Also, the screening of an endometrial smear is time-consuming, and
interpretation is difficult because of the complexity of endometrial
gland cell morphology. Many carcinoma mimics lead to false-positive
results.33,34 TABLE 4. Accuracy of Endometrial Cytologic Methods for Detecting Neoplasia*
Method | Diagnostic Accuracy†(%) | Unsatisfactory Specimen†(%) |
Brushing (Endo-Pap, Gynecyte, Endocyte, Endoscan) |
91–100 |
4–10 |
Aspiration (Isaacs cell sampler, Gravlee-jet washer) |
96–100 |
10–12 |
*Histologically verified.
†Including carcinoma and atypical hyperplasia.
†In postmenopausal women.
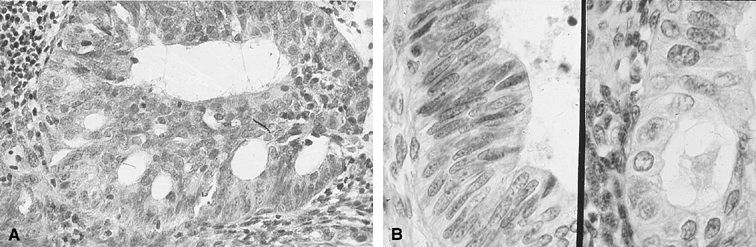
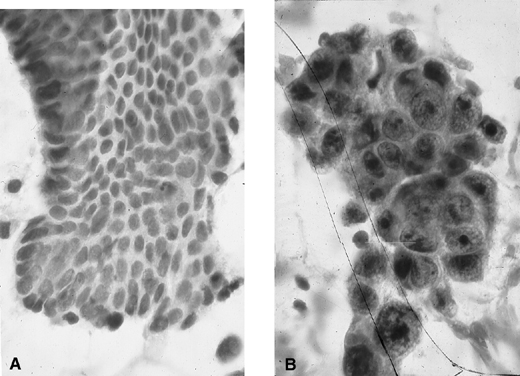 Fig. 3. Endometrial cytology obtained by endometrial aspiration. A. Hyperplastic endometrial cells with regular cytologic pattern are indistinguishable
from normal endometrial cells of the proliferative phase
of the cycle. B. Atypical endometrial cells with pleomorphic, hyperchromatic nuclei and
macronucleoli. This obese patient was asymptomatic and had a FIGO stage 1A
well-differentiated invasive adenocarcinoma. Fig. 3. Endometrial cytology obtained by endometrial aspiration. A. Hyperplastic endometrial cells with regular cytologic pattern are indistinguishable
from normal endometrial cells of the proliferative phase
of the cycle. B. Atypical endometrial cells with pleomorphic, hyperchromatic nuclei and
macronucleoli. This obese patient was asymptomatic and had a FIGO stage 1A
well-differentiated invasive adenocarcinoma.
|
HISTOLOGIC METHODS. At present, histologic sampling is the best means to diagnose either asymptomatic
or symptomatic (abnormal uterine bleeding) endometrial neoplasia. Plastic
disposable or metal reusable devices using brushing, aspiration
biopsy, suction curettage, or stroke biopsy have been used with
similarly high diagnostic accuracy (Table 5).34 The pitfalls of histologic methods lie in their relatively high cost and
degree of discomfort. The latter leads to low compliance rates for
repeat testing. Conventional curettage is much too costly yet not 100% foolproof
as far as diagnostic accuracy is concerned.42 According to current experience including our own, the endometrial devices
that seem to be the most cost-effective and are associated with the
least discomfort for patients are the endometrial aspirators.34 In cases in which tissue is not obtained with one of the low-vacuum, suction-type
aspirators, particularly in an elderly postmenopausal woman
whose endometrium is more often than not atrophic, aspirators with a
powerful vacuum suction force (e.g., Vabra aspirator; Tis-u-Trap; or
sharp-bladed, four-stroke biopsy curette) provide diagnostic tissues. TABLE 5. Accuracy of Endometrial Histologic Methods for Diagnosing Neoplasia
 We have had success in using the endometrial brush Gynecyte (Looper Surgical, Inc; European
version of Endocyte) for cytologic sampling of the
endometrium and the endometrial Pipelle (Unimar, Wilton, CT) or Endocell (Wallach
Surgical, Inc, Milford, CT) (Figs. 4 and 5) and, when appropriate, the Kevorkian curette (EuroMed, Redmond, WA) (see Fig. 5) for histologic sampling in asymptomatic and symptomatic women at risk
for endometrial carcinoma and its precursors.34 In about 10% of postmenopausal women, the endometrial cavity is
difficult or impossible to penetrate because of severe stenosis of the
external/internal os or because of internal os spasm. In these cases, placing
the patient on sequential cyclic therapy with conjugated estrogens (Premarin) (0.625 mg for 25 days) and medroxyprogesterone (Provera) (5 mg
for 11 to 12 days) for 3 consecutive months often results in
adequate dilation of the external/internal os to allow penetration of
the endometrial cavity. Another alternative is to perform TVS and assess
the thickness of the endometrium (see Transvaginal Ultrasonography
later). Finally, traction of the uterus with the endocervical Emmett’s
tenaculum or a skin (Iris) hook (see Fig. 5) is of considerable help for entering the endometrial cavity in the office. If
an endometrial aspirator of the Pipelle type is used, it is important
to move and rotate the cannula under negative action suction
force within the endometrial cavity at least six times to sample the greatest
surface area of the endometrium. In a comparison of the Pipelle
versus the Vabra aspirator, the percentage of endometrial surface mucosa
sampled with the Pipelle was 4.2% versus 42% with the
Vabra aspirator and 60% with D&C under general anesthesia.43 The difference in percentages of area sampled is likely due to the comparatively
greater suction force of the Vabra than the Pipelle device. 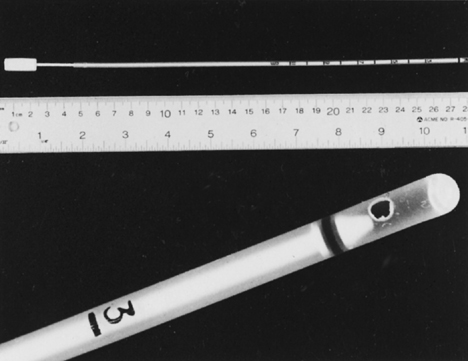 Fig. 4. Endometrial Endocell. Flexible polypropylene suction cannula with a 24-cm-long, 3-mm-wide
outer sheath and a fine-caliber piston (top). Detailed
view of the 2.5-mm distal side port through which the specimen is
aspirated (bottom). Fig. 4. Endometrial Endocell. Flexible polypropylene suction cannula with a 24-cm-long, 3-mm-wide
outer sheath and a fine-caliber piston (top). Detailed
view of the 2.5-mm distal side port through which the specimen is
aspirated (bottom).
|
 Fig. 5. Endometrial aspirators and curette. Pipelle, Z-xampler (BEI/Zinnanti, Chatsworth, CA), Uterobrush (Medscand USA, Hollywood, FL), Endocell, and
Kevorkian curette without baskets (top). Emmet tenaculum and iris hook (Cooper
Surgical, Shelton, CT) for uterine traction (bottom). Fig. 5. Endometrial aspirators and curette. Pipelle, Z-xampler (BEI/Zinnanti, Chatsworth, CA), Uterobrush (Medscand USA, Hollywood, FL), Endocell, and
Kevorkian curette without baskets (top). Emmet tenaculum and iris hook (Cooper
Surgical, Shelton, CT) for uterine traction (bottom).
|
Sampling for Histology: A Step-by-Step Guide
- Bimanually examine the uterus to determine its position.
- Clean the cervix and vagina with acetic acid or other aseptic solution.
- Insert an Emmett’s tenaculum or Iris hook (see Fig.
5) into the outer one third of the endocervical canal and pull gently
to obtain traction of the uterus.
- Insert an endometrial aspirator into the endocervical canal (see Figs.
4 and 5).
When the aspirator is at the lower uterine segment level, push and rotate
it to facilitate entering the endometrial cavity.
- When it is at the fundus, pull the plunger back rapidly and completely
in the cannula to create a high negative pressure gradient.
- Move the cannula back and forth 6 to 12 times in the endometrial cavity
and rotate it at the same time.
- Remove the cannula with the plunger pulled back (retain suction) from
the endometrial cavity. Empty the material on a lens paper by pushing
the plunger forward and place it in 10% buffered formalin tissue
fixative.
If little or no tissue is obtained, the procedure can be repeated once. If
still no tissue has been obtained, endometrial sampling can be performed
using either the Vabra or other powerful aspirators or a metal
curette (see Fig. 5). If still no tissue is obtained and the uterus is small, one can assume
endometrial atrophy or fibrous pedunculated polyps are present. TVS
or hysteroscopy, if the patient is symptomatic, may be performed. In current practice, staging of endometrial carcinoma is surgical (hysterectomy, bilateral
salpingo-oophorectomy, and pelvic node biopsy)44 and includes the histologic assessment of invasion of the endocervical
mucosa (International Federation of Gynecology and Obstetrics [FIGO] stage
IIA) versus the stroma (FIGO stage IIB) in the hysterectomy specimen. As
a result, the preoperative evaluation of the endocervical canal
is no longer necessary. The exception to the rule is a younger, premenopausal
woman in whom a fractional sampling of the uterus can determine
whether the patient has an endocervical or an endometrial primary
tumor. TRANSVAGINAL ULTRASONOGRAPHY. TVS can visualize the endometrium on a monitor when a 5-MHz probe is placed
against the vaginal fornix. The thickness of the endometrium can
be measured with precision because the endometriomyometrial junction has
a distinct halo-like appearance (Fig. 6). TVS is highly sensitive but also has high false-positive rates (low
specificity) for identifying endometrial carcinoma. Studies suggested
that specificity may be improved without jeopardizing sensitivity rates
if the cutoff values were based on length of time since menopause.41 When the endometrial thickness is 4 mm for women less than 5 years since
menopause and 3 mm for women more than 5 years since menopause, TVS
had a 97.4% sensitivity, 75.7% specificity, and 99.7% negative
predictive value. 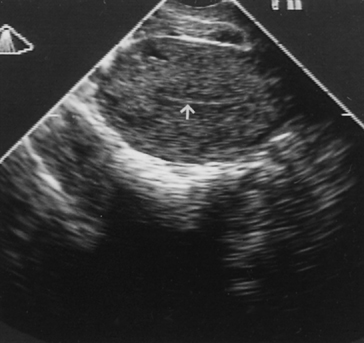 Fig. 6. Transvaginal ultrasonography of the uterus. Note the atrophic endometrial
lining (arrow). Fig. 6. Transvaginal ultrasonography of the uterus. Note the atrophic endometrial
lining (arrow).
|
MAGNETIC RESONANCE IMAGING. At present, magnetic resonance imaging and computed tomography have been
proved to be useful for obtaining preoperative data on the extent and
depth of myometrial invasion by endometrial carcinoma rather than in
the primary diagnosis of endometrial carcinoma and its precursors.45 Its role in the primary diagnosis of endometrial cancer and its precursors
remains to be determined. HYSTEROSCOPY. The value of hysteroscopy in the diagnosis and directed biopsy of a variety
of intracavitary or endometrial lesions in women with postmenopausal
bleeding has been extensively documented (see Chap. 36). If insufficient
tissue is obtained on suction curettage, or if a patient continues
to have abnormal bleeding, a formal D&C is often recommended, despite
the fact that its superiority over office procedures in the
diagnosis of cancer has not been established.42 Absolute indications for hysteroscopy have not been established (Fig. 7). When available, however, hysteroscopy is indicated in any woman with
abnormal uterine bleeding in whom an intrauterine abnormality is suspected. Other
indications include recurrent miscarriages, infertility caused
by endometrial pathology, removal of an impacted intrauterine device, and
suspected submucous leiomyomas before abdominal myomectomy. Hysteroscopy
is contraindicated in the presence of active infection and
intrauterine pregnancy. Active bleeding is a relative contraindication
to office hysteroscopy only because blood interferes with vision if
carbon dioxide is used as a distending medium. In patients who have severe
medical problems, it is prudent to perform hysteroscopy in an outpatient
setting where full monitoring and resuscitation facilities are
available.  Fig. 7. Hysteroscope with light source (left) and insufflator (right). Fig. 7. Hysteroscope with light source (left) and insufflator (right).
|
DILATION AND CURETTAGE. D&C essentially has been replaced by office-based endometrial biopsy
using flexible aspiration devices. The latter is more cost-effective
than D&C, and the diagnostic yield in symptomatic and asymptomatic
women is similar to D&C with sensitivity and specificity
rates of 90% and 95%.34 Cervical stenosis prevents successful endometrial sampling in about 10% of
cases. FALSE-NEGATIVE HISTOLOGY.
Even direct sampling of the endometrium for histology may fail to detect
adenocarcinoma. In several studies, D&C under general anesthesia
missed 10% of endometrial carcinomas.34,42
This is not surprising for, as was stated earlier, only 60% of
mean surface area is sampled with D&C versus 40% for Vabra
curettage and 4% for endometrial biopsy with the Pipelle endometrial
aspirator.43 Others found 4 of 86 (4.6%)
women with postmenopausal bleeding with endometrial carcinoma who had
either a negative endometrial biopsy result or D&C within 2 years
before cancer diagnosis.46 In another study
from Australia,47 the false-negative rate
of endometrial biopsy of focal adenocarcinomas of the endometrium was
47%.
|