The primary clinical treatment of vulvar carcinoma is surgical extirpation and it is discussed in detail elsewhere. However, the risk of local and regional recurrence and occasionally, a lack of surgical candidacy have ensured a dominant role of radiotherapy in ultimate curative treatment programs. This section elucidates the role radiotherapy plays in various clinical situations.
Postoperative Radiation Therapy
ADJUVANT TREATMENT OF THE GROIN AND PELVIS.
Previously, pelvic lymph node dissection was routinely performed in patients with a node-positive groin dissection. Because survival is markedly reduced in patients with pathologically involved inguinal lymph nodes, controversy surrounded the appropriate extent of lymph node dissection. Although the risk of pelvic lymph node involvement with the pathologically negative groin was reported to be negligible, it was postulated that in as many as 20% of patients, the pelvic nodes may harbor subclinical disease. Pelvic lymphadenectomy was supported by some even when the groin dissection is negative.12–17
Hacker and Berek showed that pelvic lymph node dissection does not contribute to survival when the groin nodes are pathologically negative. In their review of 79 patients with negative groin nodes and 16 patients with only one involved node, omission of pelvic lymphadenectomy did not affect survival.15
The addition of pelvic lymphadenectomy may be associated with increased complications.18 GOG Protocol 37 randomly assigned 114 patients with invasive vulvar cancer and positive groin nodes after radical vulvectomy and bilateral groin lymphadenectomy to receive pelvic lymph node dissection or 45 to 50 Gy of radiation therapy to the groin and pelvis. The primary site was not radiated. The estimated 2-year survival rate was 68% in the radiation group and 54% in the pelvic dissection group (p = .03). Pelvic recurrence was 6.8% in the radiation group and 1.8% in the pelvic node resection group. More significantly, groin recurrence was 5.1% in the radiation group and 23.6% in the node resection group, supporting the strong association between prevention of groin relapse and survival with radiation. The survival benefit with radiation in this study was shown for patients with clinically fixed nodes, two or more positive groin nodes, or both. Early and late morbidity was similar in both groups.9
The population sample of GOG Protocol 37 was too small to conclude that radiation was of benefit for patients with only one positive groin node. Mariana and coworkers published a review of 58 patients following radical vulvectomy and bilateral inguino-femoral groin dissection. Twenty patients had positive groin nodes and most of those who received treatment (9 of 16) had only one positive node. Sixteen patients received 45 to 50 Gy to the dissected groin with cobalt-60 (Co-60). The 64% survival rate seen in the radiated patients was comparable with results of GOG 37 and better than historical results with surgery alone.20
The need for whole pelvic radiation in patients with a single positive groin node has not been prospectively established and treatment in these cases may be individualized; however, in the presence of clinically fixed or at least two positive groin nodes, adjuvant groin and elective whole pelvic radiation is the standard of care.
ADJUVANT TREATMENT OF THE PERINEUM.
Local and nodal recurrence characterizes most failures after surgical treatment of vulvar cancer. Indications for radiation therapy of the perineum have been identified following analysis of GOG studies and by histopathologic review of patients treated with surgery alone. Heaps and coworkers concluded that margins smaller than 8 mm were associated with a higher risk of local failure.21 Review of 184 patients from GOG Protocol 36 noted that presence of tumor size larger than 4 cm and lymphovascular invasion was associated with a 21% risk of local recurrence, whereas absence of these risk factors reduced local failure to 9%.22
Faul and partners retrospectively analyzed the impact of adjuvant radiation to the perineum in 62 patients with vulvar cancer following radical vulvectomy (52 of 62) and wide local resection (10 of 62) with either positive or close (smaller than 8-mm) margins. The type of surgery was equally divided between the radiation and observation groups. The 31 patients who received post-operative radiation had 16% local recurrence whereas the 31 patients treated with surgery alone had 58% local recurrence (p < .05). The 2-year actuarial survival rate after local recurrence was 25%. The benefit of radiation was seen whether margins were close or positive. Subset analysis showed that local failure with positive margins was reduced from 75% to 42.5% after a mean dose of 57 Gy was delivered to the perineum. Local failure with close margins was reduced from 40% to 6% after a mean dose of 49 Gy was delivered to the perineum.23
The value of adjuvant perineal radiation was confirmed at the Mallinckrodt Institute of Radiology where 25 patients treated with wide local resection or partial vulvectomy received adjuvant radiation to the perineum. Local control following wide local excision was reduced from 100% to 75% if the radiation dose was less than 50 Gy, whereas there appeared to be no dose-response above 50 Gy for patients treated with partial vulvectomy. Advanced lesions (T3, T4) appeared to benefit from doses higher than 60 Gy as local control increased from 62% to 80%24,25 (p = NS).
Current recommendations for postoperative radiation treatment of the perineum include close or positive margins, depth of invasion more than 5 mm, lymphovascular invasion, or an infiltrative pattern of growth.
ELECTIVE TREATMENT OF THE GROIN.
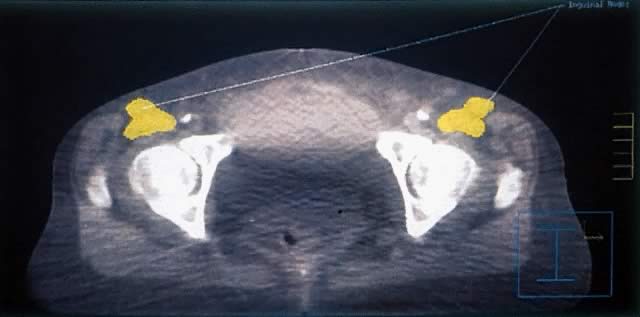
Radiation therapy may be given to the dissected groin in patients with positive inguinofemoral lymph nodes or to the undissected groin following wide local tumor excision when the risk of groin involvement is considered high. The GOG prospectively evaluated 588 patients with squamous cell carcinoma of the vulva reporting five independent factors predicting positive groin nodes: high tumor grade, suspicious groin nodes on examination, lymphovascular invasion, advanced age, and tumor thickness. Inaccuracy of clinical node assessment was illustrated by one fourth of patients with clinically negative groins (N0, N1) were subsequently found to be positive on pathologic assessment.9 Because groin recurrence is nearly uniformly fatal, prophylactic irradiation in appropriately selected patients potentially reduces both local failure and improves survival.
Standard treatment of vulvar cancer with a clinically negative groin has been radical vulvectomy or wide local tumor excision with bilateral inguinofemoral node dissection. Given that 70% to 80% of N0 patients have pathologically negative nodes, most patients undergoing groin dissection do not benefit from the procedure, which is associated with significant morbidity. A reasonable alternative has been elective groin irradiation. An early report from the University of Florida treated 6 patients with N0-1 nodes with elective bilateral groin irradiation to 45 Gy in 5 weeks and observed no groin failures or treatment complications.26 This experience led to elective inguinal lymph node irradiation for multiple pelvic carcinomas associated with inguinal metastasis. The most recent update of this experience included 18 patients with vulvar cancer treated to 45 to 50 Gy to the clinically negative groin; 81% had control of disease in the groin.27,28
Following publication of the results of GOG Protocol 37, pelvic radiation therapy and irradiation of the involved groin have become the standard of care for patients with positive groin dissections. These results and encouraging retrospective data led to an effort to omit elective groin dissection. GOG Protocol 88 prospectively randomized 58 patients with N0-1 groins postradical vulvectomy to receive groin radiation to 50 Gy or groin dissection. There was an 18.5% groin relapse rate in the radiation arm and no groin failures in the dissection arm prompting early termination of the study. Both progression-free interval and survival were significantly better in the groin dissection arm. The conclusion of the study was that radiation of the undissected groin was significantly inferior to groin dissection.29
Two explanations for the poor results in the radiation group have been examined. First, the dose of radiation was prescribed to a depth of 3 cm in all patients although inguinal lymph node depth can vary widely, contributing to underdosage of the groin at risk. Second, 20% to 25% of the patients in the radiation group could be expected to have occult groin involvement so that 50 Gy would be considered an inadequate dose. Nonetheless, elective groin radiation for the N0-1 vulvar cancer is not routinely given unless groin dissection is otherwise contraindicated.
Manavi and associates reviewed 135 patients with clinically negative groins who had been treated with simple vulvectomy. No radiation therapy was given to the primary site. Sixty-five patients received an average of 60 Gy to the undissected groin with Co-60. Seventy remaining patients were observed without radiation. Inguinal relapse was 4.6% in the radiation group and 10% in the observation group. Total survival time was similar in both groups. Significant complications occurred in 7.7% of the radiation group and 2.9% of the observation group. The study had insufficient power to evaluate the distribution of prognostic factors in the two groups but suggested that groin irradiation may be omitted in low risk patients, that is, primaries with no central location, no lymphovascular invasion, thickness of 2 mm or less, and low and intermediate grade tumors.30 With adequate dose delivery to the inguinal lymph nodes based on careful planning using computed tomography (CT), radiation may be an appropriate and less morbid substitute for bilateral groin dissection in low-risk patients but cannot be strongly advocated at this time without further prospective evaluation.
Preoperative Radiation Therapy and Organ Preservation
PREOPERATIVE RADIATION FOLLOWED BY ORGAN SPARING SURGERY: THE EARLY EXPERIENCE.
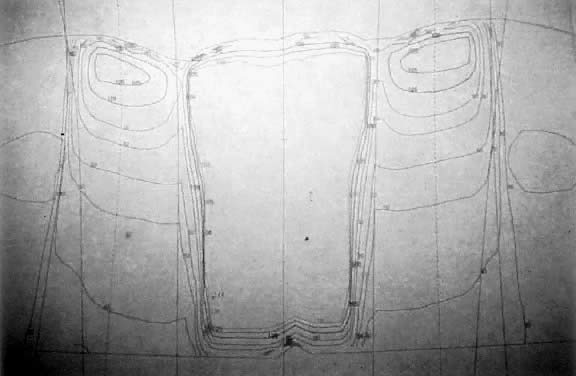
Pelvic exenteration to adequately resect locally advanced vulvar cancer is associated with major complications including wound infections, fistulas, and 10% postoperative mortality. Exenteration includes removal of the bladder and rectum. Efforts to decrease the extent of surgery, preserve rectal and bladder function, and avoid stomas led to attempted combinations of external beam radiation, brachytherapy, and surgery in selected patients.4,12,31 Berven in pioneering work in the 1940s presented evidence demonstrating the feasibility of preoperative radiation therapy with radium in 286 patients with locally advanced disease. Electrocoagulation of the lesion and teletherapy followed by en bloc resection resulted in a 38% 5-year cure rate.4 Boronow in 1973 presented 9 patients with advanced lesions treated with brachytherapy and external beam radiation followed by radical vulvectomyin place of pelvic exenteration. Only 1 patient recurred locally and survival was similar to that seen in exenteration series. Vaginal stenosis was the commonest complication.6 He updated his experience in 1982 with 26 primary cases and 7 recurrences. The whole pelvis was treated to 20 Gy followed by 20 Gy to the groin and pelvic lymph nodes blocking the midline structures. Finally, two radium applications to the vulvar primary were delivered. Disease-free survival rate with 1- to 11-year follow-ups was 65%. The disease-free survival rate for the recurrent cases was 71% with 1- to 3-year follow-up.5
Further and more recent evidence that a conservative approach to advanced disease can be offered without compromising survival was presented by Hacker and colleagues who reviewed their experience with 8 patients who needed pelvic exenteration. All patients received 44 to 54 Gy to the primary lesion and regional lymph nodes. Conservative excision was possible in 87.5%, 50% had a complete pathologic response, and 5 patients were alive from 15 months to 10 years following treatment. One patient developed bilateral hip fractures. Half these patients suffered uncomplicated moist desquamation.32
Rotmensch and colleagues in 1990 described 16 patients with stage III or IV tumors treated with 40 Gy to the primary site and 45 Gy to the groin and pelvis, then resection with radical vulvectomy and groin dissection 1 month later. The 5-year survival rate was 45%, the recurrence rate was 46%, and the visceral preservation rate was 63%.33 These early results supported preoperative radiation therapy and radical vulvectomy as a reasonable alternative to pelvic exenteration.
PREOPERATIVE RADIATION AND CONSERVATIVE SURGERY: THE ERA OF CHEMOTHERAPY.
Chemotherapy is directly cytotoxic to cancer cells and sensitizes tumor cells to the lethal effects of radiation. Chemosensitization of tumor cells was the basis of encouraging clinical work in the treatment of anal canal carcinoma in the United States and in Europe. The demonstrated ability of chemoradiation to preserve organ function in anal canal carcinoma patients, as well as the modest success with preoperative radiation alone to spare exenterative procedures in vulvar cancer, led to several efforts to improve functional outcome without compromising survival by administering concurrent preoperative chemotherapy and radiation.9 To this end, during the past 10 years a substantial body of evidence has accumulated using various chemotherapeutic agents and radiation fractionation schemes. With improved radiation-planning techniques and ever-expanding experience with chemotherapy, organ preservation in vulvar carcinoma continues to be an exciting frontier in the management of gynecologic cancers.
The addition of chemotherapy to radiation has the theoretical advantage of allowing for increased acute tumoricidal effects while reducing risk to normal tissue of late toxicity, which increases with higher doses of radiation. As tumor bulk increases as typically seen in locally advanced disease, higher total doses (more than 60 Gy) of radiation are required for disease control. Before sequencing, dosing, or timing guidelines had been established, Thomas and colleagues in the 1980s treated 33 patients at Princess Margaret Hospital with radiation therapy and concurrent 5-fluorouracil (5-FU) and mitomycin C to examine the efficacy and toxicity of combined modality therapy for advanced primary or recurrent vulvar cancer. Several clinical situations were studied that produced various radiation doses and fractionation schemes. Radiation dose to the vulva did not exceed 60 Gy. 5-FU was given as a continuous infusion over 4 or 5 days for one or two courses in most patients. Mitomycin C was also given in one or two injections. Although this approach was used in preoperative, postoperative, and recurrent settings and also in place of surgery, combined modality therapy appeared to be well tolerated without requirement of treatment breaks and allowed for less extensive surgery in selected cases.34
Substantiating and extending the findings of Thomas and coworkers, Berek and colleagues at the University of California at Los Angeles reported the results of a phase II trial exploring pathologic response and toxicity with the use of preoperative chemoradiation. Cisplatin (50 to 100 mg/m2) and 5-FU (1000 mg/m2) as a continuous infusion over 4 to 5 days were given at days 1 and 29 of radiation to 12 patients with stage III or IV disease. The pelvic lymph nodes, groin, and vulva were treated to a dose of 44 to 54 Gy. A complete pathologic response was observed in 67% of the patients and no further treatment was offered. A partial response was seen in 3 patients and no response was seen in 1 patient, all of whom had radical vulvectomy or exenteration. With a median follow-up of 37 months, 10 of 12 patients had no evidence of disease. Most patients suffered only grade 2 toxicity and there was no grade 4 or 5 toxicity.3 The strong association between a complete pathologic response and long-term local control has also been demonstrated in several other reports.35–39
The combination of mitomycin C and 5-FU in the preoperative setting was more recently reviewed at Loma Linda University where 19 patients with advanced vulvar lesions received concurrent radiation to 45 to 50 Gy to the primary, pelvis, and groin with a 96-hour infusion of 5-FU (1000 mg/m2) in weeks 1 and 5. The last few patients in the series also received mitomycin C (10 mg/m2) on day 1. With a median follow-up of 34 months, a 53% complete pathologic response was noted. Radical vulvectomy or exenteration was only required in five treatment failures. Overall, local control for the entire series was 95%. Only 3 patients required treatment breaks, all secondary to skin reaction, and there was no other significant observed toxicity.40
Similar to the experience in anal canal carcinoma, increased toxicity has been observed with higher doses of chemotherapy. In particular, mitomycin C has a potent radiosensitizing effect both on tumor cells and normal tissue. Two prospective phase I/II studies from Milan employed concurrent 5-FU and mitomycin C to spare radical surgery in advanced primary and recurrent disease. Landoni and associates treated 41 primary tumors that were either unresectable or would have required radical vulvectomy or pelvic exenteration. Radiation was given with a planned 2-week break with 36 Gy delivered before the break and 18 Gy afterward, along with 5-FU (750 mg/m2) and mitomycin C (15 mg/m2) with each course. Median follow-up was 22 months. Ten patients had a complete pathologic response and had an 80% disease-free survival rate, whereas 8 patients who could not have surgery died of their disease. Of the 23 patients with a partial response, presence of a pathologically positive groin alone or with a primary was associated with decreasing long-term disease control. Patients with only a pathologically positive groin had the best outcome compared with those with positive primary sites. Although 89% of the patients in the Landoni and associates' study completed treatment and 72% were able to undergo surgery, moderate and severe complications were common and no significant improvement in survival was noted over management with surgery alone.37 Lupi and coworkers prospectively treated 31 patients with primary and recurrent tumors requiring exenteration with a similar split-course regimen (36 Gy before a 2-week break, 18 Gy after) concurrent with 5-FU (750 mg/m2) and mitomycin C (15 mg/m2). Although the disease-free survival rate was 63% in the primary group, major postoperative complications were found in 65% of patients and there was a 14% treatment-related mortality.39 The dosage of mitomycin C in most institutions in the United States is limited to 10 mg/m2, which appears to be better tolerated. Higher doses of mitomycin C as used in the Milan studies require planned or unplanned breaks from radiation therapy, which allow for tumor repopulation and may produce an adverse impact on the efficacy of treatment.
Various chemotherapy combinations in addition to 5-FU, cisplatin, and mitomycin C have been explored in the preoperative setting based on in vitro results, although clinical results have, in some cases, been disappointing. Jaakkola and colleagues demonstrated an additive effect of paclitaxel and radiation therapy in four different cell lines of vulvar squamous carcinoma.41 Clinical results with paclitaxel in this setting awaits further investigation. Preoperative use of bleomycin (180 mg) both with and without radiation has been reported from Norway and Rome respectively. In the former, median survival was only 8 months in 20 patients who were treated for stages III and IV tumors and was only 6.4 months in 22 recurrent cases, notwithstanding the addition of 34 to 45 Gy to the tumor volume if it was thought to be inoperable after initially delivering 30 Gy.42 No significant benefit in mitigating radical surgeries was observed by Benedetti-Panici and coworkers with the preoperative use of bleomycin, cisplatin, and methotrexate without radiation.2
Massive tumors have also been successfully treated with concurrent radiation
and chemotherapy. Twelve patients from M.D. Anderson Cancer Center
with large vulvar cancers (mean size, 8.7 cm) were treated with concurrent
cisplatin (4 mg/m2  96 hours), 5-FU (250 mg/m2/day) and 40 to 50 Gy to the groin, pelvis, and primary. Eight of these
patients were able to have resection 6 weeks later and 4 of these 8 had
a complete pathologic response, all of whom on follow-up had no evidence
of disease. No treatment breaks were required and there was no significant
hematologic toxicity suggesting that higher doses of chemotherapy
could have been used.36
96 hours), 5-FU (250 mg/m2/day) and 40 to 50 Gy to the groin, pelvis, and primary. Eight of these
patients were able to have resection 6 weeks later and 4 of these 8 had
a complete pathologic response, all of whom on follow-up had no evidence
of disease. No treatment breaks were required and there was no significant
hematologic toxicity suggesting that higher doses of chemotherapy
could have been used.36
The GOG has recently published results of the largest prospective study exploring the feasibility of organ preservation with concurrent chemotherapy and radiation. Seventy-three patients with T3 and T4 tumors received split-course radiation and concurrent cisplatin (50 mg/m2) on day 1 with 5-FU (1000 mg/m2) as a continuous infusion over the first 4 days. Radiation therapy was divided into 2380 cGy courses, separated by a 1- to 2-week planned break depending on the degree of perineal reaction. Treatments were given twice daily at 170 cGy per fraction. Inoperable groin nodes and the lower pelvic lymph nodes were included in the radiation portals. Surgical excision and bilateral inguinofemoral dissection was planned in all patients. No visible tumor was seen at surgery in 33 patients. Of the remaining 38 patients with gross residual disease, only 2 were unresectable and only 5 had positive margins. With a median follow-up of 50 months, 55% of the patients were alive and without evidence of disease. Toxicity was acceptable and was confined to expected perineal reactions and wound complications.43
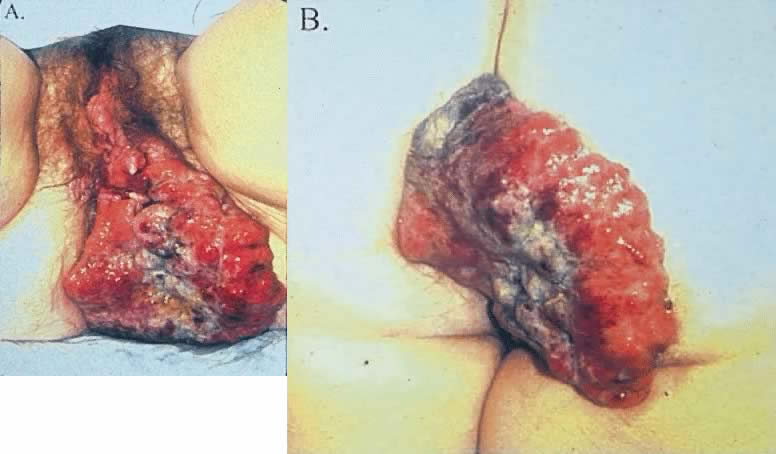
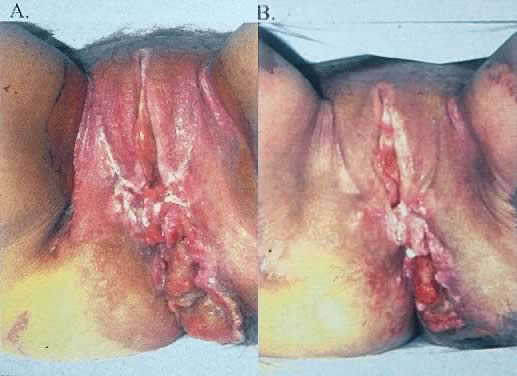
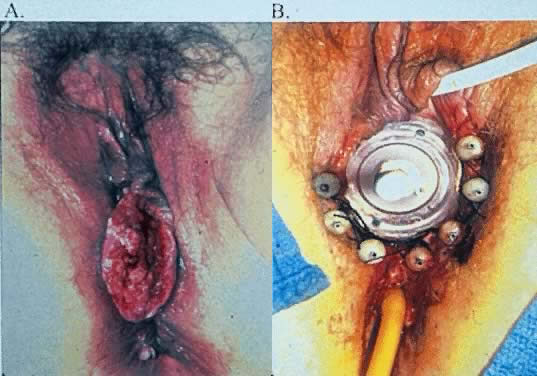
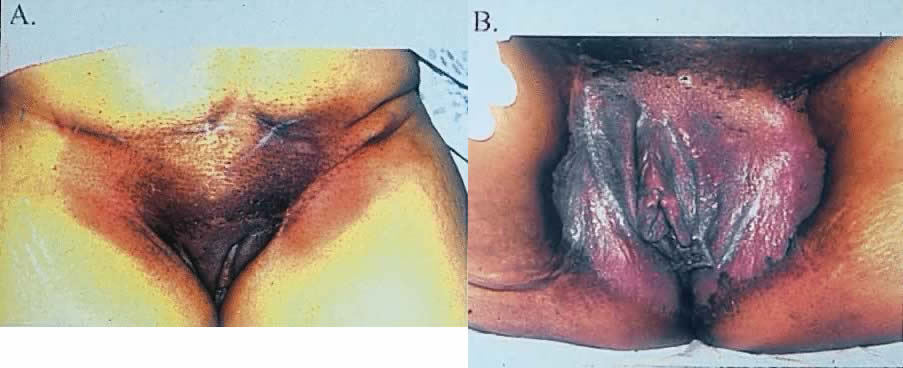
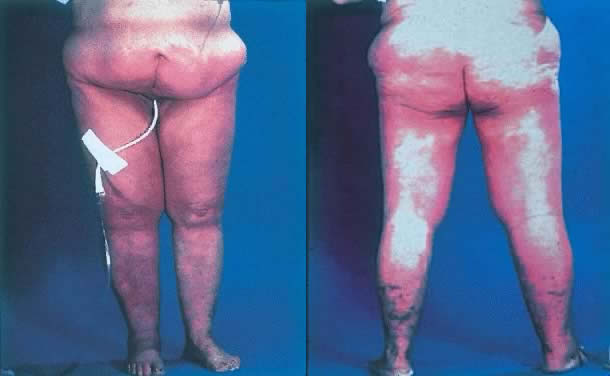
The most obvious benefit of organ preservation is improved functional outcome; however, the psychologic impact of cosmetic salvage cannot be overstated. Figure 1 shows a patient with a massive vulvar cancer. Often patients do not seek care until disease has reached an advanced stage because of embarrassment and fear. Figure 2 shows the same patient following combined chemotherapy and radiation treatment. Although preliminary data have been very encouraging, longer follow-up is needed. The clinical experience with organ preservation strategies during the past 10 years has shown efficacy and acceptable toxicity and should provide the impetus for future prospective trials.