Histologic Effect The histologic effect of progesterone in endometrial carcinoma is well
described in studies of serial biopsy specimens obtained from patients
in whom surgery or irradiation was contraindicated, as well as in studies
in which progestogens were instilled into the uterine cavity. Progesterone
causes a decrease in the atypia and pleomorphism of the glands, with
a decrease in mitotic figures and better differentiation of the
glands. The cytoplasm becomes more granular and eosinophilic, and secretory
vacuoles appear. In well-differentiated tumors, the carcinoma
is replaced by endometrial hyperplasia or by endometrial atrophy (Fig. 4). 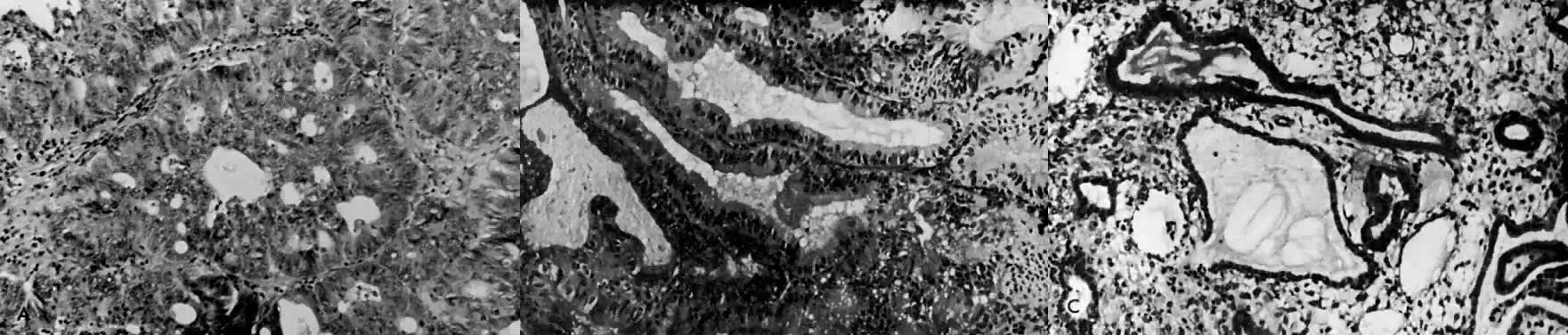 Fig. 4. A. Endometrial carcinoma, grade 1. A biopsy specimen prior to the administration
of progestogen. B. Endometrial biopsy performed 8 weeks after commencement of 400 mg medroxyprogesterone
three times per week by intramuscular injection. C. The patient was treated for 12 weeks with intramuscular medroxyprogesterone. Endometrium
is shown at the time of hysterectomy; no carcinomatous
tissue is visible. Fig. 4. A. Endometrial carcinoma, grade 1. A biopsy specimen prior to the administration
of progestogen. B. Endometrial biopsy performed 8 weeks after commencement of 400 mg medroxyprogesterone
three times per week by intramuscular injection. C. The patient was treated for 12 weeks with intramuscular medroxyprogesterone. Endometrium
is shown at the time of hysterectomy; no carcinomatous
tissue is visible.
|
It must be recognized that secretory vacuoles may be present in endometrial
carcinoma when no progesterone has been administered. Furthermore, the
neoplasm may show the histologic changes associated with progesterone, but
the tumor may progress and the patient may die. Electron microscopy is useful in differentiating endometrial hyperplasia
from neoplasia (Fig. 5). Examination of endometrial hyperplasia demonstrates numerous microvilli, an
active Golgi complex, and endoplasmic reticulum. In carcinoma
in situ and invasive cancer, these organelles tend to be present much
less frequently. Thus, light microscopy is a better means of distinguishing
endometrial hyperplasia from atypia and invasive cancer, whereas
electron microscopy may be a better choice for distinguishing glandular
hyperplasia from atypical hyperplasia. Nevertheless, we are still completely
reliant on the eye of the gynecologic pathologist to distinguish
cancer from hyperplasia, as flow cytometry and other new modalities
cannot distinguish between them. The administration of progesterone in endometrial carcinoma is associated
with an initial increase in endoplasmic reticulum, mitochondria, and
Golgi complex, as well as with glycogen accumulation (Fig. 6A and Fig. 6B). Giant autolysosomes then appear, similar to those seen in the late luteal
phase. The neoplastic cells become low cuboidal and devoid of mitotic
activity. Eventually, there is complete glandular atrophy, and there
may be stromal decidualization. 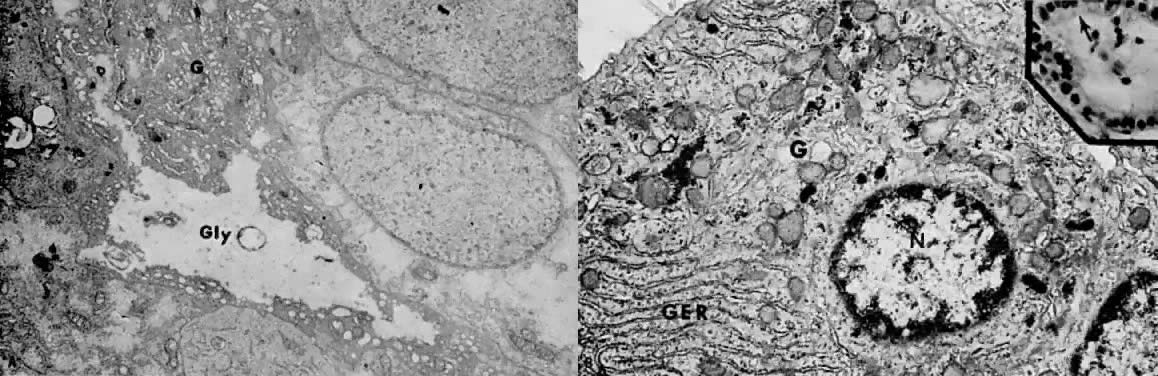 Fig. 6. A. Well-differentiated adenocarcinoma 4 weeks after treatment with medroxyprogesterone
acetate. The early cellular effects of progestin therapy
include lack of nuclear chromatin clumping, regular nuclear membranes, accumulation
of large masses of intracytoplasmic glycogen ( Gly ), and hypertrophy of the Golgi complex ( G ). These features are consistent with active synthesis of glycoproteins ( Fig. 6. A. Well-differentiated adenocarcinoma 4 weeks after treatment with medroxyprogesterone
acetate. The early cellular effects of progestin therapy
include lack of nuclear chromatin clumping, regular nuclear membranes, accumulation
of large masses of intracytoplasmic glycogen ( Gly ), and hypertrophy of the Golgi complex ( G ). These features are consistent with active synthesis of glycoproteins ( 5000). (Courtesy of Alex Ferenczy) B. Moderately differentiated, invasive adenocarcinoma 90 days after treatment
with medroxyprogesterone acetate. ( Inset) Following secretory conversion and activity, the neoplastic cells appear
reduced in size (“exhausted”) and have supranuclear vacuoles ( arrow ). Note nuclear normochromasia and lack of nuclear pseudostratification (H&E, 5000). (Courtesy of Alex Ferenczy) B. Moderately differentiated, invasive adenocarcinoma 90 days after treatment
with medroxyprogesterone acetate. ( Inset) Following secretory conversion and activity, the neoplastic cells appear
reduced in size (“exhausted”) and have supranuclear vacuoles ( arrow ). Note nuclear normochromasia and lack of nuclear pseudostratification (H&E,  350). As a result of secretory differentiation, the granular endoplasmic
reticulum ( GER ), mitochondria, and Golgi complex ( G) are hypertrophic. Owing to secretion of glycogen-rich apical cytoplasmic
substance, only minute amounts of glycogen granules ( arrow) are found at this stage of therapy ( 350). As a result of secretory differentiation, the granular endoplasmic
reticulum ( GER ), mitochondria, and Golgi complex ( G) are hypertrophic. Owing to secretion of glycogen-rich apical cytoplasmic
substance, only minute amounts of glycogen granules ( arrow) are found at this stage of therapy ( 27,000; N, nucleus).(Courtesy of Alex Ferenczy) 27,000; N, nucleus).(Courtesy of Alex Ferenczy)
|
Organ Culture Studies The addition of progesterone to organ culture of normal proliferative endometrium
induces the histologic and electron microscopic characteristics
of postovulatory endometrium in vivo.39 Glycogen accumulates beneath the nucleus and is secreted into the lumina
of the glands. Giant mitochondria appear in association with the glycogen, and
nucleolar channel systems appear in the nucleolus, as they
do between days 16 and 19 of the menstrual cycle in the ovulating female (Fig. 7). All progestational agents will induce glycogen formation and giant mitochondria, but
only those with an acyl group (CH3-CO) at position 17β will produce nucleolar channel systems in culture. So
far, no nucleolar channel systems have been observed in endometrial
carcinoma. 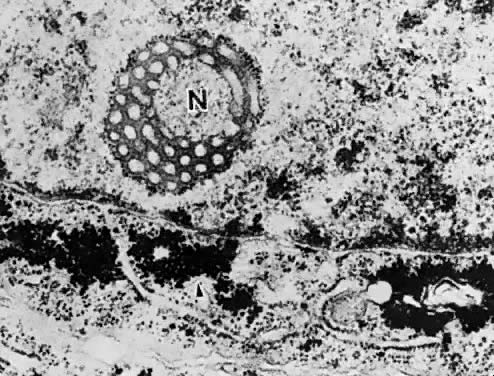 Fig. 7. Nucleolar channel system ( N) and glycogen accumulation in the adjacent cytoplasm of the endometrial
cell ( arrow ). Fig. 7. Nucleolar channel system ( N) and glycogen accumulation in the adjacent cytoplasm of the endometrial
cell ( arrow ).
|
In organ cultures of endometrial carcinoma,40, 41 low-dose progestational agents produce tumor differentiation, whereas
high doses produce tumor necrosis (Fig. 8). In some studies, estrogen was found to potentiate the necrotizing effect
of progesterone.42 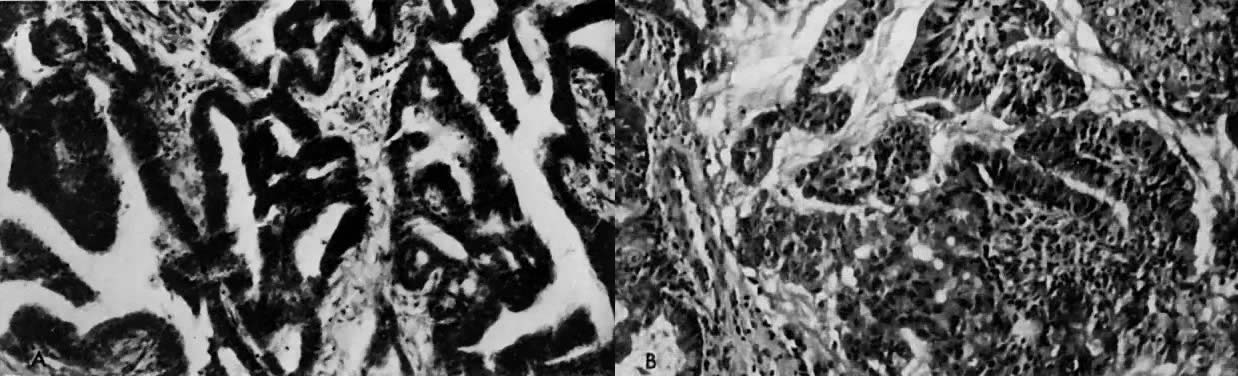 Fig. 8. A. Well-differentiated endometrial carcinoma, original tissue. B. A 72-hour organ culture with slight differentiation of the granular tissue
in the presence of low doses (10 μg/mL) of progesterone. An organ
culture in which the endometrial carcinoma was exposed to a high dose (100 μg/mL) of
progesterone showed necrosis of the tissue. Fig. 8. A. Well-differentiated endometrial carcinoma, original tissue. B. A 72-hour organ culture with slight differentiation of the granular tissue
in the presence of low doses (10 μg/mL) of progesterone. An organ
culture in which the endometrial carcinoma was exposed to a high dose (100 μg/mL) of
progesterone showed necrosis of the tissue.
|
An organ culture screening study showed that 12 of 43 patients showed tissue
necrosis; in an additional 5 patients, the culture did not survive. Thus, only 26 cultures
were available for study, of which 5 showed
necrosis with both high and low concentrations of progesterone, 6 showed
necrosis with a high dose, and 15 showed no significant effect in
culture. This incidence of progesterone sensitivity is similar to the
clinical effect seen in these patients. Although organ culture provides a good investigational tool, its clinical
application has been superseded by determination of estrogen and progesterone
binding. The practical lessons learned from organ culture sensitivity
screening are, however, very applicable to the steroid receptor
studies. First, one must be sure that carcinomatous tissue is being tested, since
the site of endometrial carcinoma is so frequently focal. It is essential
to send tissue for histologic section at the same time as the tissue
is sent for either organ culture or to test for estrogen and progesterone
receptors. Second, the tissue used for the assay must be fresh. Curettage
or even endometrial biopsy causes local tissue damage, necrosis, and
infection, and healing and repair needs to take place for
sampling to give valid results. At least 4 weeks should elapse before
further samples are obtained. Since definitive therapy is usually implemented
before this, it is suggested that samples for steroid receptor
estimation or tissue culture be obtained at the time of the primary biopsy. A mass screening program for progestogen sensitivity is now not justified. It
may occasionally be useful in cases in which recurrent disease
or metastases are amenable to biopsy, and this will be further discussed
in relation to progesterone receptor studies. Studies of Ribonucleic Acid and Deoxyribonucleic Acid Synthesis Studies of the effect of progesterone on RNA and DNA synthesis in endometrial
carcinoma42 show that progesterone at a dose of 80 mg/mL produces a mean reduction
in uptake of 39% over controls, which is a greater reduction than with
DNA synthesis. Nordqvist42 performed clinical studies with the use of radioactive thymidine and uridine. Thirteen
patients were treated with polyestradiol phosphate plus
either 17α-hydroxyprogesterone or 19-nor-17α-hydroxyprogesterone. There
was significant inhibition of DNA and RNA synthesis in 10 and 12 of 32 patients, respectively. All of these patients were given
estrogen as well as progestogen. There was no correlation between DNA
and RNA metabolism and the histologic effect in the patients studied
after 3 to 7 weeks of treatment. Monolayer Tissue Culture Studies There are several reports of normal endometrium and endometrial carcinoma
in tissue culture. Hiratsu43 reported on the successful culture of benign proliferative and secretory
endometrium and showed that estradiol stimulated growth significantly
at a dose of 0.1 mg/mL, but had an inhibitory effect at doses of 1 and 10 mg/mL. Progesterone, testosterone, and androstenedione displayed
growth-inhibitory effects on the cells within a range concentration
of 0.01 to 50 mg/mL. Liszczak and co-workers44 cultured normal human endometrial cells in monolayer cultures for up to 50 days
by a method designed to ensure maximum harvest of glandular
cells. These cells had the ultrastructure characteristics of normal endometrial
epithelial cells. The addition of progesterone to the culture
led to the appearance of abundant rough endoplasmic reticulum, a prominent
Golgi complex, and membrane-bound electron-dense bodies, findings
characteristic of secretory cells. No nucleolar canalicular systems
were induced. Such a model is the tool of choice in the study of normal
endometrium in relation to added hormones. Kuramoto and associates45 described a cloned monolayer culture of endometrial carcinoma that reproduced
the architecture of the original cancer when the cells were implanted
into a hamster cheek pouch. Histologic studies with DNA and RNA
uptake showed stimulation with estrogen and inhibition with progesterone. The
addition of high-dose estrogen, androgen, or progesterone was
inhibitory. It then became feasible to perform monolayer culture studies, by which
epithelial cells and stromal cells could be grown separately. They could
be combined in culture, thus providing an ideal means of studying the
interdependence of the epithelial and stromal elements and relating
these to the effect of various hormones. Another study approach was the
establishment of both normal endometrium and endometrial carcinoma
in nude mice (Fig. 9). Gershwin and colleagues46 and Hayakawa and co-workers47 reported that endometrial carcinoma grows well in nude mice, and they
found that fresh transplants from human tumors were established more easily
compared to cells from monolayer culture. 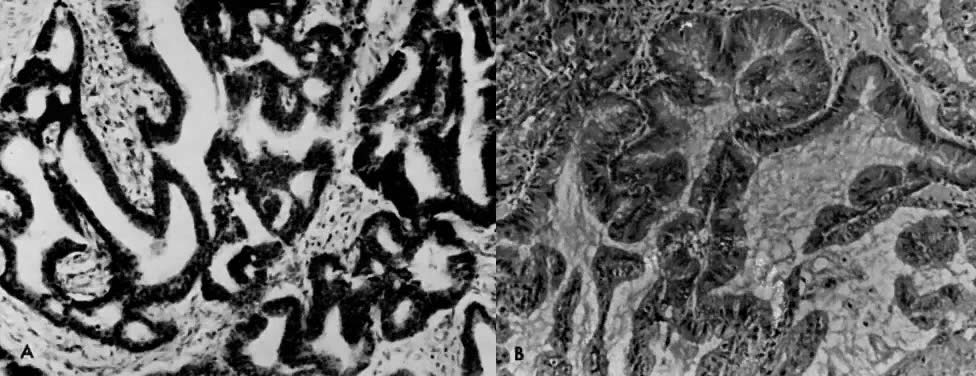 Fig. 9. Endometrial carcinoma explanted in irradiated thymectomized mice. A. Original tissue. B. Tissue grown in the immune-suppressed mouse.(Courtesy of Dr. Meirion Thomas) Fig. 9. Endometrial carcinoma explanted in irradiated thymectomized mice. A. Original tissue. B. Tissue grown in the immune-suppressed mouse.(Courtesy of Dr. Meirion Thomas)
|
By 1993, Möbus and co-workers48 were able to find reports of 28 cell lines of endometrial cancer, but
most of these were from anaplastic tumors, and few were amenable to hormone
manipulation. These authors added six new cell lines, only three
of which produced a progesterone receptor. Nevertheless, it is likely
that studies from human cells, either in vitro as monolayers or organ
cultures or in tolerant mice (e.g., nude mouse, irradiated thymectomized mouse), are more likely to lead
to meaningful data than study of adenocarcinoma in animal models (e.g., mouse, rat, rabbit). Although these animals are mammals, their hormonal
environment is quite different from that of humans. Estrogen and Progesterone Receptors In the 1980s, gynecologists sought to place estrogen- and progesterone-receptor
measurements in endometrial cancer on the same footing as it
is in the management of breast cancer. This never took hold because well-differentiated
endometrial cancer is cured much more often by surgery
and much less often by hormone administration or hormone manipulation
for all the reasons already discussed. As more high-risk endometrial
carcinomas are found to be nonresponsive to progesterone, the frequency
at which receptor measurements are performed as a routine of endometrial
cancer management will decrease. The methods of receptor measurement
are also changing. When this chapter was last revised in 1988, estrogen and progesterone receptors
were being measured biochemically on tumor specimens that had
an unknown amount of noncancerous endometrium, the presence of which
will alter the receptor value even with meticulous histologic control. During
the past 10 years, immunohistochemical assessment of steroid receptors
has become significantly more reliable. The technique has the
advantage that the receptor may be visualized in the cells' nuclei, and
one may thus be certain that the receptor is being measured in malignant
cells and not in normal cells. The concept that there is receptor
in the cytosol is now obsolete. Most gynecologic oncologists now rely
on histochemical measurements of both estrogen and progesterone receptor. Current Concept of Steroid Action Like other steroid hormones, estrogen and progesterone circulate both as
free molecules and as complexes with sex steroid-binding globulin. The
specific receptors of high affinity are restricted to target tissues. Thus, estrogen
receptors are present in the uterus, hypothalamus, and
breast and are absent in conditions of hormone insensitivity, such
as the androgen insensitivity of testicular feminization. The free steroids
diffuse in and out of all cells and are “captured” only
in “target” cells by specific receptors (Fig. 10),49 which are specialized proteins in the nucleus of the target cell that
form complexes with a particular steroid (see Fig. 10).49 These receptors are specific for the steroid and the target tissue. There
are 10,000 to 60,000 such receptors in a particular cell. Each receptor
binds its particular steroid with high affinity, showing an equilibrium
dissociation constant (Kd) of 10-9 to 10-10 mol/L. The binding of the steroid molecule to the receptor causes the
latter to be “activated” (step 3; see Fig. 10), and this allows the complex to attach to specific nuclear “acceptor” sites
on the chromatin (step 4; see Fig. 10). This process then results in the alteration of gene transcription (step 5; see Fig. 10), resulting in changes in messenger RNA and protein synthesis. The effect
of the steroid in the cell is observed 12 to 24 hours after steroid
administration. 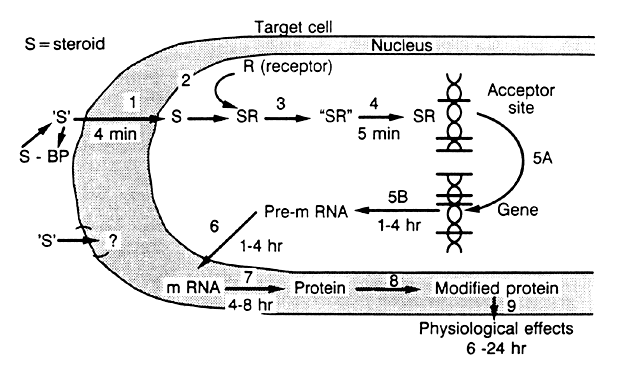 Fig. 10. Model illustrating the mechanism of action of steroid hormones in target
tissues.(Spelsberg TC, Rories CF, Rejman JJ et al: Biol Reprod 40:54, 1989) Fig. 10. Model illustrating the mechanism of action of steroid hormones in target
tissues.(Spelsberg TC, Rories CF, Rejman JJ et al: Biol Reprod 40:54, 1989)
|
Biochemical Assay Methods for Steroid Receptors Methods measuring the quantity of specific steroid receptors depend on
their ability to form complexes with radiolabeled steroids.50 The tightly bound radiolabeled steroid can be separated from free or loosely
bound ligand and counted, usually in a scintillation spectrophotometer. The
major difference between the various methods is in the principle
used to separate the free or loosely bound steroid from the steroid
most strongly associated with the specific receptor protein. For
example, with estradiol, the receptor is half saturated (Kd = approximately 10-10 mol/L at 4°C), whereas the nonspecific binding is so weak that it
is difficult to measure. Estrogen is the strongest specific steroid receptor
interaction known, but even the weaker association of progesterone
or glucocorticoid with their respective receptors have a Kd value of less than 10-8 mol/L. The Kd value of nonspecific binding is approximately 10-5 mol/L. The second distinction between the two types of receptor sites is the number
per cell. These are thousands of specific proteins per cell, compared
to millions of nonspecific proteins per cell. The most common method of separating bound from free steroid is based on
the ability of charcoal to absorb free steroid, the addition of dextran
limiting any absorption of steroid receptor complexes. The removal
of the free steroid by the dextran-coated charcoal leads to the dissociation
of weak steroid protein complexes but has only a limited effect
on higher affinity specific complexes. Polidexide (Sephadex) has been
used to quantitate estrogen receptors in animal systems. It has the
advantage of more complete separation of free and specific-bound steroid, but
it is a less simple method than dextran-coated charcoal. Sucrose-gradient
analysis was used by some investigators but is time-consuming
and expensive. Electrophoresis has been used to measure androgen receptors owing to its
ability to distinguish cellular androgen receptors and serum bound globulin. Specific Actions of Estrogen and Progesterone on Endometrium Our present understanding of the relationship between estrogen and progesterone
indicates that estrogen induces cell growth and multiplication and the formation of microvilli
and stimulates the synthesis of progesterone receptors. Progesterone reverses these processes and also inhibits the synthesis of estrogen receptors. In humans, it appears that estradiol is the active estrogen. Labeled estradiol, but not estrone, is found bound
to chromatin in human endometrium when estrone is incubated with
tissue slices of endometrium. Estradiol is converted to estrone by 17β-dehydrogenase
and estrone leaves the tissue, resulting in a decrease
in the concentration of estradiol in the tissue. The enzymatic activity
is localized in the secretory glandular epithelium. The stroma
does not show estradiol 17β-dehydrogenase activity. Thus, by inducing 17β-dehydrogenase and decreasing the number of estrogen receptors, progesterone
inhibits the proliferative effect of estradiol in
normal endometrium. Characteristics of the “Cytosol” Receptor The estrogen receptor in the cytosol of human endometrium is a protein
with a dissociation constant for estrogen of approximately 1/10 to 1/9 mol/L. In
sucrose density gradients, it appears both in the 4S and 8S
forms. Estrogen receptor binds estradiol better than estrone and estrone
better than estriol. Nonsteroidal estrogens bind to estrogen receptors
with high affinity, but do not bind to the sex steroid-binding globulin
or to plasma. The level of estrogen receptors is high in the proliferative phase and
reaches its maximum at the time of ovulation. It is very high in hyperplastic
endometrium, but low in secretory endometrium and in samples of
atrophic endometrium and in patients on oral contraceptives. The number
of estrogen receptors is directly related to the level of plasma estriol
and inversely related to the level of plasma progesterone. The
level in the endometrium is greater at the fundus than in the lower uterine
segment. The specific high-affinity progesterone receptor protein in cytosol also
has a dissociation constant of 1/10 to 1/9 mol/L and a molecular weight
of 110,000 kd. The next best competitor after progesterone is 5α-dihydroprogesterone
and deoxycorticosterone. 11-Deoxycortisol, corticosterone, and
testosterone are weaker competitors, and cortisol and
estradiol do not compete. In earlier studies, cortisol-binding globulin
could not be differentiated from specific progesterone-binding globulin, so
some reports have to be interpreted with caution. Problems in Biochemical Measurement The samples of human endometrium used for steroid receptor assay may contain
progesterone or estrogen, so the receptor binding sites may be partially
saturated with endogenous ligands. The receptors in the cell
may therefore have binding sites that are occupied and some that are free
to bind to added radiolabeled hormone. Whether all sites or only unoccupied
sites are being measured depends on the incubation conditions
and on which hormone is being measured. With estrogen, mainly the unoccupied
cytosol sites are measured, since the estrogen receptor complexes
dissociate slowly at 4°C. If the incubation is performed at higher
temperatures, a total assay of occupied and unoccupied sites may
be obtained because the estrogen receptor complex is stable at the higher
temperature. With progesterone, the rate of response dissociation
is very rapid, so an incubation at 4°C will measure both occupied
and unoccupied receptor sites. Variations in technique in part explain
the differences in the level of estrogen and progesterone receptors
that have been reported from different laboratories. Immunohistochemical Measurement of Estrogen and Progesterone Receptor Monoclonal antibodies developed for the immunocytochemical localization
of estrogen receptor51 and progesterone receptor52 have allowed identification of these receptors in biopsy and hysterectomy
specimens from patients with endometrial carcinoma. Several studies
have now compared the biochemical and immunocytochemical techniques,53 54, 55, 56 and there is general consensus that immunohistochemistry provides information
that may be more accurate and reliable than the chemical techniques, even
if archival tissue blocks are used. Parl and Posey57 enumerated the problems encountered with immunohistochemistry, including (1) delay
in fixation, causing autolysis; and (2) impure fixative, causing
alteration in receptor antigenicity. The main advantage is that
one is certain that the receptor is present in tumor cells, not in adjacent
nonmalignant tissue. The biochemical methods of course cannot
discriminate the type of epithelial cell, and the receptor value may be
inflated, particularly by the presence of hyperplastic endometrium. The
concordance level between the two methods will be significantly influenced
by the levels chosen as significant for each one, even if there
is careful histologic control with the biochemical determination. Role of Steroid Receptor Assay in Endometrial Carcinoma The introduction of techniques for detecting the presence of estrogen receptors
and progesterone receptors and actually measuring the amount
present was greeted with enthusiasm, and with the hope that the information
gained would help us choose patients for hormonal therapy. Indeed, the
available information indicates that receptors are present with
many carcinomas; however, they may also be absent. The presence of progesterone
receptors correlates with the histologic grade of the tumor, there
being a high frequency of progesterone receptors among well-differentiated
tumors and a low frequency among poorly differentiated tumors. The
receptor for estrogen appears to be present apparently independent
of the grade of the tumor. All investigators have been in agreement that both estrogen and progesterone
receptor levels are usually high in endometrial hyperplasia, with
a mean progesterone-binding capacity of 514.5 ± 421.9 femtomole (fmol)/mg
protein.58 All adenomatous hyperplasias studied had progesterone receptors, but 35% of
mixed adenomatous and cystic hyperplasias had no progesterone receptor. Of
Ehrlich and Young's59 patients, 58% had progesterone-binding globulin. Of those with grade 1 tumor, 84% had
progesterone-binding protein; with grade 2 tumor, 47%; and
with grade 3 tumor, 25%. The values of progesterone-binding globulin
corresponding to each grade are shown in Table 4. It should be noted that Young and Ehrlich defined a significant progesterone-binding
protein level as more than 50 fmol/mg protein. Pollow
and associates60 and Ehrlich and Young59 agree that there are patients with well-differentiated carcinoma in whom
no progesterone-binding protein is detectable, whereas a certain proportion
of patients with poorly differentiated tumors have significant
progesterone-binding globulin. It should also be noted that histologic
serous carcinomas and clear cell carcinomas have low levels of progesterone
receptor.5 TABLE 4. Progesterone Binding Globulin in Endometrial Carcinoma Related
to Tumor Grade
| Value | Range |
Grade 1 | 420.0 ± 584.9 | 0–2400 |
Grade 2 | 60.9 ± 54.0 | 0–196.9 |
Grade 3 | 62.9 ± 127.0 | 0–507.2 |
The values are expressed in femtomoles per milligram protein. The difference
between grade 1 and grade 2 or 3 is significant (p < 0.02)
(Young PCM, Ehrlich CE: Progesterone receptors in human endometrial cancer. In
Lippman M, Thompson B (eds): Steroid Receptors in the Management
of Cancer. Boca Raton, FL, CRC Press, 1979, vol. 1. Copyright © The
Chemical Rubber Co, CRC Press, Inc)Progesterone-binding globulin is more frequent among well-differentiated
tumors. The level of estradiol 17β-dehydrogenase is poorly correlated
with tumor grade. Several studies have found that patients given
exogenous progestogens show a decrease in both cytoplasmic progesterone
and estrogen receptor levels.61 Pollow showed that estrogen administration increased cytosolic progesterone
receptor values in relation to tumor differentiation. Ehrlich and co-workers58 and Pollow and colleagues62 gathered sufficient data to demonstrate a correlation between clinical
response and histologic grade in patients with endometrial carcinoma
who are treated with progestogens. When no progesterone receptor was detectable, response
was rare but could occur. Response was frequent when
significant progesterone levels were measured. One must conclude that in endometrial hyperplasia the effect of estrogen
on the endometrium is associated with insufficient progesterone production
and that the mechanism is probably a physiologic one. This is not
as certain in the case of endometrial carcinoma, and the only evidence
at present is that of Pollow and associates.62 Nevertheless, it is possible that the long-term beneficial hormonal effect
of progestins in endometrial carcinoma may be associated with an
exhaustion of progesterone receptors through the negative feedback mechanism
similar to that demonstrated in normal endometrium. Role of Tamoxifen Tamoxifen is a nonsteroidal antiestrogen that acts by attaching to a different
area of the receptor than estradiol and may interfere with the
binding of estradiol. In the absence of estradiol, it acts as an estrogen
agonist. It is being extensively used as adjuvant therapy in patients
with early breast cancer. There is increasing evidence, however, that
tamoxifen therapy is associated with an increased incidence of endometrial
cancer.63, 64, 65 Tamoxifen administration has also been shown to cause endometrial hyperplasia
in previously oophorectomized women.66 In vitro evidence has failed to demonstrate a stimulatory effect of tamoxifen
on endometrial carcinoma cells in culture,67 and it may have an inhibitory effect.68 The effect of tamoxifen on the uterus is well summarized by Barakat.69 From 1985 to 1995, 140 cases of tamoxifen-associated uterine cancer have
been reported. Fisher and associates70 reported that the relative risk of endometrial cancer associated with
tamoxifen was 7.5 in a study of 2843 patients with node-negative, estrogen
receptor-positive breast cancer randomly assigned to tamoxifen or
placebo. Not all tumors are necessarily well differentiated, and there
is a significant incidence of grade 3 endometrial lesions.71 Nevertheless, the beneficial effects of tamoxifen in the management of
breast cancer far outweigh the risk of endometrial cancer, especially
if patients have continuing gynecologic supervision.69 Because of its antiestrogenic properties, tamoxifen has been investigated
in trials analyzing response in metastatic and recurrent carcinoma
of the endometrium. Swenerton and colleagues72 showed objective response in four of nine patients with recurrent endometrial
carcinoma treated with tamoxifen who had previously been treated
with medroxyprogesterone. Bonte and colleagues73 used tamoxifen both in patients prior to therapy and in those with recurrent
or metastatic disease. They reported an overall remission rate
of 50% with the use of 20 mg tamoxifen twice daily and found it effective
after medroxyprogesterone had failed; however, the duration of response
was brief. Moore and co-workers74 summarized the single-agent studies in the literature and found an 8% complete
response rate and a 14% partial response rate among a total of 257 patients. However, the dose of tamoxifen varied from 20 to 40 mg
daily, many patients were pretreated, and a large proportion of patients
had grade 2 and grade 3 tumors. As with progestins, the tumors most
likely to respond are those of histologic grade 1 that are positive
for estrogen and progesterone receptors. It appears, however, that tamoxifen
may give short-term responses after progestogens have failed. Tamoxifen has been administered in combination with progestogens with or
without estrogen. This practice is based on the finding that tamoxifen, when
bound to the estrogen receptor, may stimulate the production
of progesterone receptor and therefore potentiate the efficacy of progestogen
therapy.75, 76 Carlson and associates77 treated 12 patients with tamoxifen and medroxyprogesterone and obtained
objective response in 4, with a duration of response of 7 to 24 months. Rendina
and colleagues,78 however, showed no response with this combination, but of their patients
had histologic grade 3 tumors. Thrombotic and hepatic toxicity has
been reported with combination hormonal therapy.69 It appears that there is no advantage to using tamoxifen rather than a
gestagen as primary therapy in endometrial cancer. As with the progestogens, tamoxifen
must be used with well-differentiated tumors in order
to be effective as primary therapy in metastatic or recurrent disease. The
case for tamoxifen in combination or sequenced with either estrogen
or a progestational agent is currently unsubstantiated. Other Hormonal Manipulation in Endometrial Carcinoma Wall and co-workers79 treated nine patients with endometrial carcinoma with clomiphene citrate
and noted a histologic response in two. Quinn and associates80 treated 11 women unresponsive to medroxyprogesterone with aminoglutethimide
and noted a clinical response in 1 patient. | 

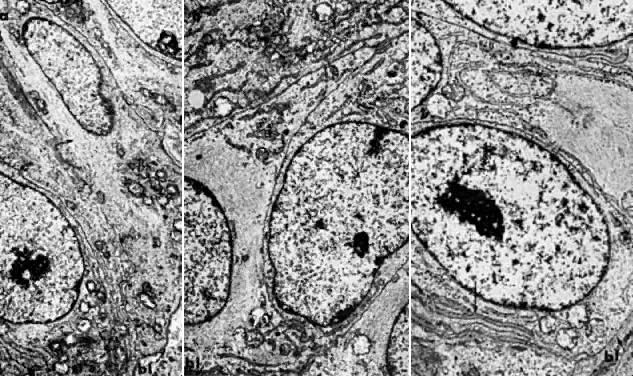
 7000). B. Carcinoma in situ of the endometrium. Note nuclear rounding, pleomorphic
organelles, and interlacing bundles of microfilaments in a perinuclear
location (
7000). B. Carcinoma in situ of the endometrium. Note nuclear rounding, pleomorphic
organelles, and interlacing bundles of microfilaments in a perinuclear
location (



