Treatment and prognosis for vaginal squamous cell carcinomas are discussed later, whereas those for all other histologies are discussed in this section.
Squamous Cell Carcinoma
Squamous cell carcinoma is the most common primary vaginal malignancy but constitutes only 1% of all gynecologic cancers (Table 3). The mean age of patients with vaginal squamous cell carcinoma initially diagnosed is 60 years (range: 25–98).10,12,19 More than 75% of these lesions occur in patients older than age 50 years.12 The cause of squamous cell carcinoma of the vagina is becoming clearer. Recent interest has focused on the association between HPV infection and multifocal carcinoma of the lower female genital tract.24 Between 20%–60% of cases of vaginal cancer have been associated with HPV DNA, usually type 16.15,16,17,20,21,32
Table 3. Approximate Percentages of Primary Vaginal Malignancies According
to Histology
| Histology | Percentage |
| Squamous cell | 88 |
| Adenocarcinoma | 05 |
| Sarcoma | 03 |
| Melanoma | 03 |
| Small cell | 01 |
VAIN has been proposed as a precursor of vaginal cancer, although the true malignant potential of VAIN is unknown. The “HPV field-effect” hypothesis also has merit, because patients with VAIN commonly had cancer in other genital organs. Women with primary vaginal carcinoma have a 20% chance for previous invasive cervical cancer and a 70% chance for previous cervical intraepithelial neoplasia (CIN).17,23 The median interval between the diagnosis of cervical cancer and the diagnosis of vaginal cancer is 14 years, with a range of approximately 6 to 28 years. Sixteen percent of these patients have a history of previous pelvic radiation.10,18
It is often difficult to accurately determine the spread into subvaginal tissues, particularly the spread of anterior and posterior lesions. Therefore, differences in clinical observations are common. This is reflected in the wide range of reported stage distributions and survival rates per stage. The best data estimate that approximately 25% of patients present with stage I, 33% with stage II, 25% with stage III, and 15% with stage IV disease (Table 2).10,12,33,34,35
The most common site for squamous cell carcinoma is the upper third of the vagina. Fifty-one percent of these lesions are found to arise from the upper third, 19% from the middle third, and 30% from the distal third. Fifty-seven percent of tumors originate from the posterior wall, 27% from the anterior wall, and 16% from the lateral wall.23,33,34,35
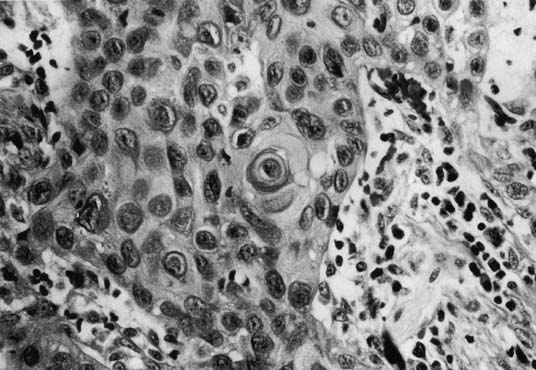
Tumor size can range from an occult lesion to a large mass measuring more than 10 cm in diameter. 35 Grossly, these tumors may be polypoid fungating masses or indurated, ulcerative plaques. On microscopic examination, the carcinoma consists of malignant squamous cells infiltrating from the vaginal epithelium and extending between the submucosa (Fig. 1). Cords of malignant cells advance into the submucosa and are usually surrounded by a large inflammatory reaction. Initially, local extension is into the submucosa, with later spread into the paracolpium and parametrium.36,37 Squamous cell carcinomas are usually moderately differentiated and nonkeratinizing. The degree of histologic differentiation, based on the amount of keratinization and number of squamous pearls, does not constitute a sound basis for assessing prognosis.24 The major prognostic factors are the stage of the tumor at the time of diagnosis and the size.16
|
Verrucous carcinoma is a distinctive variant of well-differentiated squamous cell carcinoma that rarely occurs in the vagina.38,39 This tumor typically presents as a relatively large, well-circumscribed, soft, cauliflower-like mass. Microscopically, it exhibits a papillary growth pattern with marked hyperkeratosis and broad, bulbous pegs of acanthotic epithelium that push into the underlying stroma. Cytologic features of malignancy are lacking. Because of its well-differentiated character, the microscopic diagnosis of invasive carcinoma may be difficult, especially if the biopsy specimens are superficial. Verrucous carcinoma can recur locally after surgery, but it rarely metastasizes. This behavior difference justifies treating this carcinoma as a distinct tumor entity.
Adenocarcinoma
Approximately 5% of primary vaginal malignancies are adenocarcinomas (Table 3). Whenever this diagnosis is considered, it is necessary to rule out metastatic lesions from the bowel, uterus, or ovary. The most common variant is the clear-cell adenocarcinoma, which can occur spontaneously and in women with in utero exposure to DES.6,7,8,14,40 Primary non-DES-related adenocarcinoma of the vagina is rare and occurs predominately in postmenopausal women.
DES was used extensively in the late 1940s and early 1950s to maintain high-risk pregnancies, such as those in women with a past history of abortion, diabetes, or multiple gestation.41,42,43 Approximately 5% of all pregnant women in the United States during the late 1940s and early 1950s used DES. In 1953, Dieckmann and colleagues41 reported that DES offered no improvement in fetal outcome, and its use gradually decreased until it was discontinued by the Food and Drug Administration in 1971. In the same year, Herbst and associates7 reported seven young women (ages 15–22 years) who presented to Vincent Memorial Hospital (Boston, MA, USA) between 1966 and 1969 with a diagnosis of clear-cell carcinoma or endometrioid-type adenocarcinoma with intrauterine exposure to DES. The Registry for Hormonal Transplacental Carcinogenesis and the Registry for Research on Hormonal Transplacental Carcinogenesis were established to correlate clinical and pathologic data on these unusual cancers.44
Many DES-exposed women have unusual vaginal epithelial changes such as adenosis.40,45 Vaginal adenosis is a condition in which müllerian-type glandular epithelium is present after vaginal development is complete. It most commonly involves the anterior wall and upper third of the vagina, and the classical gross appearance is that of red, velvety, grape-like clusters.7,8,14 The process may involve the surface epithelium or glands in the superficial stroma. Microscopically, the glandular epithelium can be composed of any of the müllerian epithelial cell types, but cervical-type mucus cells are most common. The glands within the lamina propria may be lined by tuboendometrial-type epithelium, which exists in approximately 25% of cases and is more common in the lower vagina. Robboy and associates45 suggested that atypical vaginal adenosis and atypical cervical ectropion of the tuboendometrial type are precursors of adenocarcinoma.
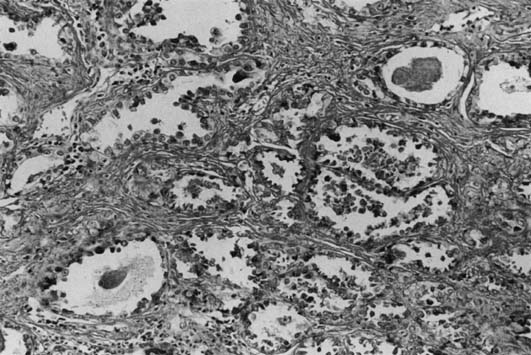
Most DES-associated tumors have occurred in women between ages 17–21 years (median age: 19 years).6,7 Clear-cell carcinoma develops in 0.1% of exposed women. The DES-associated clear-cell cervical adenocarcinomas have a predilection for the ectocervix. Most vaginal carcinomas arise on the anterior wall, usually in the upper third, corresponding to the most common site of adenosis. The tumors vary greatly in size (range: 1–30 cm). Most of the tumors are exophytic and superficially invasive.46 The larger tumors are polypoid and nodular, and the smaller tumors appear flat or ulcerated with a granular or indurated surface. Small tumors can be easily missed on colposcopic examination if confined to the lamina propria and if covered by intact, normal epithelium. These small tumors are usually asymptomatic and are detected only by palpation and directed biopsies as part of a thorough examination for a DES-exposed patient. Microscopically, they exhibit three basic histologic patterns: tubulocystic (most common); papillary; and solid.40 The tumor cells are cuboidal or columnar with clear cytoplasm and a distinct cell membrane, or they are the hobnail-type with large, atypical, protruding nuclei rimmed by a small amount of cytoplasm (Fig. 2). Glycogen is also found to be abundant.
|
Treatment can involve surgical intervention and radiation therapy.7,46,47,48,49,50,51 For stage I clear-cell adenocarcinoma in the typical young patient, surgery may be considered to preserve ovarian function.6,7,8,10,13,14 Surgery for vaginal clear-cell adenocarcinoma requires a radical hysterectomy and vaginectomy with reconstruction. The vaginectomy is performed only to the level required to obtain an adequate margin. Local excision appears inferior to radical surgery. The role of chemotherapy has not been determined.
The overall recurrence rate for clear-cell carcinoma approaches 21%, with the lungs, supraclavicular lymph nodes, and pelvis being the most common areas.7 Such cancers appear to have recurrence patterns different from those of squamous cell carcinomas, with a greater tendency to develop metastases in these distant sites.47,48,49,50,51 Although most recur within 3 years, late relapse of more than 19 years has been reported.7,46,47,48,49,50,51 Recurrent disease can be treated with radiation, surgery, or chemotherapy if widely metastatic. For central recurrences after surgery, pelvic radiation with external and interstitial therapy has been used. Pelvic exenteration appears to be more successful for patients with clear-cell carcinoma than for those with squamous cell carcinoma.47
Small-Cell Carcinoma
Small-cell carcinoma can occur in the vagina in pure form or associated with squamous or glandular elements. A high proportion of small-cell carcinoma cases show ultrastructural or immunocytochemical evidence of neuroendocrine differentiation. Scully and associates52 first reported a vaginal small-cell carcinoma confirmed by immunohistochemical staining for neuroendocrine features in 1984. Histologically, these tumors are small and round, with a rim of sparse cytoplasm around the nuclei. Growth patterns include sheets, ribbons, rosettes, and palisades. The tumors are reactive for neuron-specific enolase and cytokeratin. Peters and coworkers53 reported that patients with small-cell tumors (5 patients) were a mean age of 61 years.
Optimal therapy for small-cell carcinoma has yet to be determined. Therapeutic decisions are based on the examination results of patients with cervical or lung cancer. Because small-cell carcinoma is associated with early metastases, aggressive treatment with radiation for local control combined with systemic multi-agent chemotherapy is recommended.54,55,56
Melanoma
Primary malignant melanoma is the second most common cancer of the vagina (5% of all primary vaginal tumors) and has the worst prognosis of all vaginal malignancies.10,33 The incidence is estimated to be 0.03 per 100,000 women per year. Symptoms have usually been present for 3–6 months before a biopsy is performed. A recent review of 26 patients treated more than 30 years confirmed the previously cited clinicopathologic features.57 Most patients are white, with a mean age of 60 years (range: 38–90 years). The most common presenting symptom is vaginal bleeding, followed by a palpable vaginal mass.
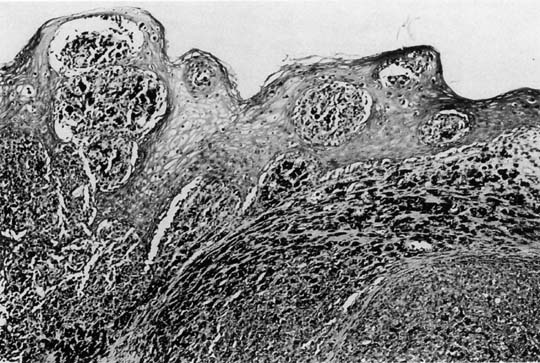
The lesions are polypoid–nodular in most cases with ulceration and a thickness greater than 3 mm.10,33 They are typically located in the distal third of the anterior vagina and appear pigmented black or dark blue. The disease is usually locally advanced on initial examination. Microscopically, these tumors are similar to those in other sites (Fig. 3).{FIG3} Poorly differentiated lesions that are difficult to distinguish from sarcomas or squamous cell carcinomas can be identified by their distinctive ultrastructural features or by immunohistochemical staining patterns. S-100 remains the most sensitive immunohistochemical marker for these tumors.57 MART-1 and tyrosinase can be helpful when S-100 is negative. Tumor depth according to the method of Breslow58 should be assessed, because depth of tumor invasion is the best predictor of survival.
|
Multifocal lesions are observed in 30% of patients, and nodal or distant spread is found in 20%. Sixty-six percent of patients (6/9) in one study59 had more that one focus of melanoma, either at initial diagnosis or at follow-up. The melanocytes probably come from aberrant melanocyte migration or melanocyte metaplasia. These tumors have poorer prognoses then cutaneous melanomas, with 5-year survival rates of 5% to 21% compared with 48%.60,61,62,63,64 The most frequent site of recurrence is local; the most common distant site is the lung.62
The only potentially curative treatment for vaginal melanoma is surgery. Most investigators have found no long-term survival difference between treatment with radical surgery and treatment with conservative therapy. Van Nostrand and colleagues in 199465 reported that radical surgery gave patients a significant improvement in the 2-year survival rate if the initial lesion was smaller than 1 cm2 (48% vs. 20%). However, the overall 5-year survival was similar. The resection must include all obvious disease, and conservative to ultraradical operations have been used.65,66,67 Regardless of the type of operation, positive histologic margins for melanoma or melanoma in situ result in a higher incidence of local failure and decreased survival rates. Irradiation alone, or in combination with surgery, has been successful in treating some patients.68
Local recurrences (usually within 2 years of initial treatment) can develop regardless of the type of surgical procedure. Recurrences can be treated with reoperation or radiation therapy. The exact role of chemotherapy in the adjuvant setting and for metastatic disease has not been determined.57,69
Endodermal Sinus Tumor
Extraovarian endodermal sinus tumors (EST) are extremely rare and generally originate in the vagina or cervix of young girls. EST of the vagina was first recognized in 1965. Because of the tumor's resemblance to the endodermal sinus structures of the rat placenta, which derive from the yolk sac endoderm, the names EST and yolk sac tumor were proposed.70 Most cases have been reported in patients younger than age 3 years.70,71,72,73 A bloody vaginal discharge is the typical clinical presentation. The disease appears to be locally aggressive and capable of metastasizing by hematogenous and lymphatic pathways. Grossly, the tumor appears as a polypoid mass that distends the vaginal lumen and may protrude through the introitus. The tumor may also appear as a sessile thickening of the vaginal wall with mucosal ulceration. Schiller-Duval bodies can be diagnosed on microscopic examination but are found in only a minority of cases. Alpha-fetoprotein can be demonstrated by immunoperoxidase staining, and serum levels can usually be used as a marker of disease.
Previously, untreated patients died within 2–4 months of presentation; however, with more effective chemotherapy, the prognosis has improved significantly. Copeland and coworkers74 reported on six patients with EST of the vagina and cervix who received excisional surgery with adjuvant vincristine, actinomycin-D, and cyclophosphamide (VAC) chemotherapy. They found that four of the six patients had been disease-free for 2 to 23 years. The Maligne Keimzelltumoren Study Group from Germany reviewed 14 patients with vaginal EST.75 The protocol consisted of neoadjuvant cisplatin-based chemotherapy followed by surgical removal of tumor (if necessary) and further cisplatin-based chemotherapy. In 10 children, the residual mass was completely resected, whereas in the remaining four only vaginoscopy was necessary, because no visible tumor remained. All children were free of disease after a median follow-up of 76 months (range: 9–150). Primary chemotherapy was also successful in the treatment of three children, as reported by Handel and collegues.76
Embryonal Rhabdomyosarcoma
Embryonal rhabdomyosarcoma is a malignant tumor of the rhabdomyoblasts characterized by two structural variances:
- a solid form and
- a multicystic grape-like form referred to as sarcoma botryoides.5 Sarcoma botryoides is a highly malignant tumor and is the most common
malignant tumor of the vagina in infants and children.77,78,79
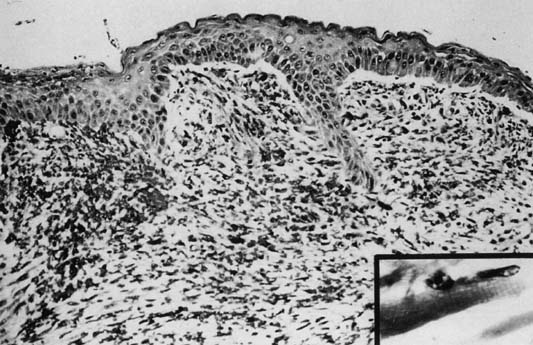
Ninety percent occur in children younger than age 5 years. Although variable in size and location, rhabdomyosarcomas have a characteristic gross appearance consisting of multiple gray-red, translucent, edematous, grape-like masses that fill and protrude from the vagina. The tumors are typically found in the vagina during infancy and early childhood, in the cervix during the reproductive years, and in the uterine corpus during the rare postmenopausal patient. Microscopically, there is a continuous zone of dense round or spindle cells (they can be layered) immediately beneath the intact vaginal epithelium. The neoplastic cells are immature rhabdomyoblasts that surround blood vessels. Elsewhere, the tumor is composed of small, dark cells that are sparsely distributed in the myxoid stroma. Some cells may show cytologic evidence of differentiation by intensely staining eosinophilic cytoplasm with cross-striation(Fig. 4).
The tumor invades adjacent structures and metastasizes to local lymph nodes and distant sites by lymphatic and hematogenous dissemination. Lymph node metastasis exceeds 25% at initial presentation. Previously, the overall 5-year survival rate was approximately 35%. Local excision combined with multiagent chemotherapy for early-stage disease has significantly improved survival.78,79,80,81,82 Neoadjuvant chemotherapy along with postoperative radiation therapy have also improved outcomes, even in older patients.81,83
Other Sarcomas and Rare Vaginal Primaries
Leiomyosarcomas,84 spindle cell sarcomas, angiosarcomas,85 alveolar soft-part sarcomas,86,87,88 fibrosarcomas, neurofibrosarcomas, and mixed mesodermal tumors of the vagina occur predominately in older patients but are rare (2% of all malignant vaginal tumors).84 Leiomyosarcomas are the most common of the adult vaginal sarcomas. These tumors usually develop along the vaginal sidewall and present as a lump that may cause problems with micturition, defecation, or intercourse. Because they are usually subdermal in location, bleeding and discharge are late symptoms.
Alveolar soft-part sarcoma of the genital tract is extremely rare, constituting 0.5% to 1% of all soft-tissue sarcomas.86,87 Patients are treated with radiation therapy after local excision and, occasionally, chemotherapy. Christopherson in 195288 described alveolar soft-part sarcoma microscopically as dense, fibrous trabeculae of varying thickness dividing the tumor into compact compartments of irregular size. Current periodic acid-Schiff (PAS) staining reveals various amounts of intracellular glycogen and characteristically PAS-positive, diastase-resistant crystalline material. These crystals are diagnostic features of this tumor.
Hemangiopericytoma has been reported to develop as an ulcerative mass on the vaginal epithelium, with bleeding occurring both spontaneously and after trauma.89 This tumor is characterized by proliferation of capillaries surrounded by a cell population derived from the myoepithelial pericyte. It can be mistakenly diagnosed as leiomyosarcoma.
The genital tract, especially the vagina, is an infrequent site for metastatic lymphomas, and it is rare for the vagina to be a primary site. These tumors can occur in premenopausal and postmenopausal patients.90 Bleeding is the most common presenting symptom, with pain and dyspareunia also occurring. Masses may be noted on routine pelvic examination.