Direct evidence has shown that oxytocin is involved or mediates several
events in reproductive physiology. Female DURING COITUS. Oxytocin is released during coitus, but the stimuli responsible for the
release are not clearly known. It may involve olfactory, visual, and
auditory pathways, or it may be an expression of the Ferguson reflex
as a result of vaginal and cervical stimulation during coitus. Circulating
oxytocin levels increase significantly with sexual arousal and at
ejaculation in males during masturbation,44 as well as during coitus.38 In women, maximum levels of oxytocin are found at the peak of orgasm.45,46 DURING PREGNANCY. Few studies have measured oxytocin in the blood or amniotic fluid throughout
pregnancy (Table 1). Using a specific and sensitive radioimmunoassay, it was found that oxytocin
is detectable in 85% of the maternal plasma taken from pregnant
women at 6 to 42 weeks' gestation, while amniotic fluid has detectable
oxytocin levels in 90% of the samples studied.20 Maternal plasma oxytocin increases significantly with gestational age. The
mean maternal plasma oxytocin level increases from 10.4 pg/ml at 8 to 9 weeks
to 26.4 pg/ml at 37 weeks and is followed by a significant
further increase to 74.2 pg/ml at 38 weeks.20 The increase in maternal plasma oxytocin throughout pregnancy may be due
to the rise in circulating estrogen levels, since the administration
of estrogens in adult males stimulates oxytocin release. The rising
levels of circulating progesterone throughout pregnancy until term could
be responsible for blocking the effect of oxytocin on an increasingly
estrogen-primed uterus and, thus, prevent premature onset of uterine
contractions. The maternal pituitary is the source of the circulating
maternal oxytocin during pregnancy although oxytocin gene is also expressed
in the decidua,47 indicating that it is a source of oxytocin during pregnancy. TABLE 1. Cumulative Data on Oxytocin Levels in Maternal Blood During Pregnancy
| Source of | Stage of | Method of Oxytocin | Levels of Oxytocin or |
Authors | Blood | Pregnancy | Determination | Oxytocin-like Substance* |
Hawker & Robertson (1957) | Arm vein | Near term | Bioassay with extraction | 1.8–85 ng/ml blood |
Gonzales-Pannizza & | | Near term | Internal tocography | 20–60 pg/ml plasma |
Sica-Blanco (1957) | | | | |
Sica-Blanco et al (1959) | | Term | Internal tocography | 20–80 pg/ml plasma |
Caldeyro-Barcia (1961) | Arm vein | Early pregnancy | Bioassay without extraction | <100 pg/ml plasma |
Hawker et al (1961) | Arm vein | Near term | Bioassay with extraction | 0.04–14 ng/ml blood |
Hawker et al (1961) | Arm vein | Near term | Bioassay with extraction | 0.24 ng/ml blood |
Kumaresan et al (1974) | Arm vein | 4–40 wk | Radioimmunoassay without | 132–330 pg/ml plasma† |
| (n = 280) | | extraction | |
Dawood et al (1979) | Arm vein | 6–42 wk | Radioimmunoassay with | 10.4–74.2 pg/ml plasma† |
| (n = 365) | | extraction | |
*”Oxytocin-like“ refers to those assays in which the end point
is uterine contraction of milk ejection.
† Values represent the range of the means for the different weeks of gestation
studied.
(Modified from Dawood MY: Contemp Obstet Gynecol 13:181, 1979)Amniotic fluid oxytocin concentration increases from a mean of 7.8 pg/ml
at 14 to 15 weeks' gestation to 43.9 pg/ml at 40 weeks and 30.8 pg/ml
at 41 to 42 weeks. Fetal urine has been shown to contain oxytocin with
a mean level of 54 pg/ml48 and probably contributes substantially to the amniotic fluid concentration. An
amniotic fluid to fetal urine oxytocin concentration ratio of 2:1 in
spontaneous labor indicates that there are other sources of amniotic
fluid oxytocin.48 Direct efflux of oxytocin from cord vessels is a possible source. Meconium, which
is rich in oxytocin,49 contributes to amniotic fluid oxytocin in special circumstances in which
enhanced uterine activity may be necessary to hasten delivery of a
fetus that is in distress. Since mRNA for oxytocin has been found in human
amnion, chorion, and decidua, these are also sources of amniotic
oxytocin.47 It is evident that with bioassay, extremely high levels of oxytocin are
detected (see Table 1), which may be partially accounted for by interference of nonoxytocin-like
substances. Radioimmunoassay measurements estimate 10 to 100 times
less oxytocin in maternal blood during pregnancy. Nevertheless, even
with radioimmunoassay, rather high concentrations are found by some investigators50 who do not use preliminary extraction of the plasma in their methods compared
with those who do.38,40 DURING LABOR AND PARTURITION. Table 2 summarizes the data available from the literature on the maternal plasma
oxytocin levels during human labor. Coch and colleagues51 found significantly higher concentrations of oxytocin in the jugular venous
blood compared with peripheral blood and concluded that the maternal
pituitary gland releases significant amounts of oxytocin during labor. Chard
and colleagues21,38 found detectable oxytocin levels in 19% to 60% of the maternal samples
during labor due to spurt release of oxytocin. They found that the percentage
of blood samples with detectable levels of oxytocin rises in
the late first and second stages of labor. We found that maternal plasma
oxytocin increases significantly from the first stage to the second
stage of labor, followed by an equally significant decline during the
third stage of labor (Fig. 2).40,52 TABLE 2. Cumulative Data on Oxytocin Concentration in Maternal Blood During
Labor
| | Source of | | |
| | Blood | | |
Author | Stage of Labor | Assayed | Method of Assay | Oxytocin Levels |
Hawker & Robertson (1957) | First | Arm vein | Bioassay with extraction | 200–2400 pg/ml blood |
Caldeyro-Barcia (1961) | First | Arm vein | Bioassay without extraction | 240 pg/ml plasma |
Hawker et al (1961) | First | Arm vein | Bioassay with extraction | 0–11, 900 pg/ml blood |
Hawker et al (1961) | Second stage (expulsive phase) | Arm vein | Bioassay without extraction | 280 pg/ml blood |
Juret et al (1961) | First stage | Arm vein | Bioassay without extraction | 60,000–100,000 pg/ml plasma |
Coch et al (1965) | First, second, third | Jugular vein | Bioassay with extraction | 600–1,800 pg/ml plasma |
| First, second, third | Arm vein | Bioassay with extraction | 50–500 pg/ml plasma |
Fitzpatrick & Walmsley (1965) | Second | Arm vein | Bioassay with extraction | 160–400 pg/ml plasma |
Vorherr (1977) | First, second | Arm vein | Bioassay | Undetectable |
Saameli (1963) | | | Indirect assessment using | 6 pg/ml plasma |
| | | oxytocin intravenous | |
| | | infusion | |
Chard et al (1970) | | Arm vein | RIA with extraction | Detectable levels in 19% |
Gibbens & Chard (1976) | First | Arm vein | RIA with extraction | Detectable levels in up to |
| | | | 60%* |
Bashore (1972) | First, second, third | Arm vein | RIA with extraction | <40 pg/ml plasma |
| | | | 180 pg/ml plasma (2 cases) |
Kumaresan (1974) | First, second | Arm vein | RIA without extraction | 70–870 pg/ml plasma |
Glick et at (1969) | First, second | Arm vein | RIA with extraction | 20–150 pg/ml plasma |
| | | | 200–400 pg/ml plasma |
Dawood et al (1978) | First, second, third | Arm vein | RIA with extraction | 40.3 ± 9.8 pg/ml plasma† |
| (expulsive phase) | | | 123.9 ± 23.6 pg/ml plasma |
| | | | 64.5 ± 13.1 pg/ml plasma |
*Oxytocin levels given are 0–25 pg/ml.
† Mean ± standard error of the means.
RIA, radioimmunoassay.
(Modified from Dawood MY: Contemp Obstet Gynecol 13:181, 1979)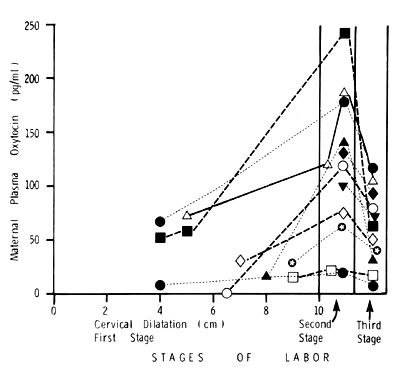 Fig. 2. Serial maternal plasma oxytocin during the first, second, and third stages
of labor in 11 women with spontaneous normal labor and vaginal delivery. In
every patient, there was an increase in plasma oxytocin from
the first to the second stage and a fall during the third stage. The
plasma samples taken during the second stage of labor were timed to coincide
with the crowning of the fetal head.(Modified from Dawood MY, Raghavan KS, Pociask C, Fuchs F: Oxytocin during
human pregnancy and parturition. Obstet Gynecol 51:138, 1978) Fig. 2. Serial maternal plasma oxytocin during the first, second, and third stages
of labor in 11 women with spontaneous normal labor and vaginal delivery. In
every patient, there was an increase in plasma oxytocin from
the first to the second stage and a fall during the third stage. The
plasma samples taken during the second stage of labor were timed to coincide
with the crowning of the fetal head.(Modified from Dawood MY, Raghavan KS, Pociask C, Fuchs F: Oxytocin during
human pregnancy and parturition. Obstet Gynecol 51:138, 1978)
|
During the first stage of labor, the mean maternal plasma oxytocin level
is 40.3 ± 9.8 pg/ml. It increases to 123.9 ± 23.6 pg/ml
during the second stage and declines to 64.5 ± 13.1 pg/ml during
the third stage. This occurs both in the individual patient and in
all the patients studied as a group. Hence, the release of oxytocin into
the maternal circulation during the second stage is accentuated by
the passage of the presenting part through the cervix and the vaginal
outlet via the mechanism of the Ferguson reflex. Because higher levels
of oxytocin are found in the jugular venous blood than in the peripheral
blood,51 the maternal posterior pituitary gland must be releasing oxytocin during
labor so as to increase the peripheral maternal oxytocin levels. FETAL OXYTOCIN. There is increased fetal plasma oxytocin concentration, as determined
in umbilical cord blood samples, when there is spontaneous onset of labor
irrespective of the mode of delivery compared with oxytocin in samples
taken from women who are not in labor when undergoing cesarean section.38,48 In our laboratory, the plasma oxytocin levels are always higher in the
umbilical artery than in the umbilical vein whether the women are in
labor or not, provided they are not given oxytocin. When the women are
in established labor, the umbilical arterial plasma oxytocin levels are
significantly higher than the umbilical venous plasma oxytocin levels (Fig. 3). Because the oxytocin levels are always higher in the umbilical artery
than in the umbilical vein, irrespective of the stage of labor and fetal
presenting part, the oxytocin must originate in the fetus and the
flow is from the fetal side to the maternal compartment. When patients
are given intravenous or buccal oxytocin to stimulate uterine contractions, levels
in the umbilical vein are higher than or similar to the
levels in the umbilical artery. This finding indicates that it is possible
to induce a reverse gradient of oxytocin toward the fetal compartment
and may indicate that in pregnant patients requiring oxytocin stimulation
of the uterus, there is an underlying deficiency in the release
of oxytocin by the fetus and possibly by the decidua, chorion, or
amnion, which accounts for the need for oxytocin administration. 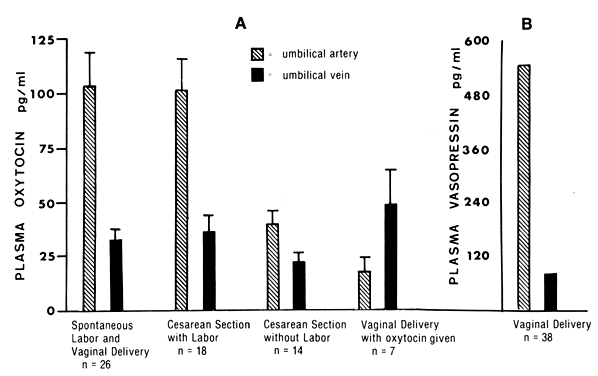 Fig. 3. A. Oxytocin levels in the umbilical arterial and venous blood during elective
cesarean section, during cesarean section after the onset of labor, and
on administration of oxytocin to the mother. Note that umbilical
arterial plasma always had higher oxytocin levels than umbilical venous
plasma except when the mother was given oxytocin. The umbilical arteriovenous
difference in oxytocin levels is significantly higher when
there is spontaneous labor than when there is no labor. (Data from Dawood
MY, Wang CG, Gupta R, Fuchs F: Fetal contribution of oxytocin in
human labor. Obstet Gynecol 52:205, 1978) B. Vasopressin levels in umbilical arterial and venous plasma from patients
in labor and patients not in labor.(Data from Chard T: Neurohypophysial hormones. In Fuchs F, Klopper A [eds]: Endocrinology of Pregnancy, 2nd ed, p 271. Hagerstown, MD, Harper & Row, 1977) Fig. 3. A. Oxytocin levels in the umbilical arterial and venous blood during elective
cesarean section, during cesarean section after the onset of labor, and
on administration of oxytocin to the mother. Note that umbilical
arterial plasma always had higher oxytocin levels than umbilical venous
plasma except when the mother was given oxytocin. The umbilical arteriovenous
difference in oxytocin levels is significantly higher when
there is spontaneous labor than when there is no labor. (Data from Dawood
MY, Wang CG, Gupta R, Fuchs F: Fetal contribution of oxytocin in
human labor. Obstet Gynecol 52:205, 1978) B. Vasopressin levels in umbilical arterial and venous plasma from patients
in labor and patients not in labor.(Data from Chard T: Neurohypophysial hormones. In Fuchs F, Klopper A [eds]: Endocrinology of Pregnancy, 2nd ed, p 271. Hagerstown, MD, Harper & Row, 1977)
|
It is still unclear whether all the oxytocin flowing from the fetal side
into the maternal compartment crosses the placenta without being inactivated
by the high concentrations of placental oxytocinase. However, it
is clear that an oxytocin gradient can be established from the fetus
to the mother or from the mother to the fetus and that oxytocin readily
crosses the placenta in the ewe53 and the baboon.54 A major role of oxytocinase in the metabolism of oxytocin has been seriously
questioned (see section on Metabolism). Since the uterus is the
principal tissue with specific oxytocin receptors and is immediately
receiving the fetal hormones after its transplacental passage, much, if
not all, of the fetal oxytocin in the umbilical artery must be readily
available to stimulate the uterus. Thus, the fetus appears to act as
an “oxytocin-injection” system to the mother, comparable
to the fuel-injection system of an automobile. Additional sources of
oxytocin such as the decidua, chorion, and amnion can also act in a paracrine
manner on the uterus.47 The umbilical plasma arteriovenous difference in oxytocin concentration
is significantly higher when the woman is in established labor than when
she is not.48 Using the arteriovenous difference in umbilical plasma oxytocin levels, the
average umbilical blood flow, and umbilical blood hematocrit levels, the
estimated mean secretion rates of oxytocin from the fetal to
the maternal compartments have been shown to be 2.75 mU/min (5.5 ng) when
there is spontaneous labor and vaginal delivery; 3 mU/min (6 ng) in
patients having cesarean section after the onset of labor; and only 0.5 mU/min (1 ng) if
cesarean section is performed without onset of labor (Table 3).48 It is noteworthy that the secretion rate of 0.5 mU/min in women who are
not in labor is consistent with the clinical observation that only ineffectual
uterine contraction, if any, will occur with the administration
of such a small dose of oxytocin but that the secretion rate of 2.75 to 3 mU/min
found in spontaneous labor will cause effective uterine
contractions at term when there has been an increase in oxytocin receptors. Since
the secretion rate of oxytocin from the fetus to the mother
during the first stage of labor, as shown in women undergoing cesarean
section with labor, is similar to that found during the second stage
in spontaneous labor and vaginal delivery, the sudden increase in
maternal plasma oxytocin observed at the second stage must be due to additional
oxytocin released from the maternal neurohypophysis. Hence, the
data available indicate that during the first stage of spontaneous
labor, fetal oxytocin plays a significant and major role while the maternal
pituitary releases additional oxytocin to increase markedly the
maternal circulating oxytocin concentration during the second stage (expulsive
phase). This increase is necessary to augment the strong uterine
contractions required to expel the fetus during the final stage of
its exit from the birth canal. TABLE 3. Estimated Secretion Rate of Oxytocin from Fetal to Maternal Compartment
Based on Umbilical Plasma Arteriovenous Difference in Oxytocin
Concentrations
| Mean ± SE Umbilical Arteriovenous | Mean ± SE Secretion Rate of |
Type of Delivery | Difference in Plasma Oxytocin | Oxytocin per Minute |
Spontaneous labor and vaginal delivery | 73.3 ± 13.3 | 5.5 ± 1 ng (2.57 ± 0.5 mU) |
Cesarean section (with labor) | 81 ± 12.4 | 6 ± 1 ng (3 ± 0.5 mU) |
Elective cesarean section (no labor) | 13.8 ± 5.1 | 1 ± 0.4 ng (0.5 ± 0.2 mU) | (Data from Dawood MY, Wang CF, Gupta R, Fuchs F: Fetal contribution to
oxytocin in human labor. Obstet Gynecol 52:205, 1978)Fetal urine has been shown to contain oxytocin.48 First-voided fetal urine obtained after delivery has a mean oxytocin concentration
of 53.8 ± 11.8 pg/ml, a higher level than that reported
by Boyd and Chard in maternal urine during labor.55 This provides further evidence for fetal participation in oxytocin release
during spontaneous labor. Little is known about the ontogeny of human neurohypophysial hormones. The
concentrations of oxytocin in the human posterior pituitary gland
of 150- to 156-day-old fetuses were determined by bioassay to be 92 to 105 ng/gland.56 Using radioimmunoassay, we found that at 14 to 17 weeks' gestation, fetal
pituitary gland had 10.2 ± 5.9 ng oxytocin/gland (mean ± SEM), increasing
to 31.6 to 38.4 ng at 20 to 26 weeks; 22.1 to 57 ng
at 32 weeks; and dramatically to 544.3 ± 33.8 ng oxytocin/gland
in 1-day-old to 5-day-old newborns.57 Thus, it is evident that the fetal pituitary has started to produce oxytocin
by the 22nd week of pregnancy, if not earlier. Vasotocin is present in the human fetal posterior pituitary gland. Extracts
of human fetal neurohypophysial tissue at 130 to 155 days' gestation
had oxytocin, vasopressin, and vasotocin. Based on tissue cultures
of fetal neurohypophysial cells, it has been shown that vasotocin is
produced and secreted by the ependymal cells.58 Thus, while oxytocin and vasopressin are synthesized in the hypothalamic
neurosecretory system, vasotocin, which has been suggested as the ancestral
molecule, is synthesized in the ependymosecretory system, which
lacks neurons. The fetal posterior pituitary gland not only is the
site of storage of neurohypophysial hormones but it also synthesizes vasotocin. Vasotocin
is also present in the fetal pineal gland, but the
significance of this is not understood. The precise stimulus that causes the release of oxytocin in the human fetus
has not been identified, and it is not known whether the release
is a primary phenomenon or secondary to the process of labor. It may be
hypothesized that stress on the fetus may cause a release of neurohypophysial
hormones, but the nature of the stress is difficult to define. It
is possible that with increasing growth of the fetus, the placenta
is unable to cope with the nutritional demands of the fetus and hence
the fetus is stressed. On the other hand, activation of the fetal neurohypophysis
during labor and delivery may be the result of acute stimulation
caused by uterine contractions to a mature fetal hypothalamoneurohypophysial
system. IN PREMATURE LABOR. Ethanol can suppress uterine activity in premature human labor. However, it
is unclear whether the alcohol affects the myometrium, the posterior
pituitary gland, or both. Evidence points to the posterior pituitary
gland because the percentage of detectable oxytocin decreases significantly
when alcohol is given during the advanced phase of the first
stage of labor.17 However, ethanol can also inhibit prostaglandin-induced uterine activity. It
is conceivable that the abnormality in the endocrine factor in
premature labor may be a deviation from the delicate balance between oxytocin, estrogens, and
progesterone, all of which influence uterine activity
either directly or indirectly by altering the sensitivity of the
myometrium to oxytocin through changes in oxytocin receptors in the
myometrium. OTHER DISORDERS ASSOCIATED WITH LABOR. There is no evidence of abnormal labor in patients with diabetes insipidus, although
it might be expected that the onset of labor would be delayed
in such patients if the maternal release of oxytocin is crucial. A
surge in plasma oxytocin during labor and the puerperium, similar
to the surge found in normal pregnancy, has been reported in a patient
with idiopathic diabetes insipidus.59 Since this disease usually limits the function of the maternal posterior
pituitary gland, the increase in oxytocin concentration in the maternal
circulation is probably of fetal, decidual, chorionic and/or amniotic
origin and may account for the ability of these patients to go into
spontaneous labor. Also in patients with idiopathic diabetes insipidus, not
all of the posterior pituitary glands may have been destroyed
and the oxytocin reserve in most of the patients is unknown. In a study on a limited number of patients, sequential maternal plasma
oxytocin concentrations appear to correlate well with the subsequent outcome
of labor.20 Patients who have plasma oxytocin concentrations of more than 10 pg/ml
throughout the second half of their pregnancies (good oxytocin secretors) develop
good rhythmic uterine contractions when they go into labor
and deliver vaginally without any difficulty. By contrast, those who
have plasma oxytocin concentrations of less than 10 pg/ml (poor oxytocin
secretors) develop uterine dysfunction during labor, which necessitates
cesarean section. DURING LACTATION. Increased oxytocin concentrations are found in the maternal blood during
suckling (Table 4). When the human infant suckles, rhythmic changes in intramammary pressure
are evoked in the contralateral breast. These changes are caused
by rhythmic contraction of the mammary myoepithelium in response to oxytocin. The
release of oxytocin in response to suckling is mediated through
impulses generated at the nipple and transmitted via the third, fourth, and
fifth thoracic nerves to the spinal cord up to the hypothalamus
to cause a release of oxytocin from the posterior pituitary gland. The
release of oxytocin then completes this milk-ejection reflex. In
addition to milk ejection, this reflex is also responsible for the uterine
contractions observed at the time of breastfeeding and is often
referred to as the “after pains.” Using radioimmunoassay, we
found that maternal circulating oxytocin levels are low, usually less
than 30 pg/ml prior to suckling. The oxytocin level increases 5 to 10 minutes
after initiating suckling, but the maximum levels reached
are less than 100 pg/ml during a 30-minute suckling episode (15 minutes
on each breast) in the first few days after delivery.60 In many instances, two peaks in the plasma oxytocin concentrations are
observed, perhaps a reflection of the suckling stimuli induced in the
contralateral breast when the infant is changed to the opposite side. The
release of oxytocin during lactation may not be exclusively due to
the suckling stimulus but may include olfactory, auditory, and other
stimuli. Thus, a mother who is breastfeeding may start to lactate at
the sound of hearing her infant cry. More information is needed on the
relationship of the neurohypophysis to the adenohypophysis in their respective
participation in the mechanism of lactation. TABLE 4. Maternal Oxytocin Levels During Lactation
| | Relationship to | Method of Oxytocin | |
Authors | Source of Blood | Suckling | Determination | Oxytocin Level |
Hawker & Robertson (1957) | Arm vein | Before suckling, during | Bioassay with extraction | 1.4 ng/ml blood |
| | suckling | | <0.86 ng/ml blood |
Hawker (1958) | Arm vein | Before, during, and 2 hr | Bioassay with extraction | 40 pg/ml blood |
| | after suckling | | 100 pg/ml blood |
| | | | 8 pg/ml blood |
Hawker et al (1961) | Arm vein | Before suckling | Bioassay with extraction | 0–8.8 ng/ml blood |
Coch et al (1965) | Internal jugular vein | During suckling | Bioassay with extraction | 400–600 pg/ml plasma |
Coch et al (1968) | Internal jugular vein | During suckling | Bioassay with extraction and chromatography | 24–50 pg/ml plasma |
Fox & Knaggs (1969) | Arm vein | Before suckling, during | Bioassay with extraction | 38 pg/ml plasma |
| | suckling | | 22–224 pg/ml plasma | (Modified from Dawood MY: Contemp Obstet Gynecol 13:181, 1979)Neurohypophysial Hormones in Peripheral Tissues Oxytocin and vasopressin have been identified in corpora lutea of women,61,62,63 subhuman primates,64,65 cows,66 sheep,67 and rabbits, and in human and rat testes.68 Higher concentrations are found in bovine and sheep corpora lutea than
in human and primate corpora lutea. Significantly higher concentrations
of oxytocin are found in corpus luteum at the midluteal phase (Fig. 4).63,64 Ovarian vein blood draining corpora lutea had a higher concentration of
oxytocin than when there is no corpus luteum,63,64,65,69 and the mRNA for oxytocin is present in corpora lutea.5,70,71 Although the role of oxytocin in the ovary and testis is not well understood, oxytocin
probably exerts an antigonadal effect and subserves as
an intragonadal modulator of luteal function.63,65 Oxytocin inhibits in vitro luteal cell progesterone production,65 shortens the luteal phase and progesterone levels when given directly
into the corpus luteum of monkeys,72 and also shortens the cycle length of some animals.73 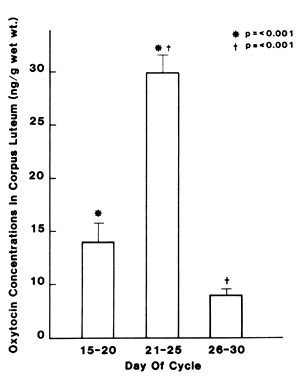 Fig. 4. Oxytocin concentrations in human corpus luteum during early (days 15–20) and
late (days 26–30) luteal phases showing a significant
increase at midluteal phase followed by a significant decrease in late
luteal phase. (Dawood MY, Khan-Dawood FS: Human ovarian oxytocin: Its source and relationship
to steroid hormones. Am J Obstet Gynecol 154:756, 1986) Fig. 4. Oxytocin concentrations in human corpus luteum during early (days 15–20) and
late (days 26–30) luteal phases showing a significant
increase at midluteal phase followed by a significant decrease in late
luteal phase. (Dawood MY, Khan-Dawood FS: Human ovarian oxytocin: Its source and relationship
to steroid hormones. Am J Obstet Gynecol 154:756, 1986)
|
Oxytocin is also present in human adrenal glands74 and the thymus and in the phrenic nerves of cats. In the adrenal gland, oxytocin
appears to regulate steroidogenesis. Thus oxytocin present
in peripheral nonneural tissues exerts a paracrine or autocrine effect. Male In the male, the function of oxytocin, if any, is unclear. Several studies
have shown that exogenous oxytocin may affect sperm transport, possibly
by an action on the smooth muscle in the seminiferous tubules. In
male rabbits, administration of methallibure, an inhibitor of oxytocin
release, reduces sperm count markedly.75 This effect is abrogated when oxytocin is given simultaneously with methallibure, indicating
that endogenously released oxytocin during ejaculation
may play a part in sperm transportation. Oxytocin is present in
the testes of several species.68 In rather high pharmacologic doses, oxytocin exerts an antigonadal effect
on the testes by inhibiting testosterone output.76 Oxytocin is released and increases significantly with sexual arousal and
also at ejaculation both with masturbation44 and with coitus.45 In women, oxytocin release during coitus could be responsible for the
increased uterine activity experienced during orgasm and may aid sperm
transport up the female genital tract. Other recorded effects of oxytocin include “insulin-like” effects
on stimulation of glucose uptake and formation of fatty acids, involution
of the bovine corpus luteum, and natriuretic effect. However, these
are pharmacologic effects of oxytocin rather than physiologic
effects. |