Planning of appropriate therapy for invasive carcinoma begins with clinical evaluation of the lesions and an assessment of the patient to determine her risk as an operative candidate. Vulvar carcinoma has a propensity to remain locally confined. Metastases occur by way of the lymphatics to the groin, a site amenable to clinical evaluation and successful treatment by inguinal lymphadenectomy. Treatment failures primarily result from failure to ensure adequate margins during excision of the primary lesion or from recurrence within the lymphadenectomy site. The latter should not occur with proper surgical technique for removal of microscopic clinically occult metastases. It is, however, a more frequent problem when extensive metastases associated with clinically suspicious or positive nodes are present before surgery. In addition to an increased frequency of local (incisional) recurrence, positive groin nodes also are associated with an increased frequency of pelvic lymph node metastasis and progression of disease to distant sites.
Squamous carcinoma of the vulva usually proceeds in a predictable fashion through its regional lymphatics, first to the inguinofemoral nodes and only then to pelvic and distant sites. Therefore, the superficial inguinal lymph nodes are the most common site for detecting metastasis. Metastasis to the contralateral nodes in the absence of involvement of the ipsilateral nodes is rare.8 Midline lesions involving the clitoris may have spread to bilateral inguinal nodes. Direct metastasis of invasive squamous carcinoma of the vulva to the pelvic lymph nodes is rare, occurring with a frequency of 0% to 3%.9
One of the most important recent developments in the treatment of vulvar carcinoma has been the individualization of treatment. Less radical surgery for early stage disease does not compromise survival. No lymphadenectomy is needed for patients with microinvasive tumors (stage IA). For unilateral lesions less than 2 cm in diameter, contralateral lymphadenectomy is not required unless lymph node metastasis is found in the ipsilateral side at the time of surgery. Performing separate incisions for inguinal lymph node dissection results in better wound healing with a reduced rate of infection.10
Another important development is the use of preoperative radiation therapy. It reduces the need for primary exenteration in advanced cancer, therefore leading to conservation of urinary and defecatory functions. It also is useful for the management of enlarged fixed and ulcerated nodes. Preoperative radiation has not been associated with significant healing problems or fistula formation. Planning of therapy should be individualized to define the least radical surgical intervention possible for achieving control of the primary lesion and the immediate regional lymphatics (the groin) while recognizing the potential for distant spread.
Staging
Clinical criteria for staging of carcinoma of the vulva were formulated and adopted in 1970 by the FIGO. A modified surgical staging system was adopted in 1989 and revised in 199511 (Table 1). The definition of microinvasive carcinoma follows the recommendations of the International Society for the Study of Vulvar Disease and the International Society of Gynecologic Pathologists.12 The staging emphasizes definition of the primary tumor by size and location, including the involvement of structures contiguous with the vulva. Status of the nodes is based on surgical evaluation of the groin. The presence or absence of distant metastasis also is considered, including cystoscopic or proctoscopic evaluation. The survival results for stages I and II are similar and excellent, approximately 90% when no positive groin nodes are present.13 Stage III lesions have a 50% 5-year survival rate, although successful treatment of 60% to 70% of patients has been reported.
TABLE 1. FIGO Staging of Vulvar Carcinoma (1995)
Stage | Clinical Findings |
0 | Carcinoma in situ |
I | Confined to vulva or perineum, |
IA | Stromal invasion |
IB | Stromal invasion >1.0 mm |
II | Confined to vulva or perineum, >2 cm; nodes are negative |
III | Any size lesion with spread to urethra, vagina, or anus or with unilateral regional lymph node metastasis |
IVA | Invasion of upper urethra, bladder mucosa, rectal mucosa, pelvic bone, or bilateral regional node metastasis |
IVB | Any distant metastasis, includes pelvic lymph nodes |
FIGO, International Federation of Gynecology and Obstetrics.
Treatment of the Primary Lesion
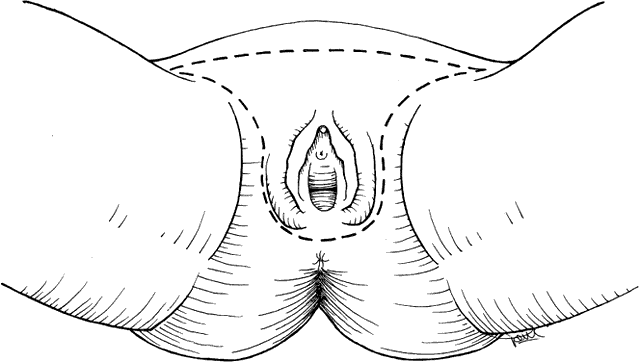
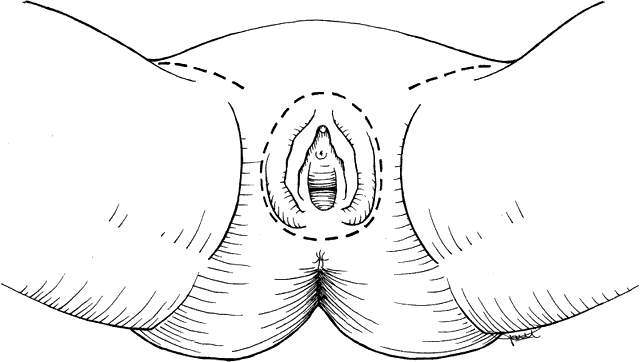
Size and location of the lesion are the primary parameters in deciding what procedure to perform. Early stage lesions are treated surgically. Advanced stage lesions are treated preferentially with chemoradiation. Pelvic exenteration with radical vulvectomy and inguinal lymph node dissection is a surgical option with significant physical and psychological morbidity as well as mortality. This procedure is rarely indicated. Treatment recommendations are summarized in Table 2. Microinvasive tumors (less than 1 mm of invasion, stage IA) may be treated by wide local excision with low risk of groin lymph node or vulvar recurrence.14,15 A 1-cm lateral margin is recommended. Lesions less than 2 cm in diameter (stage IB) may be treated successfully by wide radical excision. A 2-cm lateral margin is obtained, and dissection is carried down to the fascia of the urogenital diaphragm for the deep margin.16 When the invasion of the lesion is greater than 1 mm, ipsilateral groin lymph node dissection is recommended. This approach of radical local excision and ipsilateral node dissection works best for lesions that are lateral or posterior. Stage II lesions (greater than 2 cm in diameter) are treated by radical vulvectomy with inguinal-femoral lymph node dissection. Certain stage III lesions with extension to the lower urethra or proximal vagina may be treated the same way. Lymph node dissection is done by the separate incision technique rather than the en bloc resection performed years ago (Figs. 1 and 2). This results in decreased rate of wound separation and infection. The cosmetic results also are satisfactory for the patient. Early complications of radical vulvectomy are wound separation in up to 14% of patients and seromas of the femoral triangle in 10% to 15% of patients.17 Medical complications are significant and occur in up to 15% of patients. These include deep vein thrombosis, pulmonary embolism, and myocardial infarction. Urinary tract infections also are common. Late complications are less common. Chronic edema of the extremities and recurrent cellulitis are related to inguinal lymphadenectomy. Urinary incontinence and pelvic prolapse may require further reconstructive surgery. Osteitis pubis is rare but results in significant morbidity for the patient. Sexual dysfunction with dyspareunia from stenosis of the vagina should be managed conservatively. Poor self-esteem should be explored because the patient is not likely to express her feelings in this area. A sense of mutilation is common.
TABLE 2. Approach to the Treatment of Vulvar Cancer
Stage | Treatment |
0 | Wide local excision |
IA | Wide local excision |
IB | Wide radical excision |
| Ipsilateral inguinal lymphadenectomy |
| Bilateral if midline or clitoris lesion |
II | Radical vulvectomy with bilateral inguinal |
| Lymphadenectomy |
III | Lesions involving outside vagina or lower urethra: Radical vulvectomy with bilateral inguinal lymph node dissection |
| Lesions involving anus or rectovaginal septum: chemoradiation ± surgery |
IV | Chemoradiation ± surgery |
|
|
Inguinal Node Involvement
Careful evaluation of both the clinical and the pathologic status of the inguinal nodes is important. In the absence of inguinal node metastases, most patients with lesions smaller than 2 cm should be cured, particularly if their primary lesion is excised with wide margins negative for disease. A close surgical margin is a powerful predictor of local recurrence. Deep or lateral margins greater than 8 mm are associated with no recurrence in the study of Heaps and colleagues.18 The risk of regional lymph node metastasis is related to the primary lesion, depending on lymphatic and vascular invasion, clinical stage (tumor size), and depth of stromal invasion.18–20 When the stromal invasion exceeds 2 mm, the risk of node metastasis has been reported to be greater than 20%, whereas the risk is 40% when the thickness or invasion exceeds 4 mm.21 Although it is possible to anatomically and experimentally demonstrate the presence of pathways of direct lymphatic communication from the vulva to the pelvis, particularly in the area of the clitoris, such direct pelvic metastases have not been of clinical significance, occurring in squamous carcinoma in only up to 3% of cases.9
Clinical evaluation of the status of inguinal nodes has been subject to widespread misunderstanding and criticism because of variability in correlation between clinical impression and histologic status of the lymph nodes. In nodes that are not clinically suspicious, the frequency of metastasis varies from 12% to 43%. This wide range is not simply a reflection of variable clinical acumen, but also a statistical function. First, there is a significant variability in the frequency of metastatic inguinal lymphadenopathy reported within any series, varying from 37% to 68%. This is an uncontrollable variable reflecting the character of the population being evaluated. From review of large reported series, between 68% and 87% of the patients with clinically positive nodes will have metastatic cancer. Conversely, two thirds to three fourths of patients who have positive nodes will have palpable suspicious or clinically positive nodes before surgery. This implies that 25% to 30% of the lymph node metastases are present in unsuspicious lymph nodes. During lymphadenectomy of clinically suspicious nodes, only the enlarged nodes are removed. If frozen section confirms the presence of carcinoma, it is not necessary to proceed with full dissection of the femoral triangle. These patients require postoperative radiation. The least possible disruption of the femoral triangle results in decreased radiation complications, particularly lymphedema and wound breakdown. In a reported series of 40 patients with inguinal node metastasis, 20% had thigh or groin recurrence.22 Such recurrence occurred only in patients who had clinically suspicious or positive groin nodes before surgery. No patient with microscopic node disease, that is, metastases in nonpalpable or unsuspicious nodes, experienced recurrence in the groin.
Intraoperative lymphatic mapping and sentinel node identification has the potential to improve detection of lymph node metastasis and decrease morbidity by minimizing radical lymph node dissections. The technique of sentinel node biopsy was originally developed for selecting patients with melanoma for regional node dissection. Vital blue dye or a radioactive tracer is injected close to the tumor. The sentinel node can be identified by lymphoscintigraphy in the case of the radioactive tracer. Use of 99mTc-labeled sulfur colloid is technically feasible and may be sensitive for detecting subclinical metastasis in regional lymphatics.23 The blue dye is identified during dissection in surgery. The predictive value of intradermal injection of patent blue dye has been found to be low.24 Multicenter studies evaluating the utility of these techniques are needed.
Pelvic Lymphadenectomy
Although pelvic lymphadenectomy formerly was emphasized as an essential component of “adequate” treatment of invasive carcinoma of the vulva, this is no longer the case. First, it has been documented that direct metastasis of squamous carcinoma of the vulva to the pelvic lymph nodes bypassing the inguinal lymphatics rarely occurs.9 Second, the clinical status of the inguinal nodes does reflect the probability of pelvic node metastasis. Pelvic node involvement is not seen in the absence of ipsilateral groin metastasis. Clinically positive groin nodes are associated with pelvic node metastases in 33% of cases. Based on collected data, 19% of patients with microscopic groin metastasis also have pelvic node metastasis. This number increases with three or more microscopically positive nodes.20,22,25,26 The overall risk of pelvic node metastases is between 8.5% and 16%.27 Basically, only the patient with clinically apparent metastases in the groin nodes or in Cloquet's node is at risk for pelvic metastases. The other indication for pelvic lymphadenectomy is the presence of enlarged nodes in computed tomography scan of the pelvis. An extraperitoneal approach should be used. The absolute survival rate after positive pelvic lymphadenectomy has been reported to vary from 12.5% to 25%. Patients with positive groin lymph nodes currently are treated with postoperative radiation, which includes the pelvic nodes in the radiation field.
Radiation Therapy
The effective use of radiation therapy for the treatment of vulvar carcinoma has been demonstrated in recent prospective and retrospective studies. Relatively high doses of radiation can be delivered safely to the vulva. Locoregional control, survival rates, and complications may be improved by the use of radiation in advanced vulvar cancer. In particular, the need for exenterative surgery can be reduced with the utilization of preoperative radiation. Extrapolation from the results of chemoradiation in carcinoma of the anus has lead investigators to explore the use of cisplatin, mitomycin C, and 5-fluorouracil as radiosensitizers.28,29 Small retrospective series suggest that response rates to radiation are improved by the addition of concurrent chemotherapy. Nevertheless, the role of radiation and chemoradiation in the treatment of advanced vulvar cancer is not well defined.
The use of postoperative radiation in decreasing regional recurrences is better defined. A prospective randomized study by Homesley and associates examines the use of radiation after radical vulvectomy with inguinal and pelvic lymphadenectomy in patients who had positive groin nodes.22 For patients with clinically positive nodes, the 2-year survival rates were 59% for the group that received radiation and 31% for the group that had lymphadenectomy only. The patients with clinically positive nodes or multiple microscopically positive nodes benefited the most. For clinically negative nodes, there seems to be no significant difference in groin recurrence rates between radical lymphadenectomy and groin radiation.30
Recurrence of Disease
The number of positive nodes is the most important predictor for recurrence. Patients with one microscopically positive node have an excellent prognosis, with an overall survival rate of 90%. The presence of three or more positive nodes carries a poor prognosis with, a 5-year survival rate of 50%, even after radiation to the groin and pelvis.31,32 Recurrence localized to the vulva may be treated by local resection. Local resection may achieve disease control for an extended period or may be only palliative. Lesions limited to the vulva that can be resected with a negative margin can result in recurrence-free survival in up to 75% of cases.33 Radiation is another alternative to treat these recurrences. Central recurrence to the vagina with no evidence of inguinal node involvement may be treated by exenteration. Groin recurrence may be treated with radiation if the patient did not get radiation after the primary surgery. Groin recurrence is not associated with long-term survivors. Both local and systemic dissemination of disease occasionally have been treated successfully with chemotherapy.
 2 cm in greatest dimension; no nodal metastasis
2 cm in greatest dimension; no nodal metastasis