Studies of the gonadotropin deficiency in anorexia nervosa reveal a number of qualitative and quantitative abnormalities suggestive of a hypothalamic dysfunction. The response of luteinizing hormone-releasing hormone (LHRH) is reduced by a factor that is directly correlated with weight loss. In addition, the pattern of response in these underweight patients is more akin to the pattern seen in prepubertal children; that is, the FSH response is greater than the LH response. The opposite is usually found in the postpubertal state. The return of LH responsiveness is correlated with weight gain68,69 or can be induced by repeated injections of LHRH,70 suggesting that the pituitary gonadotropes have become sluggish owing to the lack of endogenous stimulation with LHRH. Moreover, patients who are partially recovered from anorexia nervosa tend to have exaggerated responses to LHRH.67,69 These changes have been seen in children in early puberty, suggesting that in anorexia nervosa the hypothalamic signals of the central nervous system revert to a prepubertal or a pubertal state.
Attempts to explain the neurotransmitter defect that most probably underlies the gonadotropin-releasing hormone (GnRH) abnormalities have been confusing. Naloxone, which inhibits endogenous opiates, causes increases in gonadotropins in some71 but not all72,73 patients with anorexia nervosa and in one study caused further decrease in gonadotropin levels.74 Dopaminergic mechanisms have not been specifically examined in anorexia nervosa, although in other types of hypothalamic amenorrhea an increase in LH pulse frequency has been noted with metoclopramide, a dopamine receptor blocker.75 Stress and activation of the hypothalamic-pituitary-adrenal axis has also been suggested as a possible mechanism. Cortisol is elevated, and responses to corticotropin-releasing hormone (CRH) are abnormal.76,77,78 Increased CRH is also found in cerebrospinal fluid of patients with anorexia nervosa. CRH is known to inhibit GnRH secretion. CRH may also augment dopaminergic and opiodergic inhibition of GnRH.79,80,81 The defect therefore is still uncertain, but all of these pathways are undoubtedly linked to cause dysfunction of the GnRH pulse generator.
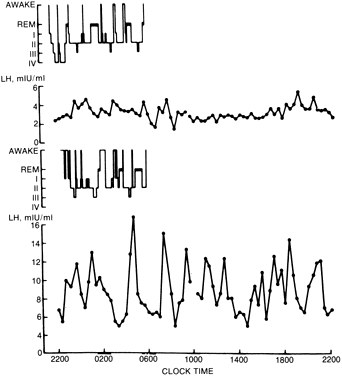
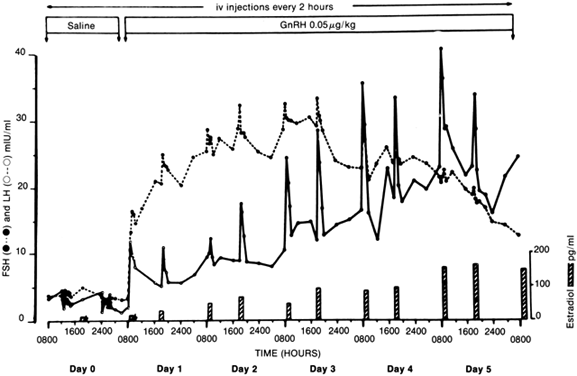
Patients with anorexia nervosa have persistent low levels of LH and FSH throughout the day and night, similar to prepubertal children. With recovery (weight gain), sleep-associated episodic secretion of LH appears, similar to that of the early pubertal child. With full recovery, the 24-hour pattern resembles that of a normal adult, with fluctuating levels without sleep-associated spikes82,83 (Fig. 1). It appears that the central nervous system mechanisms responsible for these patterns regress to a prepubertal state during the illness and reverse with weight gain. Artificially, the pattern of gonadotropin secretion can be made to revert to a normal adult-like pattern by the administration of pulsatile GnRH. If the drug is given intravenously or subcutaneously every 2 hours, a normal adult-like pattern of gonadotropin secretion results and menstrual bleeding and ovulation can be induced84 (Fig. 2). In one study, baseline plasma gonadotropin levels were lower both in patients with anorexia nervosa and in normal-weight, refed anorexia nervosa patients, compared with controls. Percentages of total gonadotropin not bound to concanavalin A (complex carbohydrate chains) were higher in anorexia nervosa patients than in controls, and in refed patients the percentage of unbound FSH was higher than in controls. On administration of GnRH, these respective percentages decreased. Although administration increased LH and FSH levels for anorexic and recovering patients, these increases were lower than they were for controls. The administration of GnRH appears to induce changes in circulating gonadotropin isoforms in subjects with and without anorexia nervosa. Furthermore, the LH response in recovering subjects and in controls is associated with the same glycosylation pattern. Therefore, the biologic activity of gonadotropin may be affected by changes produced by the administration of GnRH.84A Research indicates that neurons containing GnRH are situated in the arcuate nucleus of the hypothalamus and that axons descend into the median eminence, where they terminate. From this vascular area, a message is sent from the nerve terminals by way of the hypophyseal portal vein to pituitary gonadotropes situated in the anterior pituitary.85 These findings suggest that the amenorrhea seen in anorexia nervosa is probably caused by faulty signals reaching the medial central hypothalamus from the arcuate nucleus, the center most likely to be responsible for the important episodic stimulation of GnRH.
|
Despite the return of normal gonadotropin secretory patterns, amenorrhea persists in almost 30% of patients with anorexia nervosa.15,86 Sonographic visualization of the ovaries suggests cystic ovaries similar to those seen at adolescence.87,88 A 10-year follow-up study suggested that amenorrhea persists in 49% of patients overall, but in only 10% of those who have a normalization of weight.89
Therefore, other mechanisms, yet to be elucidated, are at fault. A likely mechanism is leptin, a hormone produced by the obese gene in mice and secreted by the adipocyte that has received considerable attention since its discovery. One reason leptin is so fascinating is that it seems to be a critical link between metabolic and reproductive pathways. Numerous studies in rodents have demonstrated that ob/ob rodents born without an active form of leptin tend to be overweight and, intriguingly, are also amenorrheic and infertile. In humans, leptin appears to modulate food intake by affecting appetite, energy requirements, and eating behavior.89A In addition, low leptin levels have been reported in patients with hypothalamic amenorrhea.89B There appear to be critical leptin levels needed to maintain menstruation.89C Furthermore, low leptin levels have been linked with low bone mass.89L
Hypoleptinemia has consistently been observed in patients with anorexia nervosa, but leptin levels increase significantly with partial weight recovery.89D,89E,89F,89G Although leptin levels correlate linearly with body mass index in recovering and normal subjects, levels uncouple in a nonlinear fashion in untreated anorexics, suggesting a threshold effect at lowest body weights.89D By comparing serum leptin levels among patients with various eating disorders, researchers have found that leptin levels are not correlated to a specific pathology but rather to individual body mass index. Therefore, leptin levels cannot be used to diagnose or develop a prognosis for anorexia.89H Although there is a high correlation between absolute body fat mass and leptin concentration, fasting behavior, regardless of body fat level, may also be correlated with low leptin.89I Several studies have shown a rapid, disproportional lowering of leptin concentration with starvation.89J,89K Anorexic patients have two factors working to lower their leptin concentration: low body fat and starvation itself. Leptin levels have been shown to normalize and perhaps peak at higher than normal values with incomplete weight gain.89E,89F This underlies the importance of leptin in the pathology of anorexia: because leptin acts to reduce food intake, the restoration of leptin concentration before full weight recovery can be extremely detrimental to the patient with anorexia nervosa. When coupled with a threshold effect at lowest weights, this characteristic response to weight gain in anorexic patients serves to perpetuate the disorder.
Of interest also are the low estradiol levels; they are partially a result of lack of ovarian stimulation, but estrogen metabolism appears to be altered. With weight loss, the metabolism of estradiol, which normally proceeds with 16α-hydroxylation, is decreased in favor of 2-hydroxylation and the formation of catechol estrogen (2-hydroxyesterone).90 The latter compound has features of an antiestrogen in that it has no intrinsic biologic activity.91 Therefore, the extraordinarily low estrogen levels seen in this syndrome are compounded by the presence of an endogenously produced antiestrogen, and, in addition, the lack of fat tissue may deny the patient extraovarian sources of estrogen (i.e. a source of estrone conversion from androstenedione).
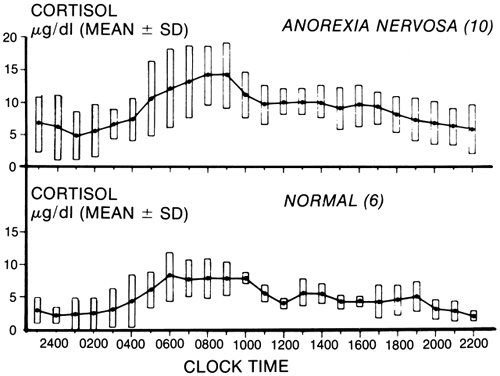
Major alterations have also been reported. Mean levels of cortisol are elevated, and random sampling may suggest an absence or even a reversal of the normal circadian rhythm. Twenty-four-hour studies have shown that both the episodic and the circadian rhythm are normal, but the cortisol levels are considerably higher than normal ( Fig. 3).92 This change, which has also been seen in malnutrition, results from a prolonged half-life of the cortisol caused by reduced metabolic clearance. As would be expected, the levels of the urinary steroids, including the 17-hydroxysteroids and the 17-ketosteroids, are usually low. Other studies have suggested that the production rates may be elevated,93 and the cortisol concentrations may exceed the binding capacity of cortisol-binding globulin (CBG). There appears also to be a decreased affinity of CBG for cortisol. Therefore, unbound cortisol may be significantly increased and available to the tissues in patients with anorexia nervosa. One would expect the higher levels of cortisol measured in these patients to suppress adrenocorticotropic hormone (ACTH). The observation that a new circadian rhythm at the higher cortisol level is established suggests that a new set point is determined by the hypothalamic-pituitary-adrenal axis. ACTH responses to CRH show blunting, and increased CRH in cerebrospinal fluid suggests activation of this axis.79,80
Despite their marked cachexia, patients with anorexia nervosa have clinical and metabolic signs suggestive of hypothyroidism. These include constipation, cold intolerance, bradycardia, hypotension, dry skin, prolonged ankle reflex, low basal metabolic rate, and hypercarotenemia.15,94 Some of these changes do suggest a compensatory hypometabolism, and studies on the circulatory system show that during maximal exercise the attainable oxygen uptake and heart rate are low in children with anorexia nervosa. The maximum oxygen consumption (V®O2max) appears to be decreased out of proportion to the circulatory and body dimensions, indicating an adaptation to the low caloric intake.95
Serum levels of thyroxine (T4), triiodothyronine (T3), and thyroid-stimulating hormone (TSH) in patients with anorexia nervosa are significantly lower than in normal persons, particularly the level of T3. Abnormal thyroid tests include low basal T3, T4, and TSH. T3 appears to increase with weight gain, but T4 and TSH remain low, and all three hormones are lower than in controls.96 The low T3 levels may be explained by an alteration in T4 conversion. In anorexia nervosa, as in starvation, the peripheral deiodination conversion of T4 is diverted from formation of the active T3 to production of reverse T3, an inactive metabolite.97 Evidence also indicates that fasting decreases hepatic uptake of T4, with a proportionate decrease in T3 production.98 The low T4 value is somewhat more difficult to explain. Low T4 euthyroidism has been seen in seriously ill patients,99,100 in whom studies indicate there is a unique dysfunctional state of deficient T4 binding with a normal free T4 availability to peripheral tissue sites. Presumably, a similar mechanism may be operative in anorexia nervosa. Secretion of TSH appears to be normal, but the peak TSH response to thyroid-releasing factor (TRF) stimulation is delayed, from 30 or 60 to 120 minutes.101 This may also reflect an altered set point for endogenous TRF regulation. Another study showed that the T4 and T3 response to thyroid-releasing hormone (TRH) is subnormal, although the TSH response is normal, suggesting chronic understimulation of the thyroid, as occurs in hypothalamic hypothyroidism.96
Other parameters of pituitary-hypothalamic function have been studied. Growth hormone (GH) levels are known to be increased in starvation or in any situation with restriction of food beyond the normal 12- to 15-hour “overnight” fast. In general, basal GH levels are significantly higher than normal in anorexia nervosa but respond normally to one or more provocative stimuli. The enhanced GH secretion in anorexia nervosa may be caused by a heightened frequency of secretory pulses superimposed on augmented tonic GH secretion. When anorexics are compared with subjects of normal weight and with obese subjects, the enhancement of GH secretion appears to reflect a complex hypothalamic dysregulation of GH release, as opposed to simply a malnutrition-induced impairment of the production of insulin-like growth factor I.96A Occasionally, low levels are seen with blunted responses to insulin hypoglycemia.15 The GH levels associated with delta-wave sleep are normal.102 The basal prolactin level is indistinguishable from normal. TRF-stimulated prolactin levels are also normal, although the time of peak prolactin is delayed.102
In addition to the endocrine abnormalities described, a number of other physiologic changes strongly suggest a hypothalamic dysfunction. One of the most impressive is the disorder of thermoregulation reported in this condition.103 When exposed to acute hypothermia or hyperthermia under controlled conditions, persons with anorexia nervosa do not show the normal adjustments. For instance, in the cold, no shivering or vasoconstriction occurs, and core temperature continues to fall. In the heat, no vasodilation occurs, and eventually the core temperature rises with no stabilization. The rate of change of core temperature per hour also correlates with the percent below ideal body weight. In addition, the absence of vasoconstriction and vasodilation prevents the “paradoxic” initial decrease in temperature seen in the heat and the rise seen in the cold. These responses, including shivering, vasoconstriction, and vasodilation, indicating loss of central temperature, are dysfunctional in the anorexic. The thermosensitive neurons in the anterior hypothalamus (which mediate vasomotor responses) and its interconnectives with a motor center for shivering in the posterior hypothalamus may be affected. Studies of thermoregulatory responses to a given metabolic heat load resulting from exercise in anorexia nervosa indicate that the altered response may involve other mechanisms. In particular, the low sweating capacity seen in this syndrome and the loss of body fat, which reduces thermal insulation, alter the ambient temperatures over which the body temperature can be maintained during exercise.104
Patients with anorexia nervosa also appear to have abnormalities of water conservation that are diagnostic of partial diabetes insipidus. This was judged by the ability to concentrate the urine maximally after 15 hours of dehydration and a further response to exogenous vasopressin. A further increase in urine osmolarity after administration of exogenous antidiuretic hormone was seen in the majority of patients with anorexia nervosa and was diagnostic of partial diabetes insipidus.103
The altered behavior patterns in this syndrome are also distinctive and include preoccupation with food and hyperactivity. Sometimes periods of gorging (bulimia) alternate with starvation and food avoidance. Altered food intake, combined with activity changes, has been seen in hypothalamic tumors and has been documented in rats with lesions of the ventromedial nucleus of the hypothalamus. These lesions have led investigators to conclude that the ventromedial nucleus can inhibit food intake and promote activity, as seen in anorexia nervosa.
Other behavioral changes include a perceptual distortion in which patients with anorexia nervosa perceive themselves to be too fat. This body image misperception can be quantitated by the use of a special apparatus consisting of two movable lights equidistant from a central point. The subject can move the lights until they appear to approximate what she perceives to be specific body dimensions or even a piece of wood. Experiments of this nature have shown that there is a direct association between the degree of the perceptual disorder and that of malnutrition and that patients with anorexia nervosa consistently overestimate body and other dimensions.105 These perceptual abnormalities may be aggravated by a high carbohydrate meal and tend to disappear with weight gain. This suggests that when the patient is severely underweight, the illness tends to be self-perpetuating, and that weight gain in itself is an important goal to break this vicious cycle. Other investigators have found, however, that these abnormalities are complexly determined and can be demonstrated in nonanorectic persons in their teens and in obese subjects. Generally, the prognosis is thought to be better in those patients whose self-reported estimates are more or less accurate after restoration of weight and are no longer influenced by an ordinary carbohydrate meal. Gross overestimation of body widths is associated with premorbid obesity, bulimia and vomiting.106
Studies have also been performed to evaluate the behavior disorder seen in anorexia as well as the perceptual abnormality. Anorexic behavior scales have been devised to aid in differentiating persons with anorexia nervosa from those who have secondary amenorrhea due to other causes. The scale may consist of a psychologic profile that is self-administered or administered by a doctor, nurse, or clinical psychologist. One study indicated that the anorectic behavior scale was very useful and accurate in distinguishing healthy female subjects from those with anorectic behavior. These scales may therefore be useful in the early diagnosis of anorexia nervosa, particularly in patients presenting with only secondary amenorrhea and little or no weight loss. In contrast to the perceptual disorder, the degree of anorectic behavior does not always correlate with the malnutrition, suggesting that the anorectic behavior is more pronounced in the better-nourished patients.107
Along with the behavioral disorder, objective changes in the brain have been demonstrated by computed tomography. Enlargement of the cortical sulci and subarachnoid spaces, as well as cerebral atrophy, has been demonstrated in a small number of cases, with reversal of the atrophy with weight gain in one.108,109 A number of recent studies suggest that changes in the brain resulting from anorexia nervosa may be irreversible. Gray matter volume deficits, unilateral temporal lobe hypoperfusion, and various localized brain dysfunctions appear to persist despite weight recovery.109A,109B,109C
Anorexia nervosa continues to be a perplexing syndrome, most likely psychogenic in nature. The large preponderance of girls from upper socioeconomic backgrounds who are affected at or near puberty suggests the importance of environmental influences. The fact that the amenorrhea may precede the development of the full-blown syndrome has often been emphasized, although in most studies amenorrhea has most often coincided with the onset of food restriction. Many of the signs and symptoms (including psychologic changes) seen in anorexia nervosa have been reported in starvation, and it is difficult to tell when “anorexia” leaves off and starvation begins to predominates.
Some of the metabolic changes—including lowered metabolic rate, a decrease in attainable oxygen uptake and maximal aerobic power (V O2max), an increase in cortisol (which stimulates gluconeogenesis and decreases peripheral glucose utilization), and a decrease in gonadotropins (with a loss of fertility)—are appropriate adaptations to starvation.
On the other hand, the early development of amenorrhea and behavioral symptoms also suggests a primary hypothalamic syndrome. The abnormalities of gonadotropin release and activity are associated with the medial central hypothalamus; those of thermoregulatory control, with the anterior and posterior hypothalamus; and those of water conservation, with the supraoptic area. The involvement of all these areas suggests a lesion too diffuse to be anything but perhaps metabolic. Although anorexia nervosa may be a primary hypothalamic syndrome, it is much more likely that this dysfunction is a result of the diffuse metabolic changes associated with starvation (Table 4).
TABLE 4. Abnormalities Suggestive of Hypothalamic Dysfunction in Anorexia
Nervosa
Abnormality | Area of Dysfunction |
Amenorrhea | |
Gonadotropin release and control | Medial central |
Thermoregulatory control | Anterior |
| Posterior |
Water conservation | Supraoptic |
| Paraventricular |
Behavioral | Ventromedial |
Altered food intake | |
Activity | |
The association of psychologic and neuroendocrine changes in patients with anorexia nervosa has led investigators to speculate that abnormalities of neurotransmission may be involved in the pathogenesis of the syndrome. Excessive dopamine and norepinephrine in particular have well-documented effects on behavior and appetite.110 In view of strong evidence depicting anorexia nervosa as a hypothalamic syndrome, it is possible to hypothesize that some of the neuropeptides, such as beta-endorphin, may have roles in mediating the abnormalities seen. These peptides have been isolated from other areas than the brain, including the gut, and it is not unreasonable to consider that the messages mediated by these neuropeptides may play a role in the alterations seen in anorexia nervosa.111,112
Evidence also indicates that some estrogens are formed in the brain and that their synthesis is favored in anorexia nervosa; an example is the catechol estrogen, 2-hydroxyestrone. Catechol estrogens may have the potential for interacting with both the catecholamine and the estrogen-mediated system in the central nervous system, thereby influencing dopamine and norepinephrine synthesis.113