Treatment is justified largely for the prevention of progression to invasive
cancer. Patients more likely to progress include those with high-risk
molecular markers. Patients who are at risk for progression include
immunocompromised HIV-positive women and transplant recipients. Other
patients may be at high risk for progression based on molecular markers, such
as multifocal disease, presence of VIN III, and markers of
angiogenesis. Pregnancy may represent a transient immunosuppressive condition. As
such, lesions that have been adequately examined on biopsy
specimens to exclude cancer may be observed for spontaneous regression. It
is imperative, however, to note the difficulty in excluding cancer
in any lesion short of its complete excision.54
Despite the uncertainty that surrounds the prognosis of VIN lesions and
their association with cancer, at this time, it is traditional to treat
all VIN lesions after a variable period of observation to establish the
diagnosis, rule out invasion, and document persistence.1,2,26,55
After cancer has been excluded, it may be appropriate under certain circumstances,
such as the third trimester of pregnancy, to observe the patient for several
months, hoping for spontaneous regression of the lesion. As previously
mentioned, there are many reports in the literature of vulvar CIS spontaneously
resolving.23,29,56
Aggressive treatment and follow-up of a large series of patients with
VIN III was reported to be highly successful in preventing invasive disease.
In a group of 102 patients with VIN III, treated and followed for 15 years,
only one case of vulvar cancer was reported.51
In another group of 113 patients analyzed between 1961 and 1993, 87.5%
of untreated patients progressed to invasive vulvar cancer, and 3.8%
of treated patients later were diagnosed with invasive vulvar carcinoma.57
As understanding of VIN increases, it is hoped that clinicians will be
able to discern which lesions can be observed and which need treatment
because of symptoms or their risk of progression. This situation is analogous
to the evolution currently taking place in the treatment of cervical intraepithelial
lesions, although the study of VIN is at an earlier stage.
One of the most important parts of treatment is delineation of the boundaries
of disease. Many patients with VIN have multifocal or multicentric disease.23,26,50
Multifocal is defined as different sites on one organ and can affect
84% of patients.50 Multiple lesions
on the vulva would be considered multifocal. Multicentric refers
to disease on different organs at the same time, such as the vulva and
the cervix. Of patients with VIN III, 35% have either the vagina
or the cervix involved with a squamous cell neoplasia.58
For this reason, all patients with VIN should have a Papanicolaou smear
and careful examination of the perineum, vagina, cervix, and anus.59
This approach is especially recommended for HIV-positive women, who may
be at higher risk for multicentric disease.33
The clinician must consider the entire extent of the abnormalities present
before initiating a treatment plan.
The main method of treating initial VIN at this time is local ablative
therapy. Before local destructive therapy is used, however, it is imperative
that invasive disease be excluded. Some authors caution against
ablative therapy with extensive VIN III because of the inability to exclude
cancer.55 There are no randomized comparative trials of the different available
modalities. Case series reports suggest, however, that efficacy rates
are approximately equal.60 Because the superficial nature of VIN is now recognized, deep resections
no longer are necessary. If the lesion is small, approximately 1 to 2 cm, a
simple local excision suffices with primary closure. Given the
frequency of multifocal disease, however, surgical excision often can
be impractical, requiring extensive removal of tissue. Cryotherapy also
may be used, although it is ill-suited for extensive lesions. For
these reasons, laser therapy is currently the most popular local ablative
method of treating VIN. Promising other therapies include photodynamic
therapy and topical immune modulators. When invasion is excluded, therapy with the carbon dioxide laser is useful
in treating large areas that have been defined clearly. Outlining
the area to be treated requires magnification with a colposcope after
application of 5% acetic acid. If the area is not marked before
laser treatment, a leading edge of thermonecrosis produces a confusing
pseudoacetowhite appearance. Guidelines for treatment depths are available
and have been correlated with gross appearance and histologic levels (Fig. 2).61 Although there is no doubt that a laser can be used to destroy neoplastic
lesions, learning the proper technique can be difficult and is best
achieved through direct instruction in the clinical setting from an
experienced physician. Even in expert hands, trying to control the exact
depth of tissue destruction is difficult, given the multiple variables
present in the clinical situation.62 In general, nonhairy vulvar areas require laser treatment to a depth of 1 mm; hairy
areas should be vaporized to less than or equal to 2 mm.47 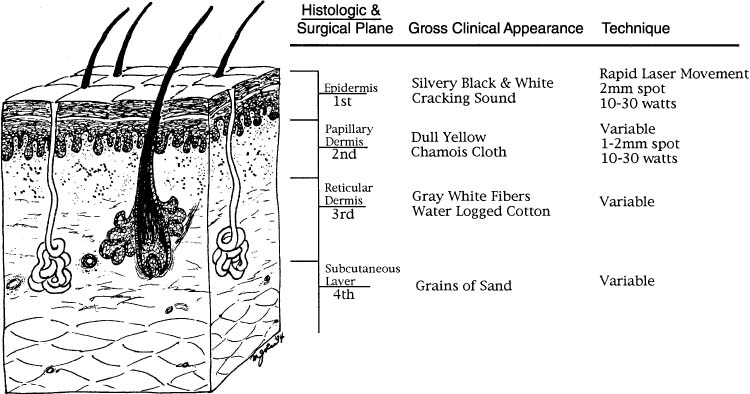 Fig. 2. Cross-sectional representation of vulvar specimen and associated clinical
features.(Data from Reid R, Elfont EA, Zirker RM, Fuller TA: Superficial laser vulvectomy: II. The
anatomic and biophysical principles permitting accurate
control over the depth of thermal destruction with the CO2 laser. Am J Obstet Gynecol 152:261, 1985.) Fig. 2. Cross-sectional representation of vulvar specimen and associated clinical
features.(Data from Reid R, Elfont EA, Zirker RM, Fuller TA: Superficial laser vulvectomy: II. The
anatomic and biophysical principles permitting accurate
control over the depth of thermal destruction with the CO2 laser. Am J Obstet Gynecol 152:261, 1985.)
|
The most challenging VIN patients to treat are those who are immunocompromised
by either transplants or HIV disease. For these patients, some
sort of long-term maintenance therapy may be effective, such as weekly
topical 1% 5-fluorouracil cream.63 Additional options include combination therapy with immunomodulators.64 In a small crossover study with no placebo control, VIN seemed to have
responded to interferon-α.65 In addition to interferons, retinoids may be another area with significant
therapeutic potential. Retinoic acid has shown efficacy in treating
cervical intraepithelial lesions and has been shown to be active on
vulvar dystrophies.66 Given the high spontaneous regression rate of VIN, however, these treatments
are considered experimental and should be used only in investigational
settings. |