Personal risk factors for endometrial cancer can be defined as those characteristics
that distinguish one individual from others of the same
demographic group living in the same area at the same time. 14 To identity these factors requires comparison of similar data from affected
and unaffected individuals. A summary of the various risk factors
to be discussed is contained in Table 2. TABLE 2. Summary of Probable Risk Factors Associated with Endometrial Cancer
Risk Factor | Approximate Risk Ratios |
Obesity | 1.8–2.4 |
Nulliparity | 2.0–3.0 |
Diabetes mellitus | 2.8 |
Prior irradiation | 8.0 |
Granulosa-theca cell tumors | 5.0 |
Exogenous estrogen therapy | 3.0–8.0 |
Late menopause (>age 52) | 2.4 | Obesity Many reports over the past 5 decades have described a high proportion of
overweight among patients with endometrial carcinoma. Unfortunately, in
relatively few studies has an entirely adequate comparison been made. In a comparison of the weights of endometrial cancer patients and controls
aged 25 to 29, Wynder and associates found that 21% of the cancer
patients were 21 to 50 lb overweight and that 9% were more than 50 lb
overweight, compared with 8% and 1%, respectively, among the controls.15 These data suggest that women 21 to 50 lb overweight have three times
the risk, and those over 50 lb overweight, have almost ten times the risk, of
developing endometrial cancer as women of normal or below normal
weight. In a Boston study controlled for age and economic status, it was found
that women in the upper third of the weight distribution had 1.8 times
the risk and those in the upper 15% of the weight distribution had 2.4 times
the risk for developing endometrial cancer compared with women
in the lower two-thirds of the weight distribution.26 When considering weight as a risk factor, it is important to remember that
no more than half of the patients with endometrial cancer in the Boston
study were in the heaviest one-third of the population.16 In other words, screening programs should not ignore the fact that many
women who develop endometrial carcinoma are not overweight. Nulliparity A positive association between nulliparity and endometrial cancer is a
long-recognized characteristic of the disease. In the Boston study cited
above, the incidence rate for nulliparas was twice as high as for women
with one child and more than three times as high as for women with
five or more children. Whether age at first pregnancy, clearly a risk factor for breast cancer, is
a risk factor for endometrial cancer is a question that cannot be
answered from data available to date. Menstrual History A late menopause has been described as a risk factor by several investigators.15,17 Elwood and Cole found a 2.4-fold increased risk of endometrial cancer
among women whose menopause occurred at age 52 or later, compared with
women whose menopause occurred at or prior to age 49. Since this risk
holds up among women in the 60 to 69 and over 70 age-groups it is much
more likely that the late menopause can be ascribed to continued ovarian
function rather than to early neoplastic or preneoplastic endometrial
changes. Diabetes Mellitus An increased incidence of impaired carbohydrate tolerance among patients
with endometrial cancer has been described for many years, but most
studies suffer from having inadequate comparison groups or none at all.18 Because of the positive association between obesity and diabetes mellitus, the
increased incidence of diabetes mellitus among endometrial cancer
patients might be dependent on obesity rather than on diabetes as
an independent variable. However, after controlling for age, body weight, and
socioeconomic status, Elwood and Cole found a 2.8 risk ratio
associated with diabetes. In a detailed prospective study of endocrine
and metabolic aspects of endometrial cancer, in which age and weight
were carefully controlled, Lucas and Yen could find no evidence of an
increased incidence of impaired carbohydrate tolerance among endometrial
cancer patients.19 It would appear that the final answer regarding the role of diabetes as
a risk factor will require similar studies in a larger group of patients
and suitably matched controls. Hypertension Again, unless age and weight are controlled as variables, no meaningful
appraisal of hypertension as a risk factor can be made. In Wynder's
study, mean blood pressure did not differ between cases and controls. In
the Boston study of Elwood and Cole, the excess risk associated
with a history of hypertension was 1.5, after controlling for age and
weight, but this was not a significant difference from unity. Polycystic Ovary Syndrome Although endometrial cancer is relatively uncommon among premenopausal
patients, it has been observed for many years that among patients under 45 who
develop endometrial cancer there is a high incidence of ovulatory
failure.20 Jackson and Dockerty found 16 endometrial carcinomas among 43 patients
with the polycystic ovary syndrome.21 In a retrospective study of endometrial hyperplasia in 97 women under
age 35, Chamlian and Taylor found that 73% were nulligravidas and 25% had
a diagnosis of polycystic ovary syndrome.22 In 14 (14%) the hyperplasia had progressed to carcinoma in a follow-up
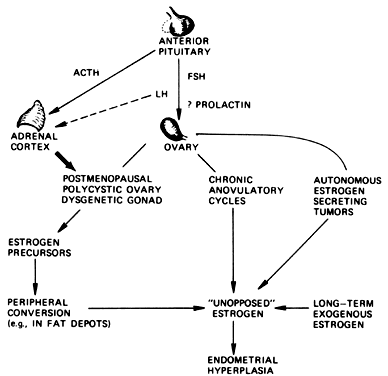
period of 1 to 14 years. The obvious association to be made is that unopposed estrogen secretion
in this group of patients induces endometrial hyperplasia and substantially
increases the risk of endometrial cancer. Cancers of Other Sites The fact that cancers of the endometrium and breast, and to a lesser extent
of the endometrium and ovary, occur in the same women with greater
frequency than can be explained by chance has been alluded to already.14,23 An association between endometrial and colon cancers has also been reported.24 Familial History Although there is some evidence for a familial aggregation of endometrial
cancer, the association is not as strong as for breast cancer and is
probably not sufficient to consider this a major high-risk factor.25,26 Radiation Exposure There is a significant risk of endometrial cancer occurring many years
after pelvic irradiation for both benign and malignant conditions. In
a study of 5749 women who had received pelvic irradiation, Wagoner and
Connelly found 16 endometrial cancers compared with an expected occurrence
of 1.8 on the basis of age and time-specific rates in the general
population.27 A positive correlation between radiation dose and subsequent cancer was
noted. It should be noted that a high proportion of corpus neoplasms after radiation
exposure are sarcomas and mixed carcinosarcomas. Granulosa-Theca Cell Tumors The relationship between feminizing mesenchymal ovarian tumors, endometrial
hyperplasia, and endometrial cancer has generated interest and controversy
among gynecologists and pathologists since the first report
of such an association by Schroeder in 1922.28 Diddle collected reports on 1189 granulosa-theca cell tumors and found
a 35% incidence of endometrial hyperplasia and a 6% incidence of endometrial
cancer,29 and Larson found a 10.3% incidence of endometrial cancer in a collected
series of postmenopausal patients with granulosa-theca cell tumors.30 Reports of endometrial cancer in several women under age 40 with granulosa
cell tumors are noteworthy,31 as is the observation that a preponderance of the tumors associated with
endometrial cancer are thecomas or mixed granulosa-theca cell tumors
rather than pure granulosa cell tumors. This is cited as morphologic
evidence of chronic estrogen stimulation of the endometrium, the theca
cell being the probable predominant site of estrogen synthesis. |