Ovarian folliculogenesis is a dynamic process marked by exponential expansion and differentiation of the granulosa cells, maturation of the oocyte, and neovascularization. Although the central roles of gonadotropins and of gonadal steroids in this explosive agenda are well accepted, the variable fate of follicles within the same ovary suggests the existence of additional intraovarian modulatory systems.1 Stated differently, it is presumed that gonadotropin action is “fine tuned” in situ, thereby accounting for observed differences in the rate and extent of development of ovarian follicles. Alterations in gonadotropin secretion cannot adequately explain the initiation and arrest of meiosis within the oocyte, the acquisition of follicular dominance, or the failure of follicular development, which leads to atresia. It is likely that the earlier stages of follicular growth, generally considered to be gonadotropin independent, may be controlled by intraovarian signaling.
The concept of local gonadal regulators originated when the embryology of the ovary was the subject of intense scrutiny. Gonadal differentiation was proposed by Witschi to result from the interaction of two morphogenic substances called cortexone and medullarine, the first of which was thought of as the stimulator of ovarian development and the latter as the promoter of testicular growth.2 Although multiple other contributors must undoubtedly be acknowledged, the notion of intraovarian regulators was promoted with special vigor by the late Cornelia Post Channing, whose pioneering experiments ushered in contemporary molecular endocrinology as it applies to ovarian physiology.3
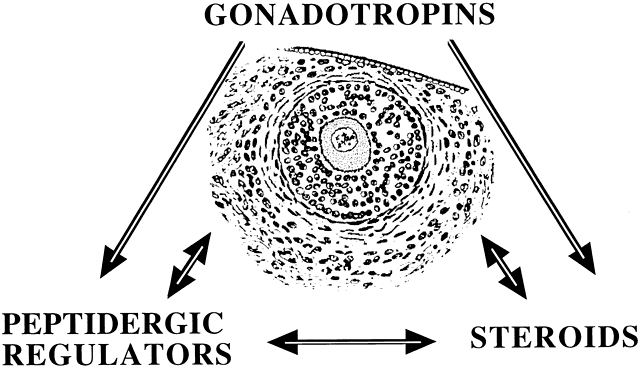
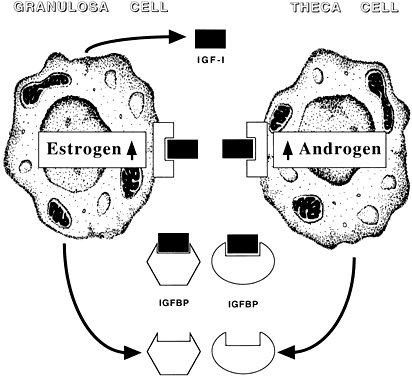
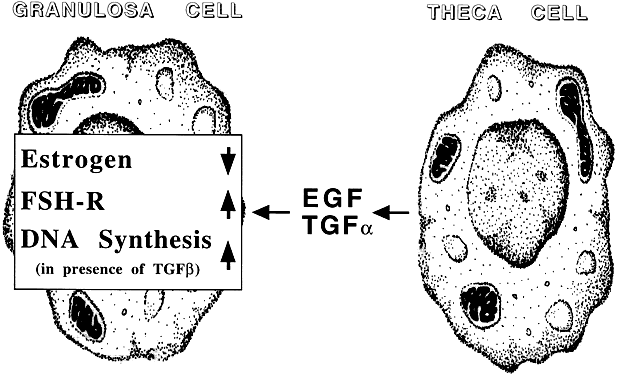
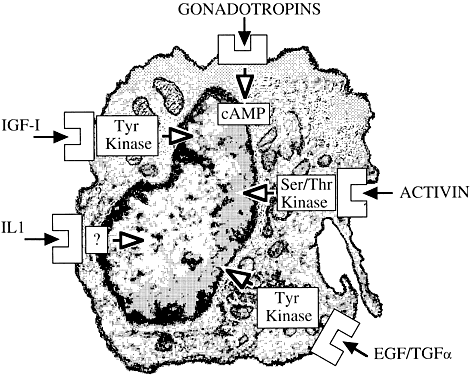
Among potential novel intraovarian regulators, growth factors, cytokines, and neuropeptides have been the subject of increasingly intense investigation. Most of these agents are not expected to act in the traditional endocrine fashion because of their local intraovarian generation (as opposed to circulatory-derived influences emanating from distant endocrine glands). Speculation favors the notion that a host of putative intraovarian regulators may engage in subtle in situ modulation and coordination of growth and function of the varied follicular cell types: oocytes, granulosa, theca, and vascular epithelium. In this capacity, a given putative intraovarian regulator may modulate the replication or cytodifferentiation of a developing ovarian cell, acting in its own right or as an amplifier-attenuator of gonadotropin action. Such putative intraovarian regulators may also be concerned with intercompartmental communication, allowing tighter linking of different cellular populations. For example, a growing body of evidence suggests that granulosa cell-derived modulators may regulate the adjacent theca-interstitial cell compartment in the interest of coordinated follicular development. In doing so, the granulosa cell may exert some control over its own destiny, in that it may regulate the very inflow of androgenic substrate from the neighboring theca. Together, gonadotropins, steroids, and locally derived peptidergic principles form a triad, which modulates the growth and differentiation of ovarian follicles (Fig. 1). According to contemporary views, potential intraovarian communication is mostly paracrine or autocrine in nature. Paracrine communication involves local diffusion of regulators from producer cells to distinct target cells within the same organ. This is a heteroregulatory phenomenon that could allow for intercompartmental communication, providing a tighter linkage of different cellular populations. In the ovary, the ability of increasing numbers of granulosa cells to produce estrogen depends on the concomitant ability of the thecal layer to provide the proper amounts of androgenic substrate. The granulosa cell, in the interest of efficient coupling, may elaborate substances (e.g. insulin-like growth factor-I [IGF-I] inhibin, activin) that could alter the function of the neighboring theca.
|
The other type of cellular communication, autocrine regulation, involves the action of a regulator on surface receptors at its cell of origin. This is a self-regulatory phenomenon wherein a single cell type modulates its own activity. In the ovary, granulosa cells elaborate substances such as IGF-I and activin that can alter granulosa cell function. Whereas steroids may be exerting intracrine (i.e. regulation within the cell of origin) effects, there is no evidence for juxtacrine (i.e. contact-dependent regulation between immediately adjacent cells) effects in the ovary.
To qualify as a bona fide intraovarian regulator, the putative agent needs to meet the minimal criteria of local production, local reception, and local action. Some evidence of indispensability to in vivo ovarian function needs to be provided. For the most part, few of the putative intraovarian regulators under study (Table 1) have satisfactorily met all of the previously described criteria (i.e. IGF-I, activin). Accordingly, the information provided later can be viewed as a prelude to what the future holds. Undoubtedly, additional information will become available with respect to the putative intraovarian regulators under consideration. It is equally certain that novel candidates will be added to this preliminary list, requiring modification of current views.
TABLE 1. Established and Putative Intraovarian Regulators
Insulin-Like Growth Factor System
IGF-I
IGF-II
IGF binding proteins
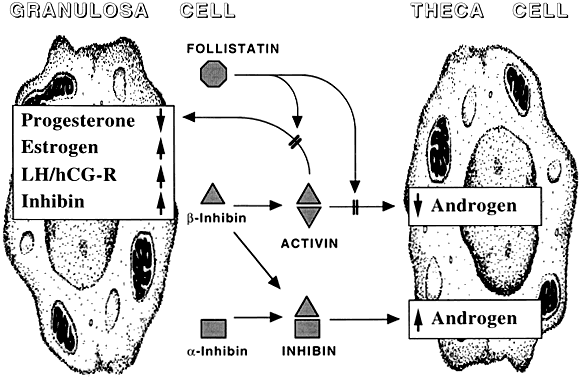
Inhibin/Activin Systems
Inhibin
Activin
Follistatin
Interleukin-1 System
Interleukin-1
Interleukin-1 receptor antagonist
IL-1 binding protein (IL-1 receptor type II)
Other Growth Factors
EGF/TGFα
TGFβ1, TGFβ2
NGF
aFGF, bFGF
VEGF
TNFα
Other Peptidergic Factors
Ovarian renin angiotensin system
VIP
Oxytocin
Endothelin
The following sections describe a select group of putative intraovarian regulators reflecting different modes of action. The principle action of each regulator is briefly listed in Table 2.
TABLE 2. Principal Actions of Intraovarian Regulators
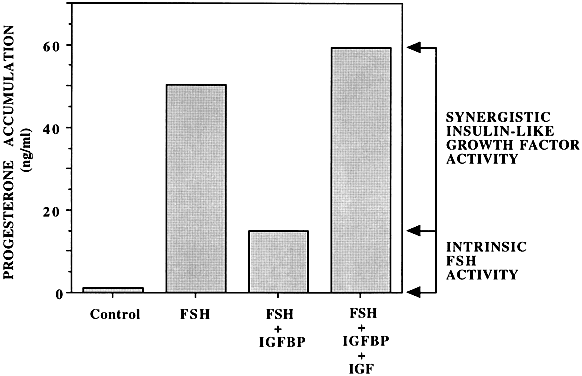
Insulin-like growth factor-I
Follicle-stimulating hormone (FSH) amplification
Follicular growth
Follicular selection
Transforming growth factor-α
Follicular maturation
Oocyte maturation
Cellular differentiation
Potentiation of gonadotropin action
Regulation of apoptosis
Transforming growth factor-β1
Follicular rupture inhibition
Follicular differentiation
Basic fibroblast growth factor
Apoptosis inhibition
Regulation of folliculogenesis
Activin
Oocyte maturation
Follicular differentiation
Early embryogenesis
Regulation of steroidogenesis
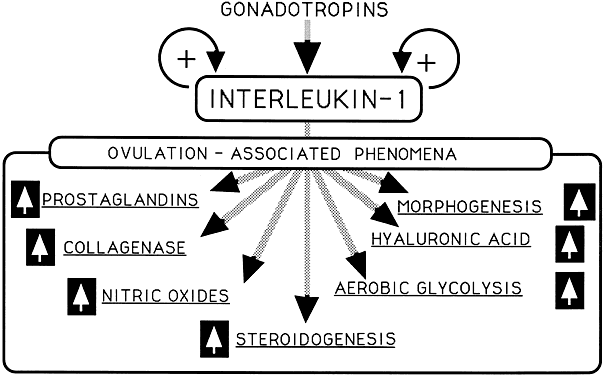
Interleukin-1 (see alsoFig. 6)
Ovulation induction
Glycolysis
Glucose transport
Tumor necrosis factor-α
Inhibits steroidogenesis
FSH antagonist
Induces apoptosis/luteolysis
Ovulation inhibition
|