Leiomyosarcomas
OCCURRENCE.
Leiomyosarcoma is the most common uterine sarcoma, representing 16% to 75% of all uterine sarcomas.5,10,11,14 Because uterine leiomyomas are so common, many researchers have attempted to establish that leiomyosarcomas result from sarcomatous changes in preexisting leiomyomas. A review of the literature reveals the frequency of this sarcomatous change to vary from 0.13% to 10% of leiomyomas, averaging 1.46%.3,14,15,16,17,18 The actual incidence is much less than this because it is impossible to identify all uterine leiomyomas and to adequately sample all leiomyomas for sarcomatous changes.
CLINICAL FINDINGS.
Because the average age of these patients is the middle 50s, leiomyosarcomas are primarily a disease of postmenopausal women.19,20,21 The majority of these patients will present with abnormal uterine bleeding. This bleeding is postmenopausal in 48% of women with leiomyosarcomas.19 Other symptoms and findings include vaginal discharge, pelvic pain, abdominal or pelvic masses, and vaginal prolapse. The majority of patients do not have the triad of diabetes, hypertension, and obesity.
Norris and Taylor suggested an etiologic relation between pelvic irradiation and uterine sarcomas. However, none of their patients with leiomyosarcomas had antecedent pelvic irradiation.22 In a report from the University of Iowa that included 24 leiomyosarcomas, only two patients had received prior pelvic irradiation: one for benign disease and one for ovarian carcinoma.19
PATHOLOGY.
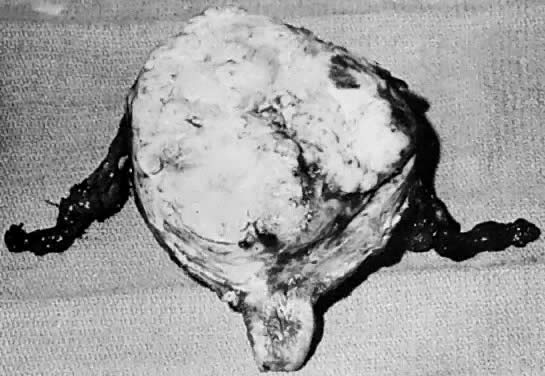
Gross.
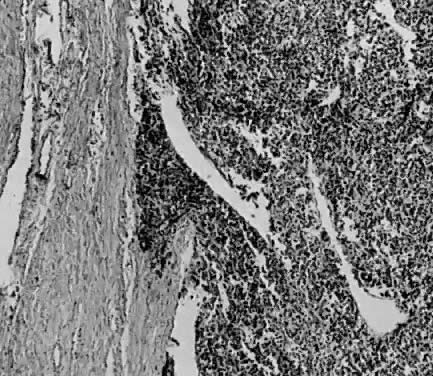
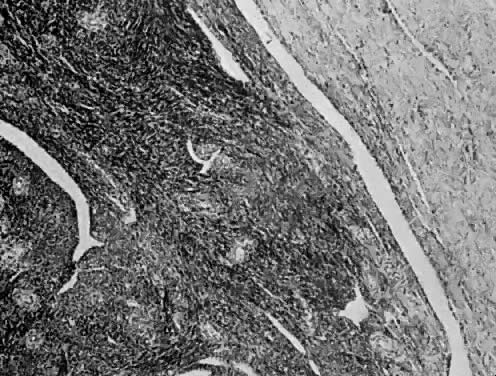
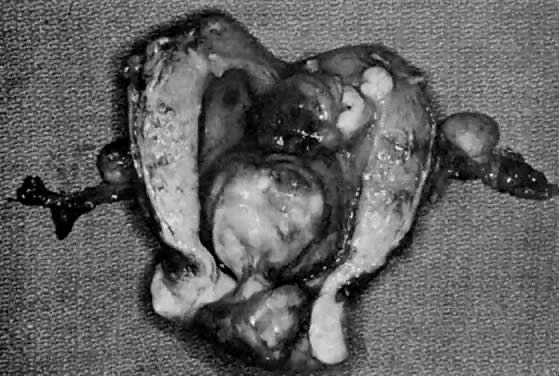
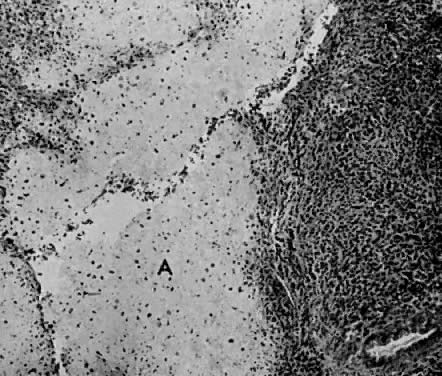
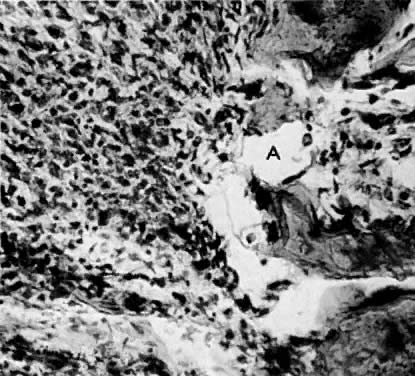
The uterus is frequently enlarged and may be regularly or irregularly shaped, and a mass may be visible through the external cervical os. The size of these tumors varies considerably and ranges from 4 to 40 cm.20 They may have a benign gross appearance and be mistaken for leiomyomas. Because of extensive growth, other tumors may have undergone necrosis and the malignancy is suspected. These tumors have been described as “unencapsulated, soft, gray-white to gray-tan, and bulging on sectioning” (Fig. 1). The whorled pattern of muscle fibers characteristic of leiomyomas is usually not visible.
|
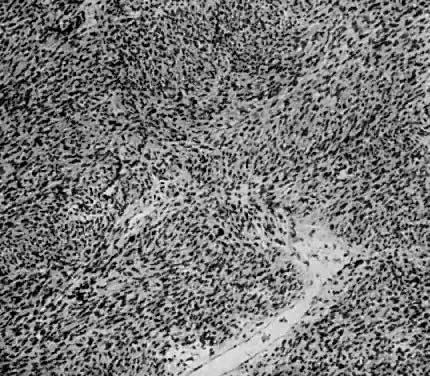
Microscopic.
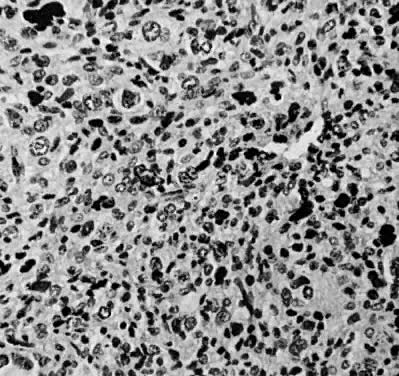
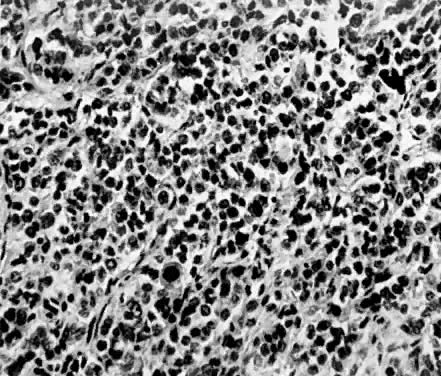
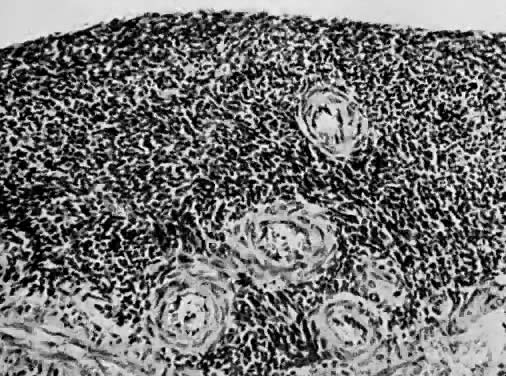
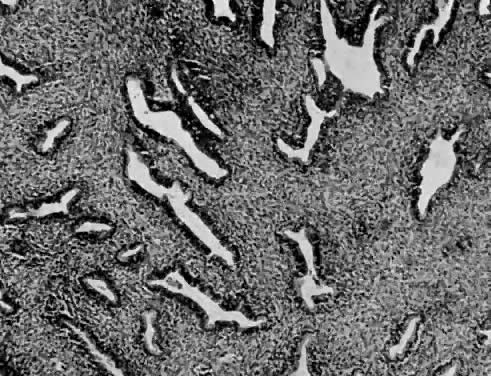
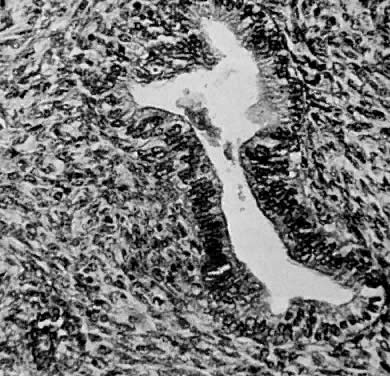
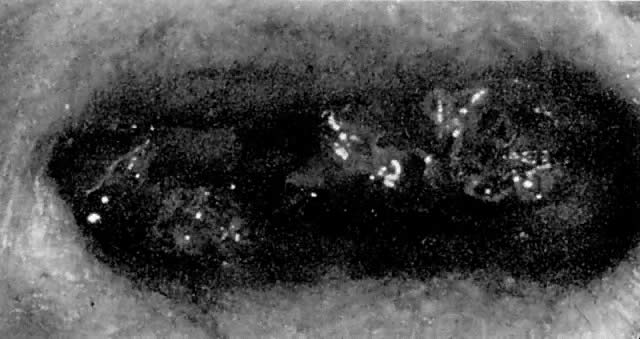
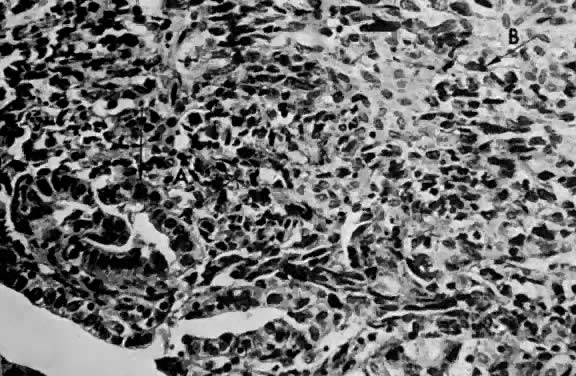
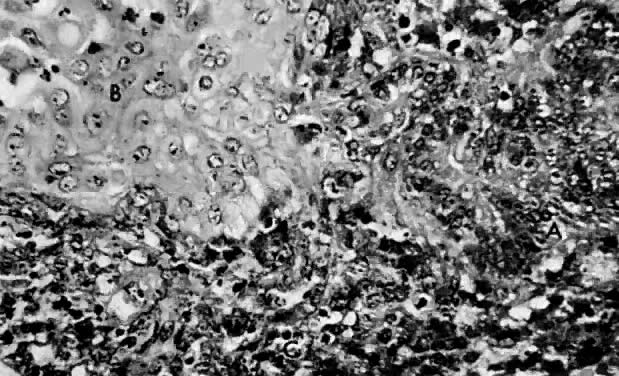
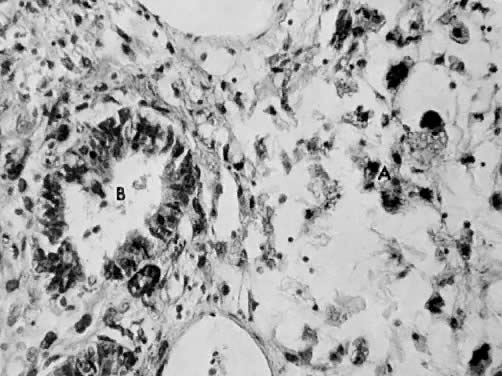
Leiomyosarcomas are composed of malignant uterine smooth-muscle cells. The cells are elongated with tapered ends. Microscopically these tumors may histologically resemble the normal uterine musculature (Fig. 2). The less differentiated the sarcoma, the less it resembles a leiomyoma. As the cellularity increases, nuclear atypism increases, the cytoplasm becomes more eosinophilic, and the number of giant cells increases (Fig. 3).
|
|
The microscopic diagnosis of leiomyosarcomas and other sarcomas has evolved slowly. Many authors have related the number of mitotic figures and the diagnosis of sarcomas.11,12,16,21,23,24 Evans was the first to document this information in 1920. He divided his cases into high, moderate, and low mitotic counts, depending on the number of mitotic figures per cubic millimeter of tissue.16 Novak and Anderson3 in 1937, Kimbrough2 in 1934, and Randall15 in 1943 reported supporting evidence. Several other diagnostic criteria have been suggested including cellularity, pleomorphism, evidence of giant cells, anaplasia, and evidence of blood vessel invasion.3,15,25 Most authors now accept 10 mitotic figures per 10 high-powered fields (HPF) as diagnostic of sarcomas. In 1981, Ellis and Whitehead discussed some of the possible flaws in this method. They stated that because of the difference in microscopes, the area of a HPF could differ by a factor of six. They suggested that for this reason the number of mitoses be expressed as the number of mitoses per square millimeter.26 Despite this admonition, most researchers agree that the mitotic count is the most reproducible and prognostically accurate method for diagnosing homologous sarcomas.27
Physicians should count the number of mitotic figures in at least 20 HPF from various areas of the tumor. The average number of these mitotic figures determines the malignant potential of the tumor. Kempson and Bari12 have demonstrated that there is nothing magical about the number 10. In their study, the patients with five to nine mitotic figures in 10 HPF did poorly. The mitotic figures can be very difficult to identify; therefore, the number counted can only be an approximation. Most researchers agree if the average number of mitotic figures per 10 HPF is 10 or greater, the tumors are definitely sarcomas.20
Atypism of the stromal cell and the presence and number of giant cells are not directly related to the diagnosis of leiomyosarcoma. However, the more undifferentiated the sarcoma, the more atypical the cells. Tumors with large numbers of mitotic figures contain more giant cells and more atypical stromal cells. Vascular invasion is an infrequent finding and is usually observed in leiomyosarcomas with more than 10 mitotic figures per 10 HPF.
PROGNOSIS.
A number of factors influencing survival have been studied:
Anaplasia28
Race18,25
Cellularity11
Pleomorphism3,11,17,18,29
Presence of giant cells12,28
Presence of mitotic figures12,20,25
Evidence of blood vessel invasion10,14,18
Amount of disease25,29,30
Menarche status14,25
Origin in leiomyomas3,17,18,28,29
Gross appearance of disease25,29
Once a sarcoma has been diagnosed, survival is directly related to the volume of tumor. The amount of disease rather than the method of therapy seems to be the most important factor affecting survival. The survival is best if the disease is confined to the uterus, and it decreases as the extent of the disease increases. The 5-year survival rate for leiomyosarcomas has been reported to vary from 0 to 68%.10,14,17,19,25,28
Spiro29 reported 77% of his patients had recurrent disease within 24 months; 88.2% of our patients had recurrences within 24 months.19 Within 24 months, 94.1% of our patients with recurrent disease died. The most common sites of recurrence are the pelvis and lungs; other sites include the liver, skin, spinal cord, ribs, and skull.19