A careful account of nutritional requirements is a prerequisite for producing
any artificial nutritional formula. This is true whether the support
is to be administered by enteral or parenteral route. An important
preliminary point is to establish clinical endpoints, which should
be used to determine when enteral feeding, PPN, or TPN should be stopped. As
has already been emphasized, this therapy should not be administered
in the cancer patient without understanding that it carries with
it substantial risks as well as benefits. It is a source of frustration
to the nutrition-care team and is certainly not in the patient's
interest for a complication to arise from a therapy that is no longer
indicated, and but for a lack of forethought should have already been
stopped. This is especially important to keep in mind when administering
TPN for an uncertain period of time in a patient in whom lean body
mass is compromised due to progression of disease. Appropriate clinical endpoints for cessation of artificial nutrition should
always include resumption of oral/enteral intake that is adequate
for absorption of energy and protein. Patients whose condition has improved
to the point where oral nutrition can resume are the most likely
to benefit from a short course of support. In a setting where nitrogen
excretion can be measured, maintenance of positive nitrogen balance
is a good clinical endpoint. If a patient is taking in more nitrogen
than she excretes in urine, feces, skin, and exhaled air, one can assume
the nitrogen is being “fixed” in new protein. If this patient
can maintain nitrogen balance without the nutritional intervention, as
determined by clinical and laboratory indices, it should be stopped. It must be emphasized that a particular patient's nutritional requirements
will be the same regardless of the route of absorption. In order
to heal, all patients need energy-producing molecules, essential nutrients
for anabolism and body processes, water, and electrolytes. Providing
these ingredients in the proper quantities and proportions is
the goal of a successful nutritional prescription. Some patients have
specific problems that make fulfilling their nutritional requirements
more challenging. Patients with large burns or wounds, complex enteric
fistulas, and renal or hepatic failure require a different protein composition
and perhaps vitamins and trace metals added to their formulas. Patients
on respirators and those with chronic obstructive pulmonary
disease need to have special attention paid to endogenous production
of respiratory CO2. This can be adjusted by changing the proportion of energy derived from
glucose, which is metabolized to CO2. The oncologist would be well advised to consult with critical-care and
nutrition specialists when devising a formulation for patients with
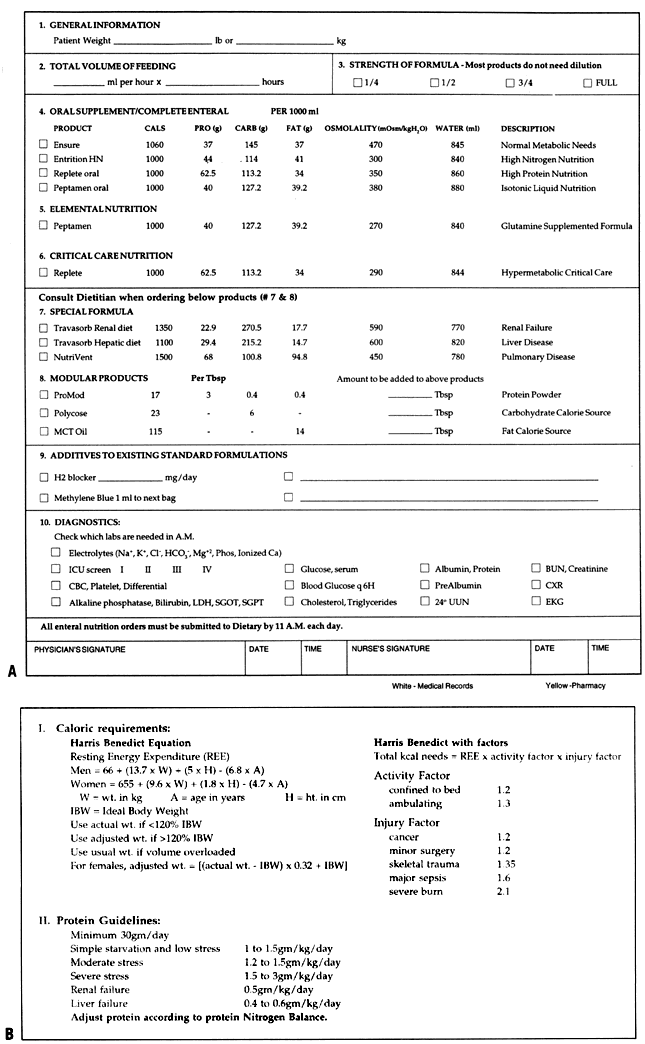
new or preexisting major organ-system insufficiency. Most hospitals use preprinted nutritional prescription forms for inpatient
therapy. This practice allows nutrition pharmacists to standardize
the preparation of enteral and parenteral solutions. Additives such as
heparin and insulin are included on the forms, and the ordering physicians
are reminded to include trace metals and vitamins on the appropriate
schedule. These forms not only facilitate preparation of the patient's
formula, but also provide guidance to clinicians about nutritional
requirements. At Magee-Womens Hospital, formulas required for
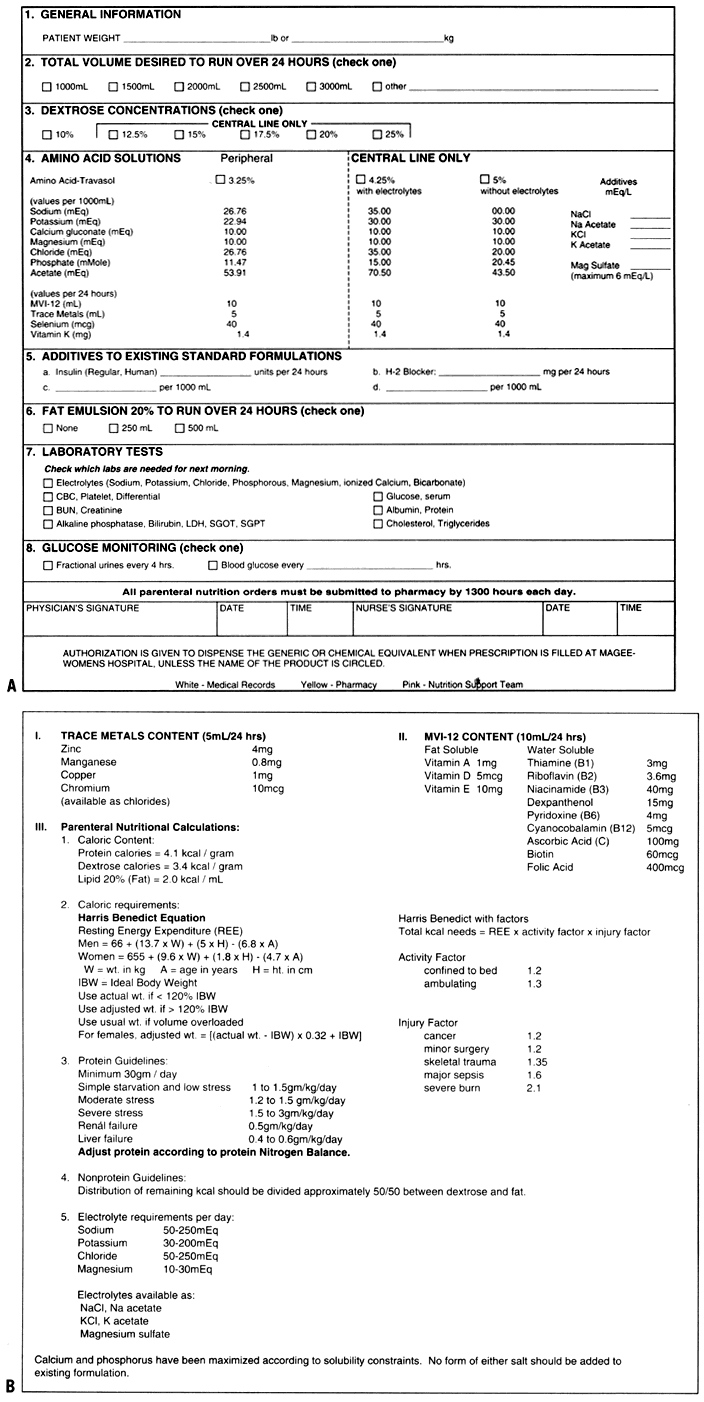
the calculation of nutritional requirements are provided on the back
of the prescription form. These forms (see Fig. 1 and Fig. 2) are currently used in the pharmacy and on the gynecologic oncology unit. Energy Requirements Preparing the nutritional prescription begins with determining a daily
energy requirement. The number of calories required to maintain homeostasis
is provided in the form of proteins, carbohydrates, and lipids. Patients
with cancer who require nutritional support have a wide range
of activity levels, and therefore have widely disparate energy substrate
requirements. To maintain lean body mass, energy as measured by calories
must be provided for anabolism to proceed and for body processes
to continue. Patients with weight loss due to inadequate ingestion of
calories should have some assessment of their basal metabolic rate. As
mentioned previously, there are several means for accomplishing this, depending
on the degree of precision required. In patients with pulmonary
artery catheters, this information can be derived from oxygen utilization
in a formula that uses cardiac output and mixed venous and
arterial oxygen saturation. A more common means of estimating nutritional
requirements is the Harris-Benedict formula. A patient with a severe
injury, however, must have her calorie requirement adjusted by the
addition or another 50% to 100% over the value derived from the Harris-Benedict
equation. Determination of standard energy requirements in gynecologic
oncology is severely limited by a paucity of data in this population. For
a patient with a significant metabolic burden attributable
to a gynecologic malignancy, one can only assume an increase in energy
requirements and make adjustments accordingly. A practical approach to energy delivery assumes that there is a given number
of kilocalories required to maintain 1 kg of lean body mass in 1 day. This
number is adjusted based on a clinical impression of the level
of metabolic stress and on consideration of the patient's list
of current problems. Patients who require nutritional support, but who
have had minor procedures, small surgical wounds, and no significant
confounding metabolic stress, are probably well served by 30 kcal/kg/day. This
should be considered the average protein requirement for patients
with primary malnutrition (i.e., nutritional support is indicated for malnutrition alone). For patients
with secondary malnutrition, such as those with ongoing severe metabolic stresses related to surgical
wounds, infections, cancer, or burns, approximately 35 to 40 kcal/kg/day
is probably required. Patients with morbid obesity, defined as more than 100 lb over ideal body weight, should have their
energy supplementation based on 25 kcal/kg/day to exploit the endogenous
energy derived from adipose reserves. In addition, this level of carbohydrate
and lipid infusion helps prevent complications due to insulin
resistance seen frequently in obese patients. Calorie counts can be
calculated from hospital tray surveys or patients' nutritional diaries
to determine whether needs are being met by oral intake. If not, consideration
should be given to starting enteral feeding. If there
is a reason to supplement parenterally, PPN or TPN should be instituted. CARBOHYDRATE REQUIREMENTS. In the nutritional prescription, the largest proportion of energy is generally
delivered as glucose (dextrose), because it is very inexpensive
and easy to mix. These solutions are available in any concentration
up to 70% (D70), and can be easily diluted to fulfill any osmolarity or fluid requirement. Because
glucose is water soluble, dextrose solutions can also be
mixed easily with electrolytes and water, facilitating determination
of osmolarity. Because glucose infusions are mixed by weight, it is simple
to calculate energy delivered by a certain volume. For example, there
are 150 g glucose for every 1 L D15, resulting in a calorie delivery of 450 g of glucose for every daily infusion
of 3 L fluid. Because every 1 g glucose provides 3.4 kcal, 1530 cal
dextrose is delivered in 3000 mL D15 each day. Dextrose infusions can also be mixed with commercial amino acid
formulas, making this source of energy the most convenient way to
deliver calories. There is, however, a physiologic limit to the amount of glucose that can
be used as a source of energy by peripheral tissues. Limitations of
cellular glucose transport mechanisms and the effects of insulin can cause
excess glucose to remain in the circulation until it is excreted. Excess
glucose can cause serious toxicities, including osmotic diuresis
resulting in dehydration, fatty infiltration of the liver, and hyperglycemic
nonketotic coma. Overinfusion of carbohydrate energy substrate
should be avoided by careful monitoring of urine for glucose, as well
as by serial blood measurements of glucose level. Even with the administration
of insulin during nutritional support, the peripheral cell
transport mechanisms may be overwhelmed, and excess glucose can become
toxic. A daily infusion of approximately 7 mg/kg/min results in fatty
infiltration of the liver and excess production of CO2. This latter toxicity is especially important in the respirator-dependent
patient receiving nutritional support. Excess CO2 can be more difficult to clear through impaired lungs, and endogenous
production can compromise respiratory function. While administering nutritional
support, one must be cognizant of potential complications related
to carbohydrate infusion. Too much carbohydrate can complicate the
healing process, negating the presumed benefit of nutritional supplementation. Carbohydrates
are required, however, because the highly metabolic
central nervous system cannot derive energy from lipids; rather, it
will use 50 to 150 g dextrose each day, and this vital nutrient
cannot be withheld from nutritional support formulas. Protein Requirements During periods of limitation of nutritional intake, the first priority
is to maintain homeostasis, and this requires a constant supply of energy. When
a patient is starved, as happens after 5 to 7 days of zero oral/ enteral
energy intake, the carbohydrate and lipid stores are depleted. In
this situation, energy for body processes must be derived from
endogenous protein, which results in the so-called catabolic state. Protein
stored in lean muscle and other tissues is shunted to biochemical
pathways that turn amino acids into energy in the form of glucose. Homeostasis
is therefore maintained at the expense of the formation of
structural and other proteins required for recovery. This impairment
of protein anabolism, which includes the formation of structural as well
as vital metabolic proteins in the form of antibodies, clotting factors, cytokines, and
enzymes, probably results in a greater susceptibility
to complications. These include failure of primary healing, delayed
respiratory competence, slow return of bowel function, renal failure, and
impaired humoral and cell-mediated immunity.4, 30, 31 The objective of protein and energy support is therefore to minimize the
complications related to acceleration of protein catabolism in the
starved state. In addition to maintaining homeostasis during the superimposed stresses
of recovery from surgery, patients with underlying metabolic derangements
require large amounts of protein-building substrate. Whereas the
average healthy person requires 0.8 g protein daily for maintenance of
every 1 kg lean body mass, an increased protein requirement is seen in
patients with cancer or other metabolic stressors. Critically ill patients, such
as those with a large tumor burden, a systemic inflammatory
response such as sepsis, or large surface area burns may require up
to 2 g protein/kg/day.32 Most malnourished cancer patients benefit from 90 to 120 g of amino acids, which
are commercially prepared as a standard solution mixed with
other parenteral components by volume. The normal ratio of nitrogen to
calories is 1:150, which is the proportion of carbohydrates and proteins
characteristic of most standard enteral formulas. This ratio must
be adjusted in patients with altered protein metabolism due to renal
or hepatic failure. Protein is adjusted on the basis of nitrogen balance. The usual starting
protein formula includes 1 g/kg/day. For a 60-kg woman, 60 g of amino
acids per day would be administered, which converts to approximately 10 g
nitrogen (1 mole nitrogen per mole of amino acid). The patient whose
protein requirement is not met by this estimate will continue to
excrete more nitrogen than she takes in, and she will not be able to build
structural and metabolic proteins. For example, if a 60-kg patient
was under greater catabolic stress than clinically suspected, she may
require 1.5 g protein/kg/day. This would correspond to 90 g of amino
acids per day, or 15 g nitrogen. The amount of nitrogen excreted in her
urine will give an estimate of the amount of nitrogen retained. When
the nitrogen given is equal to the nitrogen excreted, then homeostatic
requirements are met, but not exceeded. Obviously, if a patient must
heal a wound or deal with metabolic stress, nitrogen must be retained
in quantities sufficient to synthesize structural proteins, cytokines, growth
factors, enzymes, and immunoglobulins. This nitrogen retention
is considered a positive nitrogen balance and is prerequisite for healing
and weight gain. Determination of nitrogen loss in the urine can therefore be a clinically
useful measure in determining the adequacy of protein anabolism. Using
a standard laboratory assay for urea, urinary nitrogen can be measured
in a 24-hour specimen. The number of grams of urine urea nitrogen
can be converted to a gross estimate of the amount of excreted nitrogen
by adding 20% for respiration and loss through the skin and feces. The
goal of nutritional supplementation is met when “nitrogen in,” as
calculated from the number of grams of amino acid divided
by 6.25, is greater than “nitrogen out.” In this way, endogenous
protein production can be monitored, and the effect of the nutritional
supplementation can be gauged. This information can also be useful
in deciding when to stop nutritional therapy.5, 7, 24, 32, 33 Although most standard protein formulas are composed of relatively equal
proportions of essential and nonessential amino acids, there may be
some benefit to varying the composition of the amino acids, especially
in enteral feeding. Recent investigation has shown that specific amino
acids and precursors may be better suited to certain injuries and indications.34 For example, a high concentration of glutamine in enteral and parenteral
feeding may enable more rapid recovery of the intestine. This substrate
is used as fuel directly by the small intestine, aiding its growth
and function. Arginine, a “semiessential” amino acid, may
be especially useful in patients with cancer due to poorly characterized
immunomodulatory effects.32 Branched-chain amino acids (leucine, isoleucine, and valine) are considered
better suited to trauma patients. There are more than 30 different
amino acid preparations available.35 Amino acid proportions vary with different commercial enteral and parenteral
products.36 Oncologists should seek the advice of consultants who use nutritional
therapy more frequently. As already mentioned, the metabolism of protein also results in an energy
dividend of approximately 4 kcal/g. The number of grams of protein
may be calculated from the energy requirement in grams of nitrogen, with 1 g
nitrogen (or 6.25 g protein) for every 150 cal of energy. This
estimates the amount of energy required to utilize each gram of protein
for anabolism. Although this formula may seem cumbersome, it allows
calculation of calorie and protein needs based on excreted nitrogen, and
it can be clinically useful if nitrogen balance is measured by the
support team. Lipid Requirements Modern nutritional supplementation includes a large proportion of the daily
energy needs supplied in the form of lipid infusion. Because approximately 9 kcal/g
infused is provided, the most efficient way to deliver
energy is to use lipid emulsions. There are three principal reasons
to use lipids in nutritional support: - Energy requirements can exceed the glucose threshold, and lipids are therefore
given as a source of energy.
- Essential fatty acids are required to produce prostaglandins and steroid
hormones.
- Patients with a fluid restriction can receive more calories per volume
of lipid emulsion than by any other means; this is especially important
in patients with a history of congestive heart failure or kidney disease.
Lipid emulsions provide linoleic and linolenic acids, which are essential
nutrients. Without an oral/enteral source of linoleic and linolenic
acids, body stores of these essential nutrients will be depleted in 4 to 6 weeks.37 In addition, energy requirements are best met by using a mixed fuel system
that supplies 30% to 50% of nonprotein calories in lipid form. Lipids
can be added to the prescription as separate infusions given intermittently
or as slower infusions given with the entire nutritional formula. The
maximum recommended lipid dose is 60% of total calorie requirements. Larger
proportions may be associated with a detrimental effect
on immune function.38 Because lipid emulsions are isotonic with serum, they can be infused through
a peripheral vein, providing a large number of calories without
complications attributable to CVCs. At least 10 different commercial
lipid preparations are available for developing the nutritional prescription.35 Vitamin and Trace Metal Requirements Supplementation of vitamins and trace metals is required to provide cofactors
to enzymatic processes necessary for growth and healing. For most
patients, a standard multivitamin supplement (e.g., MVI-12) is added to the prescription as 10 mL per each 24 hours. Other
patients with specific deficiencies may require specific supplementation. In
gynecologic oncology, many patients have deficiencies based on
injury or removal of the terminal ileum due to radiation and/or surgery. This
portion of the intestine is important for enterohepatic circulation
and the absorption of vitamin B12 and fat-soluble vitamins. Because deep vein thrombosis is common in this
population, vitamin K is often iatrogenically antagonized by warfarin. In
patients who require specific replacement of vitamins, these special
circumstances must be considered, and care must be taken not to
induce a further thrombosis. Most patients will not require vitamin supplementation while in negative
nitrogen balance because their protein and energy requirements do not
exceed their vitamin stores. Only after the substrates for active metabolism
are provided will deficiencies become manifest. MVI-12 contains
water-soluble and fat-soluble vitamins, which can be administered intravenously
in patients receiving either enteral or parenteral nutrition. The
body excretes water-soluble vitamins supplied in excess, but
some fat-soluble vitamins (A, D, and E) must be titrated more carefully
to avoid toxicity. Vitamin K replacement is required to prevent coagulopathy, and
vitamin B12 is necessary to prevent macrocytic anemia and nerve damage. These vitamins
are usually given as periodic intramuscular injections, although
vitamin B12 is frequently added to the MVI-12. Chronic anemias from effects of chemotherapy
and radiation on the bone marrow can be exacerbated by nutritional
iron deficiency. As protein is supplemented, anabolic activity
may be limited by iron stores, and intramuscular iron supplementation
may be necessary. Trace metals such as zinc, molybdenum, selenium, chromium, manganese, copper, and
cobalt are given daily to prevent myriad complications from
their deficiency. Zinc deserves specific mention, as it is a nutrient
required for wound healing; it becomes especially important in the setting
of high-output enterocutaneous fistulas or diarrhea. Zinc supplements
may need to be increased in patients at bowel rest for this specific
problem, which is seen not infrequently in the gynecologic oncology
population.39 Insulin and Other Additives Patients who have been in a catabolic state due to malnutrition may not
be prepared to adapt to a sudden infusion of glucose and other nutrients. Insulin, which
is required for glucose metabolism in the cell and
lipolysis for energy production, may not be present in sufficient quantities
to begin to use the essential nutrients. Recombinant insulin is
now routinely added to intravenous nutritional supplements. A sliding
scale of subcutaneous insulin based on urinary glucose concentration
is often included in enteral feeding orders to prevent hyperglycemia. Patients on nutritional supplementation may have derangements in exocrine
and endocrine pathways that can result in gastric hyperacidity. Stimulation
of the duodenum and ileum may cause the gastric parietal cells
to produce acid by reflex. For this reason, histamine receptor (H2) blockers are an important adjunct to enteral nutrition. Changes in the
intestinal mucosa can also arise after prolonged periods of parenteral
feeding. Intravenous histamine receptor blockers may be valuable in
preventing hyperacidity in patients receiving parenteral nutrition as
well. Heparin is also frequently added to nutritional solutions to prevent catheter
occlusion or thrombosis. It is administered either by adding 1000 units/L
alimentation fluid or by flushing the catheter three times
daily with 1000 units. Care must be taken to avoid exacerbating a coagulopathy
in a patient with already compromised liver function. Electrolyte, Acid-Base, and Fluid Balance During the early clinical trials of parenteral nutrition, dehydration and
fluid overload were significant problems. Over the ensuing years, management
of fluid and electrolytes has been identified as an important
component in successful nutritional supplementation. During the initial
administration of parenteral or enteral nutrition, it is essential
to monitor fluid intake and output. Oral, enteral, and intravenous fluids
must be balanced against urinary, oral, nasogastric, fistulous, and
fecal outputs, as well as an appropriate adjustment for insensible
losses. The estimate of insensible loss must be increased by 10% to 50% for
patients with open wounds, fever, hyperventilation, or burns. Gastrointestinal
losses may need to be supplemented separately by a peripheral
intravenous line. A good rule of thumb is that specific electrolyte
derangements related to underlying conditions, such as excess gastrointestinal
or renal losses, should be replaced separate from the nutritional
prescription. In this way, nutritional requirements can be gauged
periodically and changes made less frequently. As has already been
mentioned for patients with TPN, the intravenous access line dedicated
to nutritional support should be used only for that purpose. Because
electrolyte requirements tend to change frequently, water and specific
mineral deficiencies can be adjusted several times a day by using
a separate infusion. Typical fluid and electrolyte requirements for patients receiving TPN are
shown in Table 3. These general guidelines are useful as a starting point, but electrolyte
supplementation depends on individual patient characteristics and
changes over time. For example, patients with underlying renal or cardiac
insufficiency may require less sodium than others; patients with magnesium
and calcium wasting syndromes related to cisplatin nephrotoxicity
may require extra supplementation with these minerals. Patients with
renal failure must be given potassium judiciously. In addition, acid-base
balance may be disturbed by a variety of respiratory, renal, and
gastrointestinal mechanisms that are exacerbated by cancer. In cases
of underlying acidosis due to sepsis or cancer, the indication may be
for anions to be delivered as acetate salts. This provides a better
acid-base buffer than chloride or phosphate. Sodium bicarbonate can be
given in background fluids to help regain control of physiologic pH, but
patients with acid-base derangements of this magnitude due to cancer
commonly require monitoring by critical-care specialists or nephrologists. Of
the important physiologic cations, sodium and potassium can
be delivered as phosphate, acetate, or chloride salts. In this way, anions
can be manipulated to provide extra acid-base buffers as necessary. Commercial
amino acid preparations can contain significant electrolyte
doses, and it is important to consider this when making calculations.39 TABLE 3. Calculating Minimum Fluid Requirements
Calculating Fluid Requirement for 24 Hours
24-hour fluid requirement = 100 mL/kg for the second 10 kg
+ 50 mL/kg for the second 10 kg
+ 20 mL/kg for each remaining kg
Example (for a 60-kg patient):
24-hour requirement = (100 mL 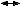 10) + (50 mL 10) + (50 mL 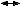 10) + (20 mL 10) + (20 mL 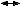 40) 40)
= 1000 mL + 500 mL + 800 mL
= 2300 mL
(Adapted from Mann WJ Jr: Nutritional Complications. In Orr JW, Shingleton
HM (eds): Complications in Gynecologic Surgery: Prevention, Recognition, and
Management. Philadelphia, JB Lippincott, 1994) |
 10) + (50 mL
10) + (50 mL