|
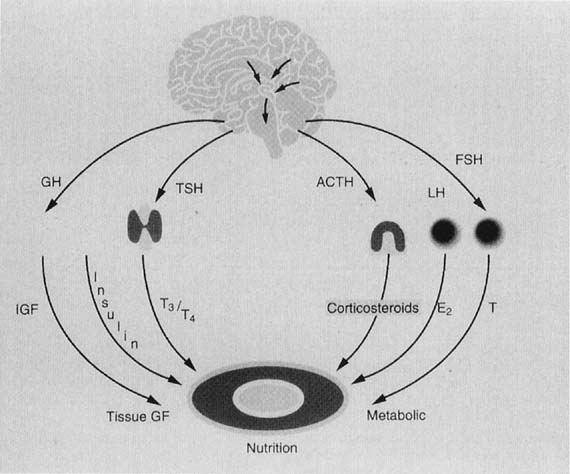
Any discussion of the physiology of puberty must incorporate knowledge
and understanding of neuroendocrinology of the hypothalamic-pituitary-ovarian
(HPO) axis. The prevalent concepts are depicted schematically in Figure
7. Puberty is one event in a dynamic process that begins in utero.
GnRH is present, stimulating the release of gonadotropins by 10 to 18
weeks’ gestation.45,46,47,48
Levels continue to rise, which results in increasing gonadal steroid production.
In the fetus and term neonate, serum estrogen levels are noted to be approximately
5000 pg/mL at term, but this also reflects the conversion of fetal and
maternal C-19 steroid precursors to estrogens by the placenta. The fetal
adrenal gland also plays a role in estrogen production throughout fetal
life. Closer to term, gonadotropin activity declines. The mechanism of
the inhibition is unclear but may involve either central factors or sex
steroid–related negative feedback. This negative feedback is, and
will continue to be, a predominant mode of control on the hypothalamic-pituitary
axis. A manifestation of in utero estrogen levels can be noted at the
time of birth, when there may be palpable breast tissue, indicative of
the end-organ effect of circulating estrogens. With the sudden loss of
maternal and placental sex steroid contributions, the neonate experiences
a resurgence of gonadotropins, and peaks or pulses may be identified for
several years. Episodic sex steroid production and ovarian cysts may result.
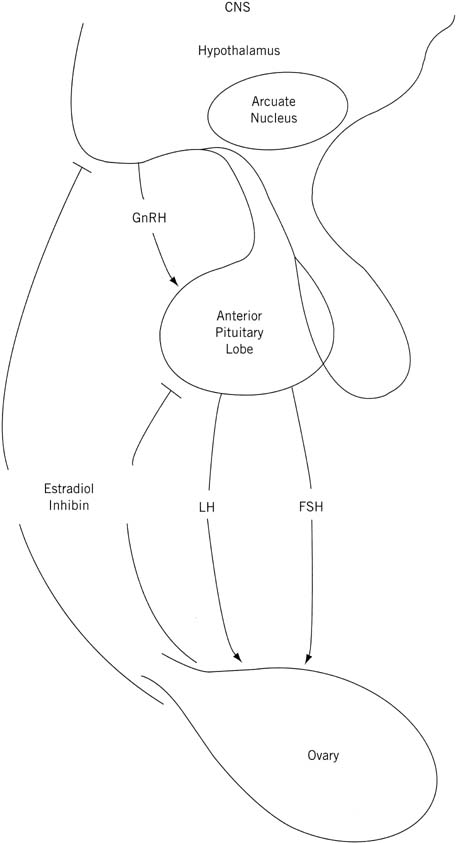 Fig. 7. Schematic representation of the central nervous system (CNS)–hypothalamic–pituitary–ovarian axis.(Lee PA, Reiter EO, Kulin HE: Neuroendocrinology of puberty. In Sanfilippo
JS, Muram D, Lee PA, Dewhurst J [eds]: Pediatric and Adolescent Gynecology, p 45. Philadelphia, WB Saunders, 1994.) Fig. 7. Schematic representation of the central nervous system (CNS)–hypothalamic–pituitary–ovarian axis.(Lee PA, Reiter EO, Kulin HE: Neuroendocrinology of puberty. In Sanfilippo
JS, Muram D, Lee PA, Dewhurst J [eds]: Pediatric and Adolescent Gynecology, p 45. Philadelphia, WB Saunders, 1994.)
|
Estradiol is the major estrogen (and sex steroid) in the female body.
This “GnRH pulse generator” undergoes a modicum of suppression
to a mid-childhood nadir, but with the advent of new fluorimetric assays,
episodic gonadotropin secretion (FSH and LH) with a circadian rhythm has
been shown during the prepubertal years.49,50,51
The axis previously thought to be quiescent is active, but it is suppressed
during the “juvenile pause.” The mechanism of this central
restraint is unknown. Various clinical clues have been reviewed and summarized
by Styne52 through analysis of hamartoma
patients and clinical states associated with increased intracranial pressure,
central nervous system neoplasms, and precocious puberty. We now know
that the prepubertal pituitary is highly sensitive to GnRH, capable of
yielding the greatest magnitude of increase in gonadotropin levels in
response to physiologically administered GnRH stimulation during this
stage of development.49,50
FSH particularly responds dramatically before puberty. The mechanism of
GnRH pulsatility and control is unknown but has been postulated to involve
catecholamines and glutamate along with dopaminergic, noradrenergic, and
opioid neurons and their interaction with the hypothalamus.53
With the onset of puberty, nocturnal gonadotropin secretory episodes increase,
primarily LH, and in essence reflect an increase in pulse amplitude.49,50,51
Properly timed pulsatile GnRH secretion must exist to effect appropriate
FSH and LH secretion.54 Frequently an LH
pulse is followed by an FSH pulse within 30 minutes, and secondarily gonadal
steroids begin to rise with pubertal onset.49,50
Whether through the concurrently increasing sex steroids and their negative
feedback influence or through inhibin, the pubertal FSH response to GnRH
is dampened over that of LH, resulting in a final LH-to-FSH ratio of 1:1
(versus 1:20 before puberty).49,50,51,55
As puberty progresses, wake-time gonadotropin pulses increase, and sleep-associated
pulses decrease to an eventual loss of diurnal variation
with more advanced puberty. Regular daytime LH pulses are followed closely
by thelarche, and the achievement of a mature LH pattern seems to
parallel the growth spurt in both sexes.56 Age of onset of menarche and estradiol levels seem to be interrelated. In
women with early menarche, there is a twofold greater follicular phase
serum estradiol level compared with women whose menarche occurs at 13 years
of age or later.57 Eventually, positive feedback mechanisms ensue, which give rise to ovarian
estradiol–triggered LH surges, ovulation, and the establishment
of regular menses (all discussed in more detail elsewhere in this
volume). Many clinical entities have contributed to the understanding
of the HPO axis as it relates to puberty and the aforementioned events:
- GnRH must be responsible for even prepubertal gonadotropin pulses
because these are abolished with the down-regulation that results from
GnRH agonist therapy.49,50,51
- The juvenile pause, or mid-childhood central restraint, must not crucially
involve gonadal steroids because patients with gonadal dysgenesis
or agenesis (Turner’s syndrome) experience the same phenomenon.58
- Anorexia nervosa patients and intensively trained (elite) athletes
can experience delayed or arrested puberty, abnormal growth, or primary
or secondary amenorrhea. They have been shown to have any combination
of decreased gonadotropins (pulse frequency and amplitude), decreased
sex steroids, and impaired gonadotropin response to GnRH. Theories to
explain such phenomena have involved crucial weight parameters and opioid
or catecholestrogen metabolism changes, none of which account for all
patients.32,59,60,61,62
Catecholestrogens are less potent than either estradiol or estrone.
They have both catecholinergic and estrogen effects. The latter effect
is minimal.
Growth hormone likely interacts with the HPO axis and has a role in puberty
and secondary sex characteristic development, but this area is fraught
with controversy and confusion. Isolated hGH-deficient patients often
experience delayed puberty with the anticipated short stature. The impact
of hGH supplementation on puberty onset, timing, and rate of progression
is a subject of great debate, however.9,13,15,19,63
There is suspicion that although hGH therapy does not trigger earlier
puberty, it may augment the rate of progression. One could argue that
such supplementation is not physiologic. Patients with McCune-Albright
syndrome who exhibit precocious puberty often are found to have hypersecretion
of hGH and normal gonadotropins.15 hGH supplementation
may facilitate ovulation induction with human menopausal gonadotropin.15
The mechanism of puberty promotion likely involves the capability of hGH
to amplify or augment gonadotropin action on the ovary, stimulating
steroid production and plausibly regulating follicular development.15 This mechanism may be effected through IGF-I as a mediator.64 The possibility still exists, however, that hGH has a direct effect on
the ovary.15 As mentioned earlier, insulin augments bioactive IGF-I by decreasing its
binding proteins, and this has implications for ovarian steroidogenesis. Insulin’s
role may not stop there, however. It also has the
ability to control sex hormone–binding proteins in an inverse
relationship. Consequently the rising insulin levels seen in adolescents (insulin
resistance) yield increased free sex hormones and likely
contribute to sexual maturation.22 Insulin also may stimulate ovarian growth. In their 1992 review, Nobels and Dewailly22 summarized the above-mentioned pubertal interactions (Fig. 8), then elegantly generated a hypothesis to explain why PCOS develops in
many women during their adolescent years (Fig. 9). The salient points are as follows: - Hyperinsulinemia and elevated levels of IGF-I, noted during adolescence, cause
theca-cell hyperplasia and excessive androgen production from
the ovaries of predisposed females.
- These intraovarian androgens lead to follicular atresia with resultant
lower estradiol levels but higher estrone levels through peripheral aromatization.
- This hormonal milieu causes a relatively exaggerated response of LH secretion
to GnRH, reinforcing the theca-cell hyperplasia.
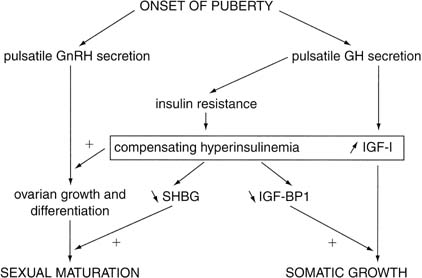 Fig. 8. Simplified scheme of the endocrine interactions that regulate pubertal
development. GnRH, gonadotropin-releasing hormone; GH, growth hormone; IGF-I, insulin-like
growth factor I; IGF-BP1, IGF binding protein 1; SHBG, sex
hormone–binding globulin.(Nobels F, Dewailly DD: Puberty and polycystic ovarian syndrome: The insulin/insulin-like
growth factor I hypothesis. Fertil Steril 58:655, 1992.) Fig. 8. Simplified scheme of the endocrine interactions that regulate pubertal
development. GnRH, gonadotropin-releasing hormone; GH, growth hormone; IGF-I, insulin-like
growth factor I; IGF-BP1, IGF binding protein 1; SHBG, sex
hormone–binding globulin.(Nobels F, Dewailly DD: Puberty and polycystic ovarian syndrome: The insulin/insulin-like
growth factor I hypothesis. Fertil Steril 58:655, 1992.)
|
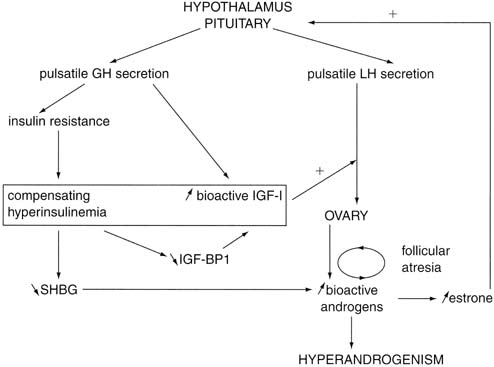 Fig. 9. Hypothetical mechanism of onset of polycystic ovarian syndrome during puberty. GH, growth
hormone; IGF-I, insulin-like growth factor I; IGF-BP1, IGF
binding protein 1; LH, luteinizing hormone; SHBG, sex hormone–binding
globulin.(Nobels F, Dewailly DD: Puberty and polycystic ovarian syndrome: The insulin/insulin-like
growth factor I hypothesis. Fertil Steril 58:655, 1992.) Fig. 9. Hypothetical mechanism of onset of polycystic ovarian syndrome during puberty. GH, growth
hormone; IGF-I, insulin-like growth factor I; IGF-BP1, IGF
binding protein 1; LH, luteinizing hormone; SHBG, sex hormone–binding
globulin.(Nobels F, Dewailly DD: Puberty and polycystic ovarian syndrome: The insulin/insulin-like
growth factor I hypothesis. Fertil Steril 58:655, 1992.)
|
On ultrasound, ovaries can (but do not always) appear polycystic, with
arrested follicles and stromal hyperplasia. In a related manner, other
authors have suggested that an alteration in hGH secretion per se sets
off the PCOS cascade.15 Regardless, many features of PCOS described earlier are noted in normal
puberty. In some patients, normalization of the insulin resistance or
increased hGH or IGF-I secretion (normally seen with attainment of final
adult height) does not occur on schedule, however, and the woman
is left with hyperandrogenism and anovulation and variable features of
obesity, acanthosis nigricans, and polycystic ovaries. Thyroid Hormones Thyroid hormones are an integral and essential prerequisite for normal
pubertal development. Evaluation of total and free thyroid hormone levels
and thyroxine (T4)-binding globulin during puberty has been addressed by Dunger and coworkers65 in their evaluation of 20 girls and 19 boys, ranging in age from 10 to 15 years. Assessment
was made at 6-month intervals; the results noted
that free T4 levels were associated with a marked decrease from 10 years of age (15.7 pmol/L) to 12.5 years
of age (13 pmol/L) before rising to prepubertal (15.9 pmol/L) levels
at 15 years of age. The nadir occurred during
pubertal developmental Tanner stages 3 to 4. Total T4 levels, although having a similar pattern, showed a slight delay in the
nadir (13 years of age, puberty stage 4). These findings were for girls
in the peripubertal years. T4-binding globulin concentrations remained unchanged in girls but fell slightly
in boys during later puberty. Theoretically, thyroid hormones are essential because they potentiate the
actions of IGF-I on cartilage66 and stimulate hGH synthesis by the anterior pituitary gland.67 One would assume that there is a significant change in the levels of free
thyroid hormone in boys versus girls during pubertal development. It
is well known that thyroid hormones play an important role with respect
to IGF-I action on cartilage growth in an animal model64 and that they stimulate hGH synthesis. When one looks at the entire spectrum
of a female’s life cycle, the continuum begins during the
neonatal stage: Thyroid hormones are elevated (i.e., at normal adult
levels) in the neonate, with a progressive decrease in levels between
ages 1 and 15 years.68 The fall is less marked or absent in the 10- to 15-year age group, and
T4 and thyroid-binding globulin levels are in the normal adult range. The
question remains as to whether or not there is a change in thyrotropin-receptor
function that is associated with pubertal development. This
question is unanswered at present. Changes in thyroid hormone levels
around puberty may reflect a change (i.e., increase) in hGH secretion
during pubertal development. There also seems to be a circannual rhythm with respect to thyroid-stimulating
hormone secretion. A peak is noted in December according to Bellastella
and colleagues.69 hGH, T4, and triiodothyronine (T3) levels did not show any similar rhythm. These investigators concluded
that before puberty, thyroid hormones play a minimal role, if any, with
regard to regulation of circannual thyroid-stimulating hormone periodicity (Figs. 10 and 11). 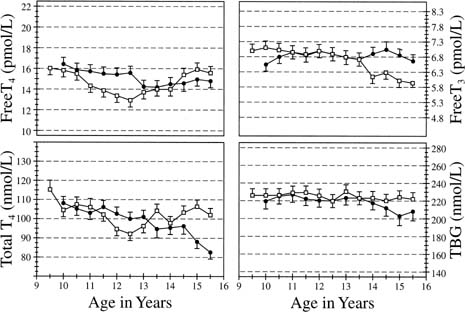 Fig. 10. Serum thyroxine (T4), free T4, free triiodothyronine (T3), and thyroxine-binding globulin (TBG) concentrations in a cohort of 19 girls
and 19 boys between the ages of 9.5 and 15.5 years. The values
represent means ± SEM. The y-axes represent the adult normal ranges.(Dunger DB, Perkins JA, Terence P, et al: A longitudinal study of total
and free thyroid hormones and thyroxine binding globulin during normal
puberty. Acta Endocrinol 123:305, 1990.) Fig. 10. Serum thyroxine (T4), free T4, free triiodothyronine (T3), and thyroxine-binding globulin (TBG) concentrations in a cohort of 19 girls
and 19 boys between the ages of 9.5 and 15.5 years. The values
represent means ± SEM. The y-axes represent the adult normal ranges.(Dunger DB, Perkins JA, Terence P, et al: A longitudinal study of total
and free thyroid hormones and thyroxine binding globulin during normal
puberty. Acta Endocrinol 123:305, 1990.)
|
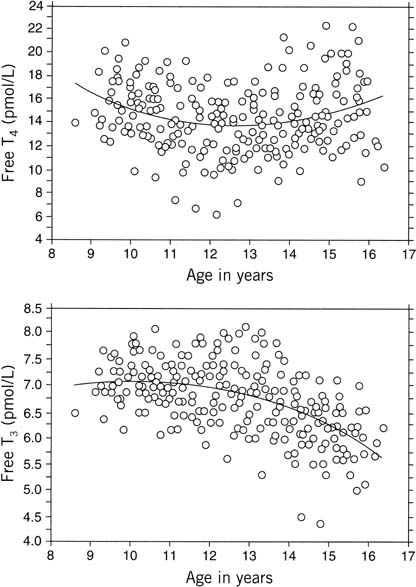 Fig. 11. Sequential measures of serum free thyroxine (FT4) and free triiodothyronine (FT3) concentrations against age in 19 girls. Individual values are plotted
together with a line showing the quadratic regression of free T4 or free T3 against age (A). FT4 = 50 − 5.7 A + 0.2 A2; FT3 = 2.9 + 0.83 A − 0.04 A2.(Dunger DB, Perkins JA, Jowett TP, et al: A longitudinal study of total
and free thyroid hormones and thyroxine binding globulin during normal
puberty. Acta Endocrinol 123:305, 1990.) Fig. 11. Sequential measures of serum free thyroxine (FT4) and free triiodothyronine (FT3) concentrations against age in 19 girls. Individual values are plotted
together with a line showing the quadratic regression of free T4 or free T3 against age (A). FT4 = 50 − 5.7 A + 0.2 A2; FT3 = 2.9 + 0.83 A − 0.04 A2.(Dunger DB, Perkins JA, Jowett TP, et al: A longitudinal study of total
and free thyroid hormones and thyroxine binding globulin during normal
puberty. Acta Endocrinol 123:305, 1990.)
|
Onset Not only is the mechanism of mid-childhood central restraint of the GnRH
pulse generator still a mystery, but also the basis for the eventual
loss of this inhibition (i.e., onset of puberty) is still elusive. Many
etiologic theories and factors have been proposed, such as body fat
composition; nutritional status; exercise and fitness level; stress; cortisol; endogenous
opiates; sex steroids; inhibin and activin; melatonin, light, and
climate; hGH, IGF-I, and insulin; thyroid hormone; prolactin; and
vitamin D. Some hypothalamic role generally is agreed on, and it seems that genetics
likely is involved. Any theories also must account for the initial
nocturnal predominance of the LH pulsatilityA brief discussion of some
of the most notable discoveries is included in this chapter. For further
details, see elsewhere70 (Figs. 12 and 13). 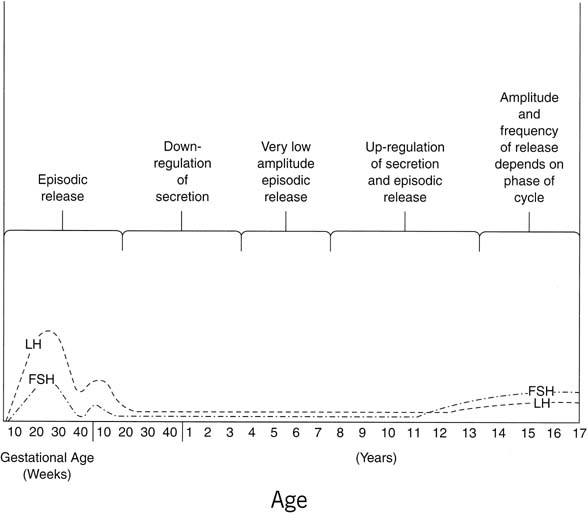 Fig. 12. Profile of relative mean levels of circulating luteinizing hormone (LH) and
follicle-stimulating hormone (FSH) during fetal life, infancy, childhood, and
puberty. Episodic release, first apparent before birth, persists
into early infancy, becomes less frequent during childhood, then
resurges to stimulate puberty.(Lee PA, Reiter EO, Kulin HE: Neuroendocrinology of puberty. In Sanfilippo
JS, Muram D, Lee PA, Dewhurst J [eds]: Pediatric and Adolescent Gynecology, p 54. 2nd
ed. Philadelphia, WB Saunders, 2001.) Fig. 12. Profile of relative mean levels of circulating luteinizing hormone (LH) and
follicle-stimulating hormone (FSH) during fetal life, infancy, childhood, and
puberty. Episodic release, first apparent before birth, persists
into early infancy, becomes less frequent during childhood, then
resurges to stimulate puberty.(Lee PA, Reiter EO, Kulin HE: Neuroendocrinology of puberty. In Sanfilippo
JS, Muram D, Lee PA, Dewhurst J [eds]: Pediatric and Adolescent Gynecology, p 54. 2nd
ed. Philadelphia, WB Saunders, 2001.)
|
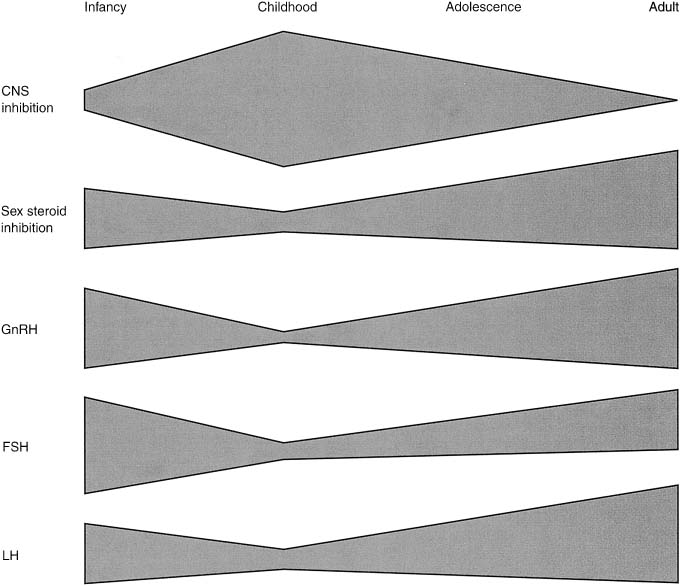 Fig. 13. Schematic mechanism for the control of the onset of puberty. Central restraint
of hypothalamic function is maximal in mid childhood. Sex steroid
inhibitory regulation of gonadotropin-releasing hormone (GnRH) is
present in infancy, early childhood, and adulthood. Pulsatile secretion
of GnRH is depressed in mid childhood, increasing with the onset of
puberty. Follicle-stimulating hormone (FSH) secretion is high in the infant
female, declines, and increases again at puberty. Luteinizing hormone (LH) secretion
is modest in infancy, is minimal in childhood, and
increases significantly during adolescence.(Root AW: Puberty in the female: Normal and aberrant. In Wallach EE, Zacur
HA [eds]: Reproductive Medicine and Surgery, p 100. St Louis, CV Mosby, 1994.) Fig. 13. Schematic mechanism for the control of the onset of puberty. Central restraint
of hypothalamic function is maximal in mid childhood. Sex steroid
inhibitory regulation of gonadotropin-releasing hormone (GnRH) is
present in infancy, early childhood, and adulthood. Pulsatile secretion
of GnRH is depressed in mid childhood, increasing with the onset of
puberty. Follicle-stimulating hormone (FSH) secretion is high in the infant
female, declines, and increases again at puberty. Luteinizing hormone (LH) secretion
is modest in infancy, is minimal in childhood, and
increases significantly during adolescence.(Root AW: Puberty in the female: Normal and aberrant. In Wallach EE, Zacur
HA [eds]: Reproductive Medicine and Surgery, p 100. St Louis, CV Mosby, 1994.)
|
The contribution of body fat, lean body mass, and body mass index to
the onset of puberty or age of menarche is also a controversial topic.71,72,73
Frisch’s71 critical weight theory
does not explain findings in all patients. There is great variation, making
absolute thresholds unreliable, but there does seem to be a trend toward
earlier puberty in moderately overweight girls and delayed puberty in
severely underweight or malnourished or obese girls. Anorexia nervosa
patients and intensively trained athletes can experience delayed (or arrested)
puberty and are found to exhibit decreased gonadotropins, dampened gonadotropic
pulsatility, and decreased levels of sex steroids. Intertwined and often
indistinguishable are the roles of diet, exercise, and psychological stress,
which all are apparently capable, in their extremes, of affecting the
age of puberty onset.53,59
Closely linked is the finding of increased cortisol in amenorrheic athletes,53
but in terms of a critical role in triggering normal puberty, cortisol
levels do not change dramatically with puberty.
There is a progressive increase in circulating levels of DHEA and DHEA-S
with sexual maturation, but only speculation exists as to a specific
role of ACTH or adrenal androgens in the onset of puberty. Even the exact
impetus for adrenarche remains a mystery. The chronologic alteration
in circulating DHEA and DHEA-S levels has been addressed by Hopper
and Yen,74 who evaluated boys and girls and adult subjects. Their research identified
a progressive and parallel increase in serum DHEA and DHEA-S in boys
and men. Their work identified an earlier rise for DHEA (age 13) than
for DHEA-S (age 14) in boys. In addition, from the age of 8 through
adulthood (in boys), there was a 2.6-fold increase in circulating levels
of DHEA and a 7.7-fold increase in DHEA-S. In girls, this parallel
rise in DHEA and DHEA-S was not as apparent; however, there was an abrupt
increase in DHEA levels between 11 and 12 years of age. These researchers
hypothesized that adrenal androgens may play a role in the “initiating
factor for the central nervous system program” related
to onset of pubertal development. The change in sensitivity
to negative feedback at the level of the pituitary may be representative
of “steroid modulation” at this central level, which
is associated with increased pituitary hormone output and the related
events regarding sexual maturation (Fig. 14). 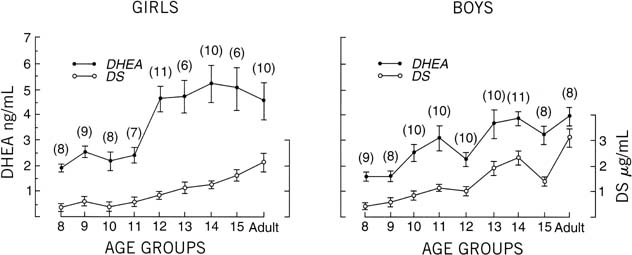 Fig. 14. Serum concentrations of dehydroepiandrosterone (DHEA) and dehydroepiandrosterone
sulfate (DS) in girls and boys. Vertical lines indicate ± SE. The
number of individuals in each age group is shown in parentheses.(Hopper BR, Yen SSC: Circulating concentrations of dehydroepiandrosterone
and dehydroepiandrosterone sulfate during puberty. J Clin Endocrinol
Metab 40:458, 1975.) Fig. 14. Serum concentrations of dehydroepiandrosterone (DHEA) and dehydroepiandrosterone
sulfate (DS) in girls and boys. Vertical lines indicate ± SE. The
number of individuals in each age group is shown in parentheses.(Hopper BR, Yen SSC: Circulating concentrations of dehydroepiandrosterone
and dehydroepiandrosterone sulfate during puberty. J Clin Endocrinol
Metab 40:458, 1975.)
|
Under normal pubertal developmental circumstances, adrenarche and gonadarche (gonadal
maturation) are closely linked. In pathologic situations, there
can be a wide disarray, however, with respect to each of these
entities.
Any excitement over a potential onset role of endogenous opiates, which
rise during stress and may suppress GnRH or LH, was dampened with the
discovery that naloxone (opiate antagonist) does not affect the initial
peripubertal sleep-associated LH pulsatility.70,74
Sex steroids themselves are unlikely culprits in the puberty trigger given
that agonadal and gonadal dysgenesis patients experience comparable increased
LH pulsatility patterns with puberty onset.58
No convincing role or consistent relationship has been identified for activin, inhibin, prolactin, or melatonin (with or without pineal gland), the
last-mentioned being the most controversial. Because of its nocturnal
secretory predominance and because it is produced by the pineal
gland, which has been implicated in the pubertal onset of seasonal breeding
animals, melatonin is a reasonable candidate in the mechanism of
puberty control.75 It has been hypothesized that declining levels of melatonin could result
in increased GnRH secretion during puberty. To date, however, no obvious
relationship between melatonin blood levels and the onset and progression
of normal puberty in girls has been found. With this concept
in mind, research involving evaluation of plasma melatonin rhythm in
humans, primarily from 24-hour serum sampling, has implicated the role
of melatonin in disorders of the hypothalamic-pituitary-gonadal axis. Specifically, delayed
puberty, precocious puberty, and hypothalamic amenorrhea
all seem to have a direct link to altered plasma melatonin profiles (Table 6).76 Under normal circumstances, however, melatonin has less of an active role
with respect to pubertal onset.77 TABLE 6. Studies of Plasma Melatonin in Human Puberty: Normal Subjects
| Results |
Investigators | Subjects | Sampling Time | No Change with Puberty | Change Present with Puberty |
Daytime Concentrations |
Silman et al (1979) | 51 healthy boys and girls, 11.5–14 years; all stages of puberty | Single sample: 1100–1300 | No changes in plasma melatonin in girls | Boys in stage 1 have higher plasma melatonin than all other stages |
Lenko et al (1982) | 79 girls, 83 boys, healthy, 9–16 years; all stages of puberty | Single sample: Girls 0800–1100 Boys 1300–1500 | No changes in plasma melatonin by sex or pubertal stage | |
Nocturnal Concentrations or 12–24-hr Profiles |
Arendt (1978) | Normal children; six prepubertal, three pubertal | Two samples: 1200, 2400 | No change in plasma melatonin between pubertal and prepubertal children | |
Fevre et al (1978) | Four normal pubertal boys, 12–17 years | Multiple samples: 20-min intervals for 24 hr | Nocturnal increase in LH and melatonin with a positive correlation between
both hormones | |
Ehrenkranz et al (1982) | Boys with constitutional short stature, eight prepubertal, 9–13 years
and seven pubertal, 13–15 years; five normal adult males | Multiple samples: 3-hr intervals for 24 hr | No difference in concentration or timing of plasma melatonin among groups | |
Waldhauser et al (1984) | 38 boys, 20 girls, 1–18 years; hospitalized in a pediatric or otolaryngological
unit | Two samples: 0700–1000 2300–0100 | | Plasma melatonin decreases as a function of pubertal stage. Inverse correlation
between nocturnal melatonin and LH concentrations |
Attanasio et al (1985) | Normal children, 21 girls, 17 boys, 1–18 years hospitalized for
minor illness or diagnostic evaluations; all stages of puberty | Multiple samples: 3-hr intervals from 2100 to 0600 and single sample 1200 | | Decline in 0300 melatonin concentration with age and pubertal stage from
infancy to stage 5 |
Cavallo (1992) | Normal children, 5–17 years; 30 boys, 32 girls; all stages of puberty | Multiple samples: 1-hr samples by constant withdrawal from 1800 to 0800 | No change in duration of nocturnal melatonin surge or time of peak with
puberty; wide individual variation in melatonin concentrations | Decline in nocturnal melatonin peak with age and pubertal stage, but no
significant relation with pubertal stage |
LH, luteinizing hormone.
Cavallo A: Melatonin and human puberty: Current perspectives. J Pineal
Res 15:115, 1993.
In boys approaching puberty, an abrupt fall in the concentration of melatonin
with advancing development suggests that this hormone may play
an integral role in the physiologic onset of puberty in boys (Fig. 15). It is hypothesized that melatonin acts directly on the gonads to suppress
function.78 Overall the effects seem to be predominant in boys compared with girls. 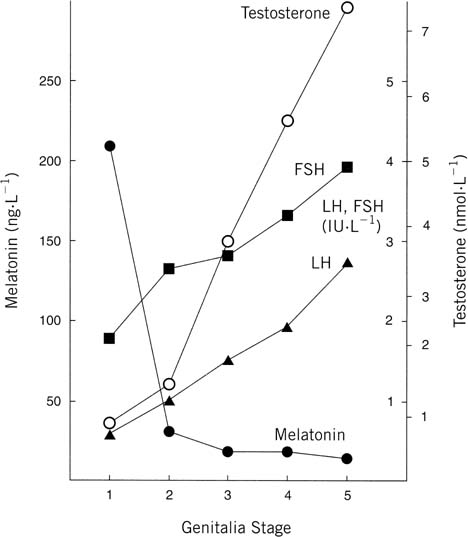 Fig. 15. Genitalia stage. Mean concentration (calculated from log transformed data) of
melatonin (•), follicle-stimulating hormone (FSH) (▪), luteinizing
hormone (LH) ( Fig. 15. Genitalia stage. Mean concentration (calculated from log transformed data) of
melatonin (•), follicle-stimulating hormone (FSH) (▪), luteinizing
hormone (LH) ( ), and testosterone (○) in
serum samples from school-age boys. The values are grouped for each
stage of puberty at which they were obtained. Stages: 1, testes, scrotum, and
penis the same size and proportion as in early childhood; 2, enlargement
of testes and scrotum, change in texture and some reddening
of scrotal skin; 3, further growth of penis, mainly in length but some
increase in breadth; 4, further enlargement of penis in length and
breadth and development of glans; 5, genitalia adult in size and shape.(Silman RE, Leone RM, Hooper JL: Melatonin, the pineal gland and human
puberty. Nature 282:301, 1979.) ), and testosterone (○) in
serum samples from school-age boys. The values are grouped for each
stage of puberty at which they were obtained. Stages: 1, testes, scrotum, and
penis the same size and proportion as in early childhood; 2, enlargement
of testes and scrotum, change in texture and some reddening
of scrotal skin; 3, further growth of penis, mainly in length but some
increase in breadth; 4, further enlargement of penis in length and
breadth and development of glans; 5, genitalia adult in size and shape.(Silman RE, Leone RM, Hooper JL: Melatonin, the pineal gland and human
puberty. Nature 282:301, 1979.)
|
With respect to pubertal aberration and melatonin secretion, there seem
to be higher melatonin concentrations in association with delayed puberty.79
From the opposite perspective (i.e., precocious puberty), there is a lower
day-night increment in melatonin levels compared with controls. The overall
conclusion is that lower circulating melatonin levels are associated with
precocious puberty.80,81
Most researchers would concur that the role of the human pineal gland
with respect to pubertal development continues to be speculative. In large
part, many of these studies are difficult to conduct because of the effects
of light exposure and overall lack of understanding at the receptor level
with respect to specific (i.e., direct) effects of melatonin on the reproductive
system.
hGH has been implicated as having a role in the onset of puberty. After
all, the increased hGH pulse amplitude noted with early puberty is secreted
mainly during the first hours of sleep, as is GnRH and LH initially. hGH
is a puberty promoter, as outlined earlier; however, puberty
occurs in girls with hGH deficiency, making hGH an unlikely major factor
in the initiation of puberty. Many genes have been identified and are involved in reproduction. They
are outlined in Table 7. TABLE 7. Genes Involved in Reproduction and Their Mutation
Gene | Chromosome Location | Mutations | Phenotype |
GnRH | 8p11 | None | ? IHH |
KAL | Xp22.3 | Inactivating | IHH and anosmia |
DAX-1 | Xp21 | Inactivating | AHC and IHH |
GnRH receptor | 4q21.2 | Inactivating | IHH |
α-Subunit | 6q | None | ? IHH and hypothyroidism |
LH-β | 19q13.3 | Inactivating | Isolated LH deficiency |
hCG-β | 19q13.3 | None | ? |
FSH-β | 11P13 | Inactivating | Isolated FSH deficiency |
LH/hCG receptor | 2p21 | Activating | Familial precocious puberty; no effect on females |
| | | Inactivating | Undermasculinization of genetic males; secondary amenorrhea in females |
FSH receptor | 2p21 | Inactivating | Premature ovarian failure in females; oligospermia in males |
| | | Activating | ? Increased sperm |
GnRH, gonadotropin-releasing hormone; KAL, Kallmann syndrome; DAX-1, dosage-sensitive
sex reversal. Adrenal hypoplasia congenital, critical region
of X chromosome, gene 1; LH, luteinizing hormone; FSH, follicle-stimulating
hormone; hCG, human chorionic gonaotropin; AHC, adrenal hypoplasia
congenital; IHH, idiopathic hypogonadotropic hypogonadism.
|
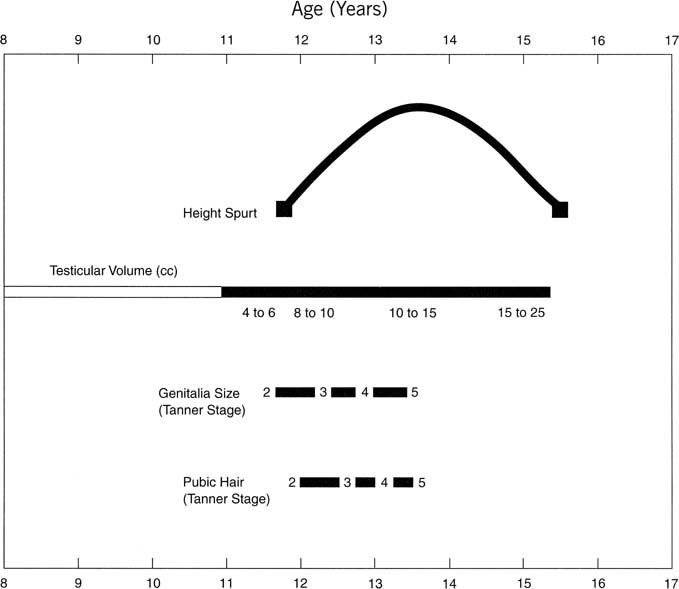
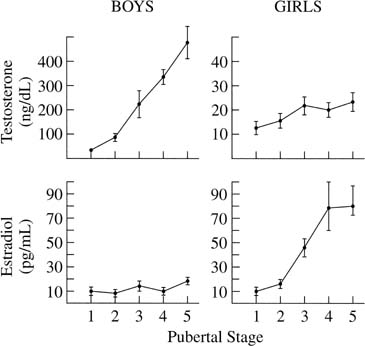









 ), and
), and