Radiation can be delivered by means of external sources (EBRT), implanted irradiation (brachytherapy), or radioactive fluid. This section discusses EBRT and brachytherapy. Radioactive fluid instillation is occasionally done intraperitoneally with P32, most commonly as adjuvant therapy in ovarian cancer. Some work has been done using P32 in patients with endometrial carcinoma with positive cytology82; this work will not be discussed further, however, because data are somewhat limited.
EBRT is used to irradiate areas thought to be at risk for disease recurrence, including the whole pelvis, the whole pelvis plus the para-aortic nodal region, and the whole abdomen. EBRT is produced by cobalt machines or linear accelerators. As the energy of radiation increases, the beam penetration also increases, making it possible to limit the peripheral radiation needed for delivery of a desired dose at depth. Because the pelvis has a relatively thick separation, higher energy beams are preferred.
Whole-abdominal irradiation is used to irradiate the entire abdominal contents. With modern radiation machines, this usually can be accomplished with a single setup, treating with an anterior and posterior field. The total whole-abdominal dosage is usually limited to 2000 to 3000 cGy in fractions of 100 to 150 cGy per treatment. Vital organs may need to be shielded to limit the radiation dose. The kidneys should be shielded to limit the dose to approximately 1800 cGy; liver shielding should also be considered if the dose exceeds 2500 cGy. Whole-abdominal irradiation in endometrial cancer is usually followed by a boost to the pelvis, preceded in many situations by a para-aortic nodal boost.
Treatment of the para-aortic nodes can be accomplished with either separate fields matched to the pelvic field or in continuity with pelvic radiation fields. I prefer to use a single field to avoid problems of matching. The para-aortic nodes can be treated with a two-field or four-field technique, generally to a total dosage of 4000 to 4500 cGy at 170 to 180 cGy per fraction. If a two-field technique is used, care must be taken to ensure that the dose to the spinal cord is limited to less than 4500 cGy. If a four-field technique is used, the location of the kidneys must be verified to avoid exceeding kidney tolerance.
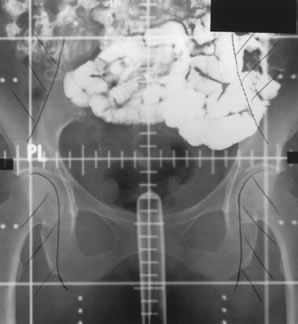
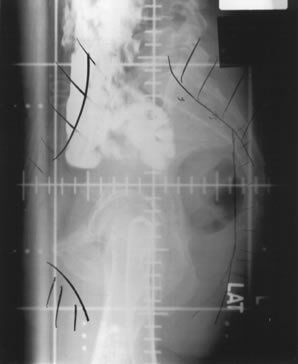
Whole-pelvic irradiation can be accomplished by either a two-field or four-field technique. To avoid excessive maximal dosages, the two-field technique should be used only with high-energy beams. The two-field technique uses opposed anterior and posterior fields (Fig. 1). The upper border of the field is generally placed at the L4-5 or L5-S1 interspace. If there is no disease extension into the vagina, the lower border should encompass one half to two thirds of the vagina. The lateral borders should be placed approximately 1.5 cm lateral to the bony pelvic rim. A marker should always be placed to indicate the location of the vaginal cuff/cervix or the most distal aspect of tumor extension. The four-field technique allows lateral shielding of structures that cannot be shielded in the anteroposterior field. This is my preferred method of pelvic irradiation. In the four-field technique, the upper and lower field borders are identical to those in the two-field technique. The anterior border of the lateral field is placed at or anterior to the anterior pubic symphysis. The posterior border is placed at the S2-3 interspace unless tumor extension necessitates larger fields. An example of a lateral field is shown in Figure 2.
|
|
Pelvic radiation therapy technique is extremely important in treatment outcomes, especially in reducing short-term and long-term toxicity.83 Barium should be given at the time of simulation to document the position of the small bowel.84 Attempts to reduce the small bowel in the radiation field include placing the patient in the prone position with a full bladder with or without abdominal compression. Patients should always be treated with a full bladder to move as much of the small bowel as possible out of the pelvic field. The total pelvic radiation therapy dosage is discussed later.
Brachytherapy refers to the placement of a radioactive source in or near the desired treatment volume. This allows a higher local radiation dose and spares surrounding normal tissues. The two main forms of delivery of brachytherapy are the LDR and the HDR techniques. The LDR technique uses isotopes that deliver radiation with a dose rate of approximately 40 to 100 cGy/hr to the prescribed target. HDR brachytherapy, which delivers approximately 200 cGy/min, can be performed on an outpatient basis. There is a significant biologic difference between LDR and HDR brachytherapy: HDR delivery has a higher “effective” radiation dose for the same nominal LDR dose. Therefore, the delivered HDR doses must be adjusted lower to give the same effective LDR treatment.
The isotopes used in LDR treatment typically include cesium-137 or radium-226. Radium-226 has fallen out of favor because of radiation safety issues. Cesium-137 has a half-life of 30 years, allowing reuse of a source over a long period, although periodic calibration to allow for decay is necessary. HDR treatments typically use an iridium-192 source that needs frequent recalibration and replacement. Iridium-192 can also be used as an LDR isotope.
Typically, in most gynecologic applications of brachytherapy, the sources of radiation are left in place temporarily and then removed. This is the case in most LDR applications and all HDR applications. Permanent LDR brachytherapy procedures have a limited use in gynecologic malignancies and are not discussed further.
The sources of radiation are, in almost every case, afterloaded into a hollow radiation carrier. This permits some planning before determining the strength of radioactive isotope to use and significantly reduces radiation exposure during placement. The carriers used for afterloading can be divided roughly into those used to treat the intact uterus and those used after surgery.
The uterus may be treated with a tandem alone, as is done in treating cervical cancer. Treating the uterus with a tandem alone may underdose the thicker sections of the myometrium. Heyman14 originally described using multiple radium capsules packed into the uterus to stretch and thin the wall to improve the dose distribution. Simon and Silverstone85 later developed afterloading capsules to decrease radiation exposure during placement.
Brachytherapy dose is defined either in terms of actual dosage delivered or in terms of total milligram-hours, which is simply derived by multiplying the total milligrams of equivalent radium by the total number of hours of the implant. The doses of radiation used when delivered before surgery with planned hysterectomy typically range from 2500 to 4000 mg-h to the uterus using a tandem or Simon-Heyman capsules and colpostats to deliver 1900 to 2000 mg-h (6000 to 6500 cGy vaginal surface dose) to the upper vagina. In some patients, 50 Gy of postoperative EBRT is added, with the whole-pelvic dosage limited to approximately 2000 cGy by the addition of a midline shield. When definitive radiation is delivered without planned hysterectomy, uterine milligram-hours range from 3000 to 10,000, depending on whether EBRT is also delivered.1–6 Although not commonly reported, the point A dose (i.e., the dose defined as 2 cm superior and 2 cm lateral to the external os) is approximately 7500 to 8500 cGy.1,3 HDR is generally delivered in a fractionated manner, with an attempt to deliver biologically equivalent dose to the traditional LDR implants.
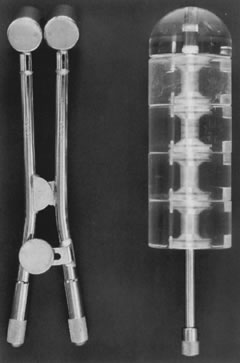
Posthysterectomy vaginal brachytherapy is generally delivered with a Delclos vaginal cylinder or with ovoids from a Fletcher-Suit apparatus (Fig. 3). The dose delivered with brachytherapy alone tends to be prescribed at the vaginal surface. Doses range from 6000 to 7000 cGy.37,42,43 I currently prescribe 7000 cGy using cesium-137 with Fletcher-Suit ovoids. The use of postoperative high-dose brachytherapy is becoming more common, allowing outpatient treatment. A common dose schedule is 2100 cGy divided into three fractions of 700 cGy and prescribed to 0.5 cm from the vaginal mucosal surface.41
|
The ideal timing of postoperative radiation therapy is not known. There is support for initiating postoperative irradiation within 6 weeks after surgery. A higher local failure rate was seen with a delay of longer than 6 weeks.86 Given the time needed to initiate treatment planning, patients for whom postoperative irradiation is being considered should be referred immediately to the radiation oncologist to prevent nonmedical delays in the initiation of therapy.
The need for a vaginal-cuff boost after postoperative EBRT recently has been questioned by a number of investigators.86,87 I continue to use a vaginal-cuff brachytherapy boost in all of my patients, and numerous large studies have consistently used vaginal-cuff boosts with excellent long-term results.40,41 In addition, at least one nonrandomly assigned review noted improved local control with the addition of a vaginal-cuff boost to postoperative EBRT.88 The number of absolute vaginal-cuff recurrences prevented by a vaginal-cuff boost is probably small; however, I have seen few recurrences or complications using this technique (abstract in press). In patients receiving postoperative EBRT, we currently boost the vaginal cuff with a mucosal surface dose of approximately 2000 to 3000 cGy after completion of 4500 to 5040 cGy pelvic radiation therapy. We have also recently initiated HDR vaginal-cuff boost and have used a dose of 500 to 600 cGy vaginal surface dose for three fractions. To limit bowel toxicity, we generally reduce the pelvic field slightly after 4500 cGy has been given to treat only the true pelvis. Occasionally, the pelvic dose is limited to 4500 cGy if the treatment-planning small-bowel barium study notes excessive bowel in the pelvic field. Other institutions deliver a higher total vaginal mucosal dose and limit the midpelvis external dose by using a midline shield.40