Histology and Ultrastructure of the Early Corpus Luteum
During the first several days after ovulation, there are important changes
in histoanatomy, histology, and ultrastructure that describe the transformation
of the mature follicle into the corpus luteum.4,13,14,15
At first, there is little to distinguish the histology of the corpus luteum
from that of the late preovulatory follicle.4,14
The granulosa cells are not yet luteinized, or lipid-laden, and this layer
remains avascular. Theca cells remain large and lipid-laden, and vessels
of the theca are engorged. There may be a small amount of bleeding into
the central cavity and, in early specimens, this feature may be the chief
clue that ovulation has occurred.
Mitoses in the granulosa layer are seen in this early phase, and luteinization
of granulosa cells (enlargement and accumulation of intracellular lipid)
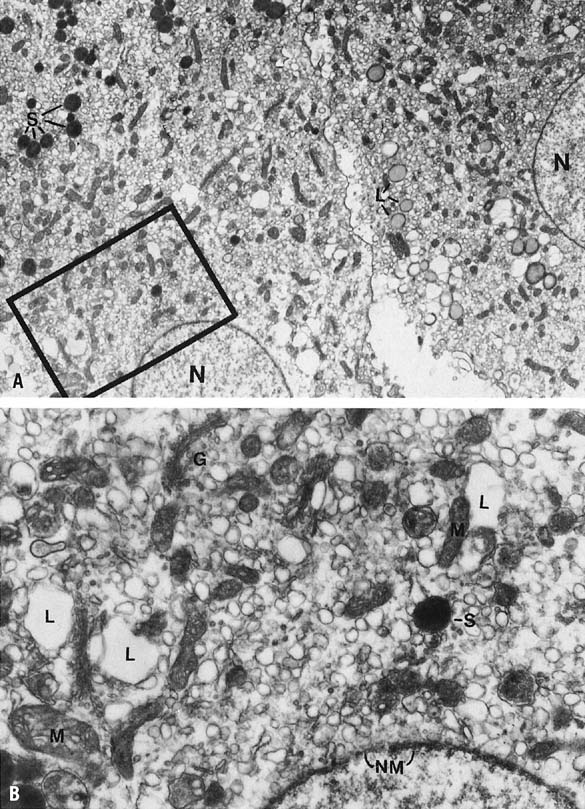
begins in earnest. Ultrastructurally, this change in granulosa lutein
cells is accompanied by an accumulation of cytoplasmic lipid droplets,
the appearance of a well-developed granular endoplasmic reticulum, and
tubular cristae in the mitochondria—all changes typical of actively
steroidogenic cells. There is organization of the cell into a peripheral
zone of tubular endoplasmic reticulum, with few lipid droplets and mitochondria,
and a central zone of mitochondria and well-dispersed Golgi, often with
intervening parallel arrays of rough endoplasmic reticulum.13,15,16,17
This geographic arrangement may allow for accession and presentation of
cholesterol from the lateral cell border to the mitochondria and for steroid
to be passed to the Golgi.16,17
The transformation of the follicular granulosa cells to the granulosa-lutein
cell mass of the corpus luteum is accompanied by the formation of new
vessels that develop rapidly over the next few days. The formation of
new vessels supports luteal function and export of luteal products and
is mediated by vascular endothelial growth factor (VEGF), acting in a
paracrine fashion.18,19,20
Locally elaborated VEGF drives endothelial cell proliferation, and endothelial
cells comprise more than 50% of the cell population of the corpus
luteum.21,22 Cell proliferation
in the corpus luteum is limited almost exclusively to vascular endothelial
cells (85% of mitotically active cells are endothelial cells) and
is greatest in the young corpus luteum (Fig.
2).21,23,24
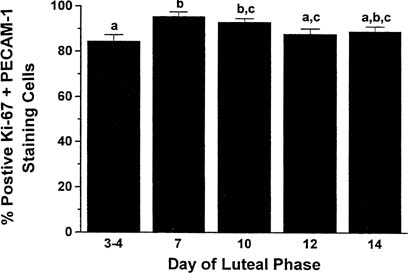 Fig. 2. Percentage of luteal cells staining positive for cell proliferation (K-67 antigen) and
the marker for endothelial cell type (PECAM-1) throughout
the luteal phase in the nonpregnant rhesus monkey. Throughout the
life span of the corpus luteum, proliferative activity resides primarily
in the vascular cells.(Christensen LK, Stouffer RL: Proliferation of microvascular endothelial
cells in the primate corpus luteum during the menstrual cycle and simulated
early pregnancy. Endocrinology 137:367, 1996.) Fig. 2. Percentage of luteal cells staining positive for cell proliferation (K-67 antigen) and
the marker for endothelial cell type (PECAM-1) throughout
the luteal phase in the nonpregnant rhesus monkey. Throughout the
life span of the corpus luteum, proliferative activity resides primarily
in the vascular cells.(Christensen LK, Stouffer RL: Proliferation of microvascular endothelial
cells in the primate corpus luteum during the menstrual cycle and simulated
early pregnancy. Endocrinology 137:367, 1996.)
|
Steroid Secretion by the Early Corpus Luteum
Development of the extraordinary steroidogenic productivity of the corpus
luteum is realized within a few days of ovulation. Acquisition of substrate
is accomplished by low-density lipoprotein (LDL) receptor–mediated
binding and internalization of very-low-density lipoprotein–associated
cholesterol.25,26,27,28
Cholesterol molecules thus acquired next must reach the inner mitochondrial
membrane, a step mediated by the sterol transfer protein StAR (steroidogenic
acute regulatory protein).29,30,31
Finally, at the inner mitochondrial membrane, cytochrome P-450 side-chain
cleavage (P-450scc) enzyme effects the synthesis of pregnenolone from
cholesterol. LH acts via the cAMP second messenger system, in synergy
with insulin, to enable each of these three key steps: increased expression
of LDL receptors, expression of StAR protein, and of P-450scc enzyme.32,33
Expression of StAR is mediated by the transcription factors steroidogenic
factor 1, CCAAT/enhancer binding protein beta, and other as yet uncharacterized
transcription factors and coregulators.31
StAR mRNA expression is greatest in the newly formed corpus luteum (Fig.
3).34 Pregnenolone, the immediate product
of side-chain cleavage of cholesterol, is transformed into progesterone
by the enzyme 3β-hydroxysteroid dehydrogenase, Δ 4-5-isomerase
located in smooth endoplasmic reticulum. The transport of newly formed
progesterone from the cell is thought to be a process of passive diffusion35;
however, an active secretory mechanism for steroidogenic cells has been
proposed.36,37
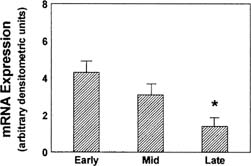 Fig. 3. mRNA expression from Northern blot determinations of the 1.6-kb StAR mRNA
in human corpora lutea of different ages. Arbitrary values determined
by normalization to 28s rRNA.(Devoto L, Kohen P, Gonzalez RR, et al: Expression of steroidogenic acute
regulatory protein in the human corpus luteum throughout the luteal
phase. J Clin Endocrinol Metab 86:5633, 2001.) Fig. 3. mRNA expression from Northern blot determinations of the 1.6-kb StAR mRNA
in human corpora lutea of different ages. Arbitrary values determined
by normalization to 28s rRNA.(Devoto L, Kohen P, Gonzalez RR, et al: Expression of steroidogenic acute
regulatory protein in the human corpus luteum throughout the luteal
phase. J Clin Endocrinol Metab 86:5633, 2001.)
|
Progesterone concentrations are 10-fold higher in the peritoneal fluid
than in peripheral blood in the days immediately after ovulation, probably
as a result of impeded vascular export from the corpus luteum.38 The rate of secretion through the peritoneal compartment may be substantial
because there is ample vascularized surface area for absorption, but
progesterone entering this compartment is largely metabolized before
reaching the peripheral circulation because of the hepatic first-pass
effect of the splanchnic circulation.39 Peritoneal fluid levels of progesterone decrease as the luteal phase advances, presumably
as more steroid finds its way into the circulation
directly through newly forming vascular channels in the corpus luteum. This
interpretation is supported by the finding that the maximum tissue
levels of progesterone are found in the early corpus luteum and decline
thereafter.40
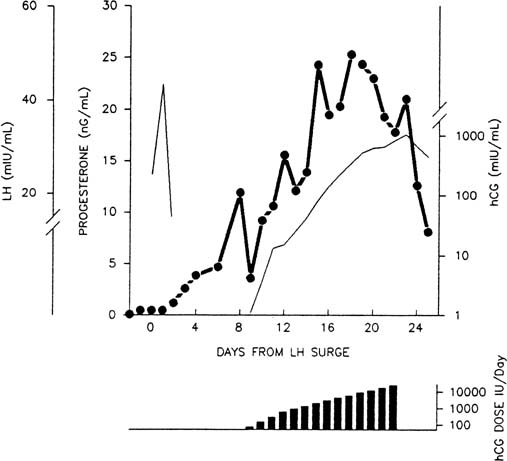
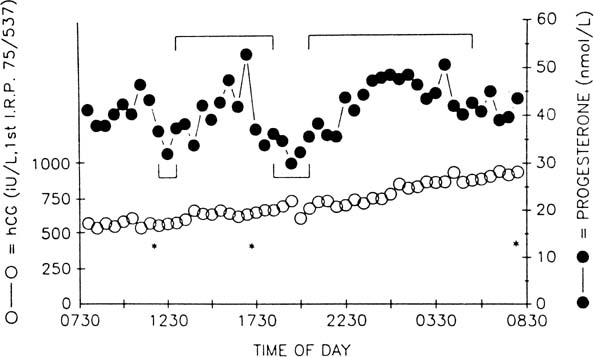
The pattern of progesterone secretion into the bloodstream by the early
corpus luteum is dominated by a basal or tonic secretion, with little
in the way of a pulsatile response to the episodic secretion of LH by
the pituitary (Fig.
4).41 Progesterone secretion of the
early corpus luteum is augmented little by hCG administration.42,43,44
This picture correlates with the functional predominance of the larger
of the two functional and morphologic cell types identified in vitro
in preparations of dispersed luteal cells.
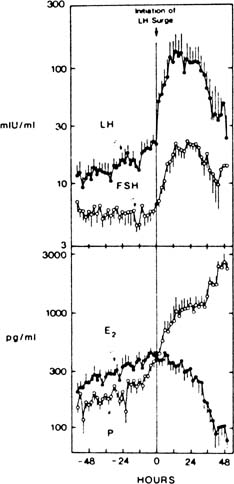
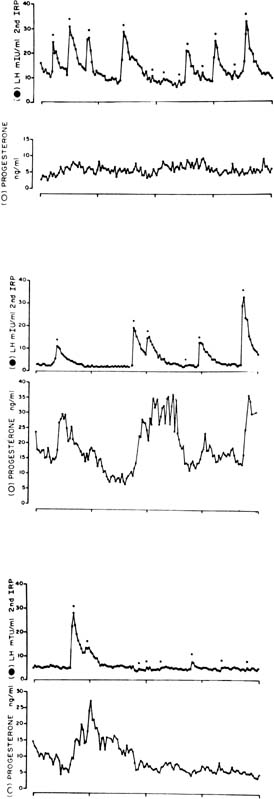 Fig. 4. Plasma concentrations of luteinizing hormone (LH) and progesterone during 24 hours
of blood sampling in women studied 2 (upper panel), 8 (middle panel), and 10 (bottom panel) days after the LH surge. Asterisks indicate significant LH pulsations. Progesterone
levels were correlated with LH levels on days 8 and 10 after
the surge but not on day 2 after the surge. The LH-evoked increments
in progesterone levels increase relative to interpulse progesterone
levels as the luteal phase progresses.(Filicori M, Butler JP, Crowley WF: Neuroendocrine regulation of the corpus
luteum in the human. J Clin Invest 73:1638, 1984. Reproduced with
permission of the American Society of Clinical Investigation.) Fig. 4. Plasma concentrations of luteinizing hormone (LH) and progesterone during 24 hours
of blood sampling in women studied 2 (upper panel), 8 (middle panel), and 10 (bottom panel) days after the LH surge. Asterisks indicate significant LH pulsations. Progesterone
levels were correlated with LH levels on days 8 and 10 after
the surge but not on day 2 after the surge. The LH-evoked increments
in progesterone levels increase relative to interpulse progesterone
levels as the luteal phase progresses.(Filicori M, Butler JP, Crowley WF: Neuroendocrine regulation of the corpus
luteum in the human. J Clin Invest 73:1638, 1984. Reproduced with
permission of the American Society of Clinical Investigation.)
|
Isolated luteal cells studied in vitro can be grouped into two
populations, determined by size, that exhibit different secretory physiology.44,45,46,47
The large cells possess a higher basal rate of progesterone secretion
than the small cells, but they are indifferent to hCG stimulation, are
devoid of LHRs, and show enhanced estrogen biosynthesis when stimulated
by FSH. By contrast, hCG markedly increases progesterone synthesis by
small luteal cells, which do have LHRs, but in which estrogen secretion
is not enhanced by FSH. These two cell types exhibit functional parallels
with the theca and granulosa cells of the preovulatory follicle and may
correspond to the granulosa-derived and theca-derived components of the
corpus luteum.16,44,45
In vivo, the overall function of the corpus luteum is LH dependent,
as shown by prompt luteolysis after administration of antisera to LH47
or gonadotropin-releasing hormone (GnRH) antagonists or agonists48,49,50,51
or after withdrawal of pulsatile GnRH in GnRH-dependent individuals.52,53
Clinically, when ovulation is induced with pulsatile GnRH for hypothalamic
amenorrhea, maintenance of luteal function requires continued, pulsed
GnRH administration or administration of hCG.54,55
Peptide Secretion by the Early Corpus Luteum
Inhibin has been identified as a secretory product of luteal cells in
vitro.56,57 Levels
of inhibin A begin to rise in the late follicular phase, peak in the mid
luteal phase, and decline with luteal demise. Inhibin B exhibits high
levels in the circulation in the late follicular phase and early luteal
phase, and these decrease thereafter. Circulating levels of inhibin during
the luteal phase are accounted for by the corpus luteum58
and roughly parallel progesterone levels.59
Granulosa luteal cells express mRNA for the inhibin alpha subunit to a
greater extent than the mRNAs for beta A and beta B subunits, which is
consistent with luteal production of inhibin A, inhibin B, and free inhibin
alpha subunit.60,61 The LH-hCG
dependence of luteal inhibin secretion has been confirmed in vitro56
and in vivo.49 The luteal phase depression
of FSH levels may reflect in part the FSH-lowering effect of inhibin.62
This function would serve to avert the chaotic consequences of the superimposition
of active folliculogenesis on luteal function.63
In addition to modulation of gonadotropin secretion, inhibin may exert
paracrine influences on luteal function and development.
Abnormalities of the Early Corpus Luteum
The postovulatory rise in progesterone levels is delayed in some women,
constituting a specific defect in the onset of luteinization. This special
form of luteal dysfunction has been associated with a lag in the progression
of secretory change in the endometrium and infertility.64,65,66
Hemorrhage into the central cavity of the corpus luteum is seen normally, but
occasionally it can be excessive and warrant surgical attention
because of pain or hemoperitoneum. Such clinical events can occur at
any time during the life span of the corpus luteum of the cycle and during
early pregnancy. Anticoagulant therapy is the sole known antecedent
for these rare, otherwise sporadic events.67 |