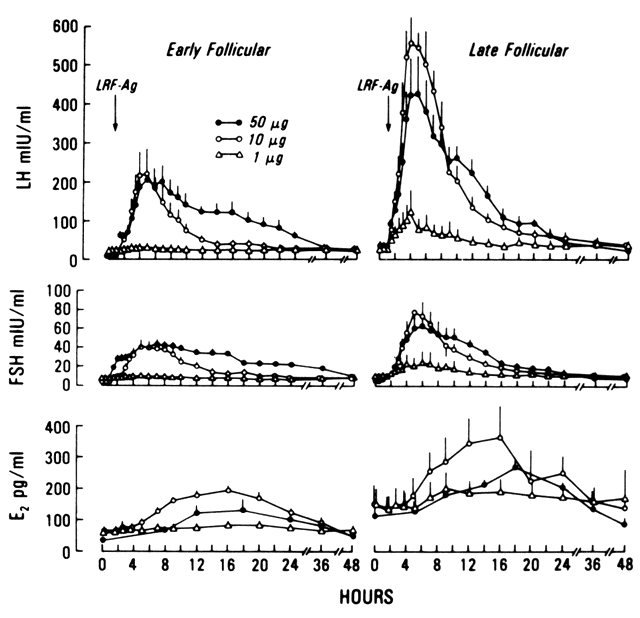
The sustained release of LH and FSH depends on the pulsatile stimulation
of the gonadotroph by GnRH. For instance, within a narrow pulse range, increases
in GnRH pulse frequency result in a reduction in gonadotropin
secretion, regardless of the magnitude of the pulse (Fig. 4).32 This paradoxic effect is believed to result from a reduction (down-regulation) in
the number of pituitary GnRH receptors.17, 33 At lower frequencies, however, a self-priming effect operates as well. 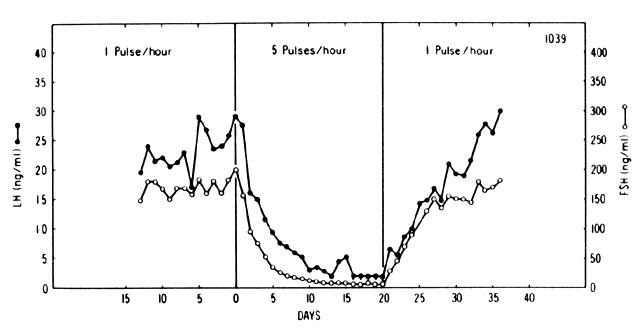 Fig. 4. Pituitary response to pulsatile and continuous administration of GnRH.(Belchetz PE, Plant TM, Nakai Y et al: Hypophysial responses to continuous
and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 202:631, 1978. Copyright 1978 by the AAAS) Fig. 4. Pituitary response to pulsatile and continuous administration of GnRH.(Belchetz PE, Plant TM, Nakai Y et al: Hypophysial responses to continuous
and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 202:631, 1978. Copyright 1978 by the AAAS)
|
Accordingly, the provision of GnRH heightens the pituitary response to
a subsequent similar stimulus. In the ovariectomized and hypothalamus-pituitary disconnected ewe, a decrease
in GnRH pulse frequency results in decreased LH pulse amplitude, secondary
to a reduction in the amount of releasable LH.34 The frequency of GnRH pulses has a qualitative effect as well. For instance, in
monkeys, changes in the frequency of GnRH pulses alters the
FSH/LH ratio, whereas changes in the amplitude of the pulses have a minor
effect.35 For example, the pulsatile administration (every 2 hours) of GnRH to hypogonadotropic
men coincides with the release of LH and the α-subunit
common to both gonadotropins, but not with the release of FSH. When
GnRH is administered every 30 minutes, the release of α-subunits
becomes erratic and baseline LH increases, although the coincidence
with α-subunit pulses is lost. In addition, FSH decreases to barely
detectable levels, resulting in an increased LH/FSH ratio.36 In contrast to the critical importance of the frequency of GnRH pulses, changes
in the amplitude seem to have a lesser role.11 Nevertheless, in ovariectomized sheep, the pulse amplitudes of GnRH and
LH are highly correlated.37 In women of reproductive age, a single bolus of exogenous GnRH elicits
an acute release of LH and, to a lesser extent, of FSH. When GnRH is given
at 2-hour intervals, a dose-response relationship exists between
GnRH (from at least 10 mg and up to 300 mg doses) and LH.38 The response is detected 5 minutes after intravenous injection of GnRH
and reaches its peak between 30 to 45 minutes later. In response to GnRH, the
pituitary may release prolactin as well. Increased circulating
prolactin following a 4-hour infusion of GnRH was first reported in
a group of patients suffering from anorexia nervosa, who had gained weight
during the week preceding the test.39 A similar effect was described in normal women, in whom the finding was
more pronounced during the peri-ovulatory period.40 Because the prolactin increment following infusion of GnRH correlates
with both the FSH response41 and with the LH response,42 the observed increase is believed to be the consequence of a paracrine
interaction between gonadotropin and prolactin. However, elevations of
prolactin in response to GnRH administration have been documented in 7 of 10 women
during the midtrimester of gestation, despite the lack
of gonadotropin response to GnRH. This nonspecific prolactin response
could also have been explained by the positive effect of high circulating
levels of estrogens during gestation on prolactin production and release, thus
sensitizing the lactotroph to nonspecific stimuli.43 Moreover, GnRH sensitizes the lactotrophs to thyrotropin-releasing hormone
action in normal women.44 Finally, GnRH may bind to somatotrophic and thyrotrophic cells as well, resulting
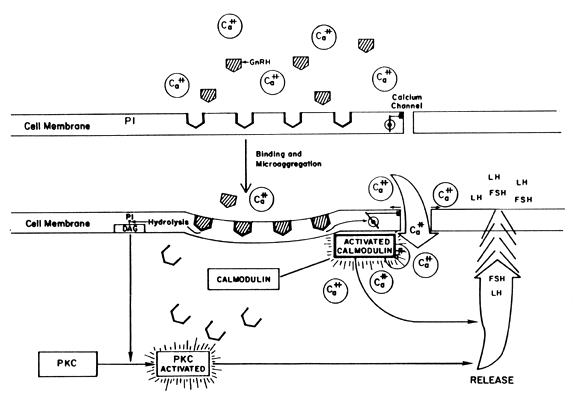
in actions not yet clarified.15 Self-Priming Action of GnRH Gonadotropin-releasing hormone induces two separate phenomena in the pituitary. On
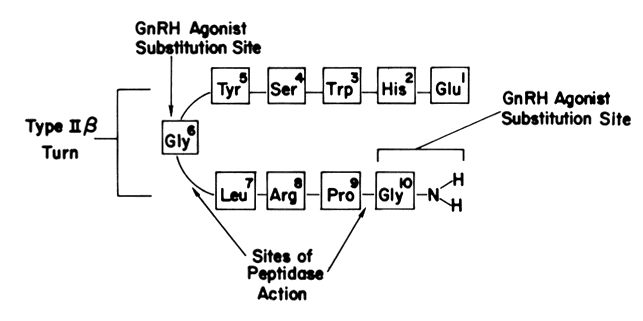
the one hand, GnRH primes (sensitizes) the gonadotroph to
subsequent stimulation by increasing the number of its own receptors (Fig. 5). A similar phenomenon of increased pituitary responsiveness to GnRH is
involved in the maturation of the hypothalamic-hypophyseal axis during
puberty,45 a phenomenon that is used to diagnose true precocious puberty. On the
other hand, GnRH triggers the release of LH and FSH. Both the “sensitization” and
the “release” effects are dose-dependent, but
functionally separate with the lower doses promoting priming, the
higher doses favoring release. The responses of FSH parallel those
of LH but on a much smaller scale.46 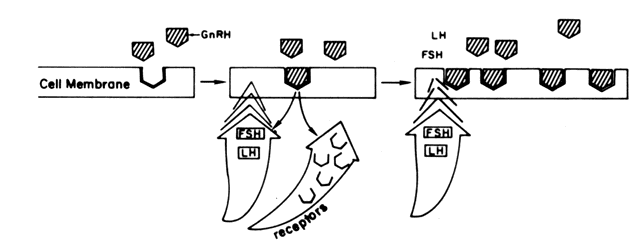 Fig. 5. Results of the binding of GnRH to its pituitary receptors. Fig. 5. Results of the binding of GnRH to its pituitary receptors.
|
One way by which the endocrine milieu modulates the pituitary response
to GnRH is by regulating the number of GnRH receptors rather than by altering
their affinity to GnRH. Indeed, in the rat, pituitary responsiveness
correlates well with the number of GnRH receptors.18 In this connection, estradiol seems necessary to unmask the self-priming
effect of GnRH.47 In the rat, low levels of prolactin are also necessary for GnRH to increase
its own receptor number. Nevertheless, a different mechanism may
be at play as well because both bromocriptine, a dopaminergic agent that
decreases prolactin release, or estradiol alone can sensitize the
pituitary to GnRH without necessarily increasing GnRH receptors.48 More recently, it has been demonstrated that inhibitors of PKC may inhibit
the self-priming actions of GnRH.49 Furthermore, it has been postulated the effects of the sex steroids on
the pituitary response may be directly related to an effect on PKC, independent
of LH and Ca++ influx.50 Effect of Estrogens on the Pituitary Response to GnRH In women, estrogens, in addition to unmasking GnRH self-priming, appear
to protect from pituitary desensitization. In this connection, estrogen
is antagonized by progesterone.51 Moreover, estradiol, at physiologic doses, increases the pituitary response
to GnRH.52 In contrast, the provision of pharmacologic doses of estradiol to normally
cycling women, resulting in serum levels of 700 to 1200 pg/mL, induces
a marked diminution in pituitary responsiveness to even high doses (300 mg) of
GnRH.53 The modulation of the pituitary response to GnRH by estradiol is duration-dependent. For
instance, the administration of estradiol at doses
resulting in serum levels of 100 to 200 pg/mL enhances the LH response
to 100 mg of GnRH, provided the preceding exposure to estrogen was at
least 84 hours in duration.54 The pituitary responsiveness is concentration-dependent and duration-dependent. Thus, LH
and FSH response to GnRH is proportional to the dose
of estradiol administered for 6 days. Minimal estradiol concentrations, resulting
in increased gonadotropin responsiveness to 100 mg of GnRH, are 90 pg/mL
for LH and 145 pg/mL for FSH.55 Interestingly, the administration of estradiol benzoate to normal women
at a dose resulting in serum estradiol concentrations similar to those
seen at midcycle can trigger an LH surge similar to that observed prior
to ovulation. The above studies, however, do not accurately reflect
the in vivo situation because the GnRH doses evaluated are not physiologic
and the peptide is not given in a pulsatile fashion. The ovariectomized
monkey with hypothalamic lesions in which gonadotropin secretion
is re-established by chronic pulsatile GnRH administration serves
as a better model to study the role of estrogen in modulating pituitary
sensitivity to GnRH. The administration of estradiol to such animals
results in an initial decline in gonadotropin levels, followed by a
discharge of LH and FSH. This suggests that estradiol exerts both a negative
and a positive feedback action at the level of the pituitary gland.56 To explain the observed effects of GnRH on the pituitary release of gonadotropins, the
existence of two pools of gonadotropins has been postulated,38 namely, a readily “releasable” pool and a “reserve” pool. The
former reflects pituitary “sensitivity” to
a discrete GnRH stimulus. The latter represents intracellular gonadotropin
not immediately available for release. The so-called capacity of
the pituitary is defined as the amount of gonadotropins released in response
to a larger and, therefore, more prolonged stimulation with GnRH. In
this context, pituitary capacity consists of the releasable and
reserve pools, as well as the amount of newly synthesized LH as the pituitary
is continuously challenged.57 To assess the sensitivity of the gonadotroph or the status of the “releasable
pool,” submaximal doses of GnRH must be used. Because
the plasma clearance of exogenous GnRH is reduced by at least 50% when
the dose is increased from 10 to 100 mg,58 the stimulus provided by relatively high doses of GnRH can be long enough
to evoke a release from the reserve pool as well. When 10 mg of GnRH
is administered every 2 hours for 10 hours, the LH response to the
first dose reflects the releasable pool. The integrated concentrations
of gonadotropins over time or the area under the LH and FSH curve during
the 10-hour period reflects the reserve pool because the initial releasable
pool is considered exhausted. Interpreted in this manner, the
administration of estradiol benzoate to mimic follicular phase levels
of estradiol results in increased releasable and reserve pools.59 Estrogens, however, seem preferentially to increase the reserve pool. For
instance, as the follicular phase progresses and estradiol levels
increase, the disparity between the response to 10 mg (releasable pool
response) and the response to 150 mg (releasable plus reserve pools) increases
in favor of the latter.60 In contrast, hypogonadal women have increased sensitivity to GnRH,59, 60 particularly if the baseline estradiol levels are less than 15 pg/mL.61 Such patients respond similarly to low or high doses of GnRH, indicating
the absence of an adequate reserve pool.60 This condition is reversed by the administration of estradiol,61 again confirming that estradiol preferentially increases the reserve pool. Similarly, increased
sensitivity is seen in postmenopausal patients. When
estradiol is replaced in these women, the acute releasable pool
is decreased at the second day of replacement but recovers on the fifth
day, describing a U-shaped curve. The reserve pool (measured as the
pituitary response to the second and third 10 mg dose of GnRH given
every 2 hours) increases on the fifth day of estrogen replacement.62 The addition of progesterone at the end of the estradiol treatment further
amplifies the response to a GnRH stimulus. As mentioned before, in
addition to the effects described, estrogen may influence the pituitary
response in more ways than one. For instance, estrogen inhibits pituitary
desensitization due to the down-regulation of receptors that follows
the continuous administration of GnRH, an effect that is antagonized
by progesterone.51 Finally, estrogen administration to oophorectomized ewes has produced
a LH pulse profile, suggesting a more prolonged LH response to each GnRH
pulse.63 A similar observation was made in women during the periovulatory period.29 Effect of Progesterone on the Pituitary Response to GnRH Progesterone given after the administration of estrogens further amplifies
the estrogen-enhanced responsiveness of the pituitary to GnRH.59, 64 In fact, progesterone, but not 17α-hydroxyprogesterone, is able to
trigger an LH and FSH surge in most normal estrogen-primed women.64, 65, 66 Indeed, the small preovulatory rise of progesterone may play a role in
the generation of the midcycle LH surge. The above notwithstanding, progesterone
antagonizes estrogen as it reduces the extent of pituitary
desensitization in response to a continuous GnRH infusion.51 Interestingly, combination oral contraceptives (containing both estrogens
and progestins) do not appear to affect the pituitary sensitivity
to GnRH.67 Other Modulating Factors of the Pituitary Response to GnRH The FSH and LH response to GnRH may be modulated by dopamine as well. Hyperprolactinemic
women, presumably dopamine deficient, display an increased
LH and FSH response to GnRH. Exogenous dopamine infusion decreases
this response in these patients as it does in normal women.68 Neuropeptide Y, a putative modulator of GnRH release,69 has been shown to enhance GnRH-stimulated LH release.70, 71 Gonadotropin surge attenuating factor (GnSAF) is a proposed modulator of
the midcycle gonadotropin surge, which is found in follicular fluid.72 It has been suggested that GnSAF attenuates both the primed and unprimed
response of the pituitary to GnRH.73, 74 One investigator has gone so far as to speculate that the self-priming
phenomenon is, in fact, a result of the interplay between GnRH and GnSAF.75 Nonetheless, the very existence of GnSAF and the physiologic role for
this agent are unclear. Inhibin has been shown to inhibit the GnRH-stimulated secretion of FSH, as
well as to diminish the LH pulse amplitude,76 independent from the GnRH stimulation protocol.77 Acute intake of alcohol appears to diminish the response of LH and free α-subunit, but
not FSH to the GnRH stimulus in men.78 Age brings about an impaired LH secretory capacity in men. While baseline
parameters remain unchanged, the bioactive/immunoactive ratio of LH
is decreased in response to an exogenous GnRH stimulus in older men.79 Additionally, older postmenopausal women have a decreased sensitivity
to estrogen feedback as evidenced by the response to a clomiphene citrate
challenge than do recently postmenopausal women.80 The significance of these findings remain to be determined. Finally, short-term fasting increases the sensitivity of the gonadotroph
to GnRH in men and women, an action independent of the level of plasma
glucose.81 Changes in the Pituitary Response to GnRH During the Menstrual Cycle Two mechanisms may play a role in the control of the hypothalamic-pituitary
axis, which culminates in the midcycle LH surge and subsequent ovulation. The
first, as previously described, is the modulation of the
pituitary response to GnRH by sex steroids. The second may be the influence
of these steroids on the GnRH pulse generator. Although the GnRH
pulse generator appears to play only a permissive role in gonadotropin
release, the gonadotropin pulse pattern varies during the cycle according
to the hormonal status (i.e., high frequency, low amplitude LH pulses during the follicular phase
and low frequency, high amplitude LH pulses during the luteal phase). The
female rhesus monkey exposed to estradiol benzoate displays increased
GnRH amplitude before and during an estradiol-triggered LH surge compared
with nonestrogen exposed controls. It is, therefore, conceivable
that, although not essential, an estradiol-triggered midcycle GnRH
surge may play a role in the generation of the midcycle LH surge.28 Similarly, the high levels of preovulatory estradiol may be responsible
for the preovulatory increase in pulse frequency and amplitude of LH
and thus, by inference, of GnRH, as observed in normal women.29 By the same token, during the luteal phase, the luteal reaction in LH
pulsatility appears to result from the action of estradiol and progesterone
at the level of the GnRH pulse generator, rather than the pituitary
gonadotrophs.31 Aside from steroidal interactions at the level of the hypothalamus, gonadotropin
release is modulated by altering the pituitary response. Before
demonstrating the influence of sex steroids on the pituitary response, the
effect of 100 to 150 mg of GnRH was shown to vary during the
menstrual cycle. While a more pronounced response is described during
the late follicular phase, various reports disagree over whether the response
is greater in the luteal phase82, 83 or in the late follicular phase.84 It is evident that the maximal sensitivity to GnRH occurs at midcycle, a
phenomenon modulated mainly by the circulating levels of estradiol. As
the follicular phase progresses, there is a change in the pituitary
response to large (150 mg), but not to smaller (10 mg), doses of GnRH,38 indicating that estradiol exerts a preferential effect on the reserve
pool. Additionally, the self-priming effect of GnRH may be an important
determinant of pituitary sensitivity. Thus, the administration of 10 mg
of GnRH at midcycle results in a tenfold increase in pituitary sensitivity.38 A U-shaped curve best describes the pituitary response to GnRH during
the follicular phase (Fig. 6). During the early follicular stage, when circulating estradiol levels
are at their lowest, both the releasable (sensitivity) and reserve pools
appear depleted. However, at even lower estradiol concentrations (i.e., hypogonadism), pituitary sensitivity and capacity appear to increase,61, 62 perhaps due to an increased GnRH output, resulting in a self-priming effect. On
the other hand, levels of estradiol consistent with the midfollicular
and late-follicular phase increase pituitary sensitivity. More
important, however, is the stimulatory effect of estrogens on the gonadotrophic
capacity. Consequently, the observed negative feedback action
of estrogens on the pituitary is exerted more at the level of gonadotropin
release rather than at the level of gonadotropin synthesis.56 During the follicular phase, transfer of gonadotropins from the reserve
to the releasable pool may occur (Fig. 7). The LH surge thus may be the consequence of multiple factors including
estrogens acting in concert with perhaps the amplifying action of progesterone59, 64, 65, 66, 85; the estrogen-induced self-priming effect of GnRH86; the prolongation of GnRH-dependent LH pulses in the presence of estradiol34; and the possible, but not crucial, estrogen effect on GnRH release.28 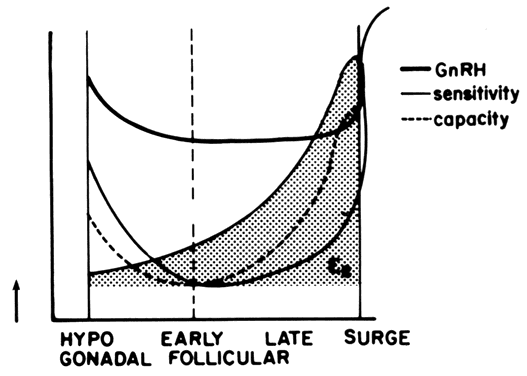 Fig. 6. Pituitary sensitivity and responsive capacity to GnRH according to the
estrogenic milieu and throughout the follicular phase of the menstrual
cycle.(Modified from Yen SSC, Lein A: The apparent paradox of the negative and
positive feedback cortisol system on gonadotrophin secretion. Am J Obstet
Gynecol 126:942, 1976) Fig. 6. Pituitary sensitivity and responsive capacity to GnRH according to the
estrogenic milieu and throughout the follicular phase of the menstrual
cycle.(Modified from Yen SSC, Lein A: The apparent paradox of the negative and
positive feedback cortisol system on gonadotrophin secretion. Am J Obstet
Gynecol 126:942, 1976)
|
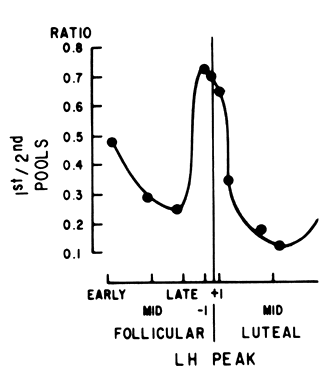 Fig. 7. Ratio between the first (releasable) pituitary gonadotropin pool and the
second (reserve) pool in response to GnRHthroughout the menstrual cycle. (Hoff JD, Lasley BL, Wang CF,Yen SSC: The two pools of pituitary gonadotropins: Regulation
during the menstrual cycle. J Clin Endocrinol Metab 44:302. Copyright 1977 by the Endocrine Society.) Fig. 7. Ratio between the first (releasable) pituitary gonadotropin pool and the
second (reserve) pool in response to GnRHthroughout the menstrual cycle. (Hoff JD, Lasley BL, Wang CF,Yen SSC: The two pools of pituitary gonadotropins: Regulation
during the menstrual cycle. J Clin Endocrinol Metab 44:302. Copyright 1977 by the Endocrine Society.)
|
In recent years, a great debate has raged over whether or not GnRH plays
a pivotal role in the midcycle gonadotropin surge. In support of this
notion is the demonstration that GnRH antagonists can effectively prevent
the midcycle LH surge.87, 88, 89, 90 Additionally, there exists the finding that the frequency of multiactivity
unit volleys emanating from the region of the hypothalamus decreases
just before the LH surge, a phenomenon most likely related to the
preovulatory E2 surge.91, 92 In this regard, however, evidence also exits refuting a role for GnRH
in the midcycle surge. For instance, it has been known for some time that
women with hypogonadotropic hypogonadism treated with pulsatile GnRH
do not require additional GnRH for the LH surge to occur.93 More importantly, GnRH pulse frequency (as evidenced by LH and free α-subunit
pulse frequency) does not change at midcycle.94 Clearly, the role of GnRH in the midcycle gonadotropin surge is not well
understood at this time. In summary, during the follicular phase, estradiol amplifies GnRH action
at the pituitary level by increasing the reserve pool while at the same
time inhibiting the release of gonadotropins. In the late follicular
phase, however, increasing amounts of estradiol may directly increase
both GnRH pulse frequency and amplitude and the transfer of LH from
the reserve into the releasable pool. This results in the paroxysmal
LH surge despite the inhibitory action of estradiol on LH release. |
 ).
).