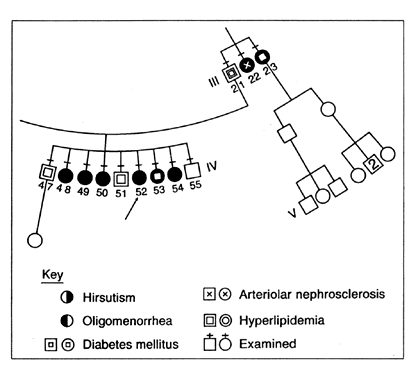
1. Moller DE, Flier JS: Insulin resistance: Mechanisms, syndromes, and implications. N Engl J Med 325: 938, 1991 2. Reaven GM: Role of insulin resistance in human disease. Diabetes 37: 1595, 1988 3. Rich-Edwards JW, Manson JE, Hennekens CH, Buring JE: The primary prevention of coronary heart disease in women. N Engl J Med 332: 1758, 1995 4. Davidson MB: Clinical implications of insulin resistance syndromes. Am J Med 99: 420, 1995 5. Archard C, Thiers J: Le virilisme pilaire et son association a l'insuffisance glycolytique (diabete
des femmes a barbe). Bull Acad Natl Med 86: 51, 1921 6. Kahn CR, Flier JS, Bar RS et al: The syndromes of insulin resistance and acanthosis nigricans: Insulin-receptor
disorders in man. N Engl J Med 294: 739, 1976 7. Flier JS, Kahn CR, Roth J, Bar RS: Antibodies that impair insulin receptor binding in an unusual diabetic
syndrome with severe insulin resistance. Science 190: 63, 1975 8. Elsas LJ, Endo F, Strumlauf E et al: Leprechaunism: An inherited defect in a high-affinity insulin receptor. Am J Hum Genet 37: 73, 1985 9. Reddy SSK, Kahn CR: Epidermal growth factor receptor defects in leprechaunism: A multiple growth
factor resistant syndrome. J Clin Invest 84: 1569, 1989 10. Rabson SM, Mendenhall EN: Familial hypertrophy of pineal body, hyperplasia of adrenal cortex and
diabetes mellitus. Am J Clin Pathol 26: 283, 1956 11. Taylor SI, Underhill LH, Hedo JA et al: Decreased insulin binding to cultured cells from a patient with the Rabson-Mendenhall
syndrome: Dichotomy between studies with cultured lymphocytes
and cultured fibroblasts. J Clin Endocrinol Metab 56: 856, 1983 12. Kobberling J, Dunnigan MG: Familial partial lipodystrophy: Two types of an X linked dominant syndrome, lethal
in the hemizygous state. J Med Genet 23: 120, 1986 13. Dunaif A, Givens JR, Haseltine F, Merriam GR (eds): Polycystic Ovary Syndrome, pp 1 - 392. Cambridge, MA, Blackwell Scientific, 1992 14. Chang R: Polycystic Ovary Syndrome. Proceedings of the Sereno Symposia
USA. New York, Springer, 1996 15. Polson DW, Wadsworth J, Adams J, Franks S: Polycystic ovaries: A common finding in normal women. Lancet 1: 870, 1988 16. Givens JR: Familial ovarian hyperthecosis: A study of two families. Am J Obstet Gynecol 11: 959, 1971 17. Wilroy RS, Givens JR, Weser WL et al: Hyperthecosis--an inheritable form of polycystic ovarian disease. Birth Defects 11 (5): 81, 1975 18. Givens JR: Familial polycystic ovarian disease. Endocrinol Metab Clin North Am 17: 1, 1988 19. Burghen GA, Givens JR, Kitabchi AE: Correlation of hyperandrogenism with hyperinsulinemia in polycystic ovarian
disease. J Clin Endocrinol Metab 50: 113, 1980 20. Barbieri RL, Ryan KJ: Hyperandrogenism, insulin resistance, acanthosis nigricans: A common endocrinopathy
with unique pathophysiologic features. Am J Obstet Gynecol 147: 90, 1983 21. Chang RJ, Nakamura RM, Judd HL, Kaplan S: Insulin resistance in non-obese patients with polycystic ovarian disease. J Clin Endocrinol Metab 50: 113, 1983 22. Dunaif A, Futterweit W, Segal KR, Dobrjansky A: Profound peripheral insulin resistance, independent of obesity, in the
polycystic ovary syndrome. Diabetes 38: 1165, 1989 23. Sorbara LR, Tang Z, Cama Z et al: Absence of insulin receptor gene mutations in three women with the polycystic
ovary syndrome. Metabolism 43: 1568, 1994 24. Ciaraldi TP, El-Roeiy A, Madar Z et al: Cellular mechanisms of insulin resistance in polycystic ovarian syndrome. J Clin Endocrinol Metab 75: 577, 1992 25. Dunaif A, Xia J, Book C-B et al: Excessive insulin receptor serine phosphorylation in cultured fibroblasts
and in skeletal muscle: A potential mechanism for insulin resistance
in the polycystic ovary syndrome. J Clin Invest 96: 801, 1995 26. Godsland IF, Walton C, Felton C et al: Insulin resistance, secretion, and metabolism in users of oral contraceptives. J Clin Endocrinol Metab 74: 64, 1992 27. Cohen JC, Hickman R: Insulin resistance and diminished glucose tolerance in powerlifters ingesting
anabolic steroids. J Clin Endocrinol Metab 64: 960, 1987 28. Polderman KH, Gooren JG, Asscherman H et al: Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab 79: 265, 1994 29. Buffington CK, Givens JR, Kitabchi AE: Opposing actions of dehydroepiandrosterone and testosterone on insulin
sensitivity. Diabetes 40: 693, 1992 30. Mortola JF, Yen SCC: The effects of oral dehydroepiandrosterone on endocrine and metabolic parameters
in postmenopausal women. J Clin Endocrinol Metab 71: 696, 1990 31. Dunaif A, Green G, Futterweit W, Dobrjansky A: Suppression of hyperandrogenism does not improve peripheral or hepatic
insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab 70: 699, 1990 32. Elkind-Hirsch KE, Valdes CT, Malinak LR: Insulin resistance improves in hyperandrogenic women treated with Lupron. Fertil Steril 60: 634, 1993 33. Moghetti P, Tosi F, Castello R et al: The insulin resistance in women with hyperandrogenism is partially reversed
by antiandrogen treatment--evidence that androgens impair insulin
action in women. J Clin Endocrinol Metab 81: 952, 1996 34. Poretsky L: On the paradox of insulin-induced hyperandrogenism in insulin-resistant
states. Endocrinol Rev 12: 3, 1991 35. Willis D, Mason H, Gilling-Smith C, Franks S: Modulation by insulin of follicle-stimulating hormone and luteinizing hormone
actions in human granulosa cells of normal and polycystic ovaries. J Clin Endocrinol Metab 81: 302, 1996 36. Andersson B, Marin P, Lissner L et al: Testosterone concentrations in women and men with NIDDM. Diabetes Care 17: 405, 1994 37. Weiss DJ, Charles MA, Dunaif A et al: Hyperinsulinemia is associated with menstrual irregularity and altered
serum androgens in Pima Indian women. Metab Clin Exp 43: 803, 1994 38. Fox JH, Licholai T, Green G, Dunaif A: Differential effects of oral glucose-mediated versus intravenous hyperinsulinemia
on circulating androgen levels in women. Fertil Steril 60: 994, 1993 39. Nestler JE, Strauss JR III: Insulin as an effector of human ovarian and adrenal steroid metabolism. Endocrinol Metab Clin North Am 20: 807, 1991 40. Plymate SR, Hoop RC, Jones RE, Matej LA: Regulation of sex hormone-binding globulin production by growth factors. Metabolism 39: 967, 1990 41. Nestler JE: Editorial: Sex hormone-binding globulin: A marker for hyperinsulinemia
and/or insulin resistance? J Clin Endocrinol Metab 76: 273, 1993 42. Legro RS, Muhleman D, Comings D et al: D3 receptor polymorphisms is associated with hyperandrogenic chronic anovulation
and clomiphene citrate failure among female Hispanics. Fertil Steril 63: 779, 1995 43. Carey AH, Waterworth D, Patel K et al: Polycystic ovaries and premature male pattern baldness are associated with
one allele of the steroid metabolism gene CYP17. Hum Mol Genet 3: 1873, 1994 44. Adams J, Franks S, Polson DW et al: Multifollicular ovaries: Clinical and endocrine features and response to
pulsatile gonadotrophin releasing hormone. Lancet 2: 1375, 1985 45. Givens JR: Polycystic ovaries: A sign, not a diagnosis. Semin Reprod Endocrinol 2: 271, 1984 46. Lee PJ, Patel A, Hindmarsh PC et al: The prevalence of polycystic ovaries in the hepatic glycogen storage diseases: Its
association with hyperinsulinemia. Clin Endocrinol 42: 601, 1995 47. Dunaif A, Graf M, Mandeli J et al: Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired
glucose tolerance and/or hyperinsulinemia. J Clin Endocrinol Metab 65: 499, 1987 48. Robinson S, Kiddy D, Gelding SV et al: The relationship of insulin insensitivity to menstrual pattern in women
with hyperandrogenism and polycystic ovaries. Clin Endocrinol 39: 351, 1993 49. Legro RS, Fox J, Dunaif A: Hyperandrogenic cycling insulin sensitive sisters: An
additional familial PCOS phenotype (abstr). Proceedings of the 10th
International Congress of Endocrinology, June 12 - 15, 1996, San
Francisco. The Endocrine Society, 1996 50. Carmina E, Koyama T, Chang L et al: Does ethnicity influence the prevalence of adrenal hyperandrogenism and
insulin resistance in polycystic ovary syndrome? Am J Obstet Gynecol 167: 1807, 1992 51. DeFronzo RA: The triumvirate: Beta-cell, muscle, liver: A collusion responsible for
NIDDM. Diabetes 37: 667, 1988 52. Lapidus L, Bengtsson, Larsson B et al: Distribution of adipose tissue and risk of cardiovascular disease and death: A 12 year
follow up of participants in the population study of women
in Gothenburg, Sweden. Br Med J 29: 1257, 1984 53. Folsom AR, Kaye SA, Sellers TA et al: Body fat distribution and 5 year risk of death in older women. JAMA 269: 483, 1993 54. Bray G: Obesity: Basic considerations and clinical approaches. Dis Mon 35: 449, 1989 55. Grasing CC, Wild RA, Parker IJ: Vulvar acanthosis nigricans: A marker for insulin resistance in hyperandrogenic
women. Fertil Steril 59: 583, 1993 56. Dunaif A, Green G, Phelps RG et al: Acanthosis nigricans, insulin action, and hyperandrogenism: Clinical, histological, and
biochemical findings. J Clin Endocrinol Metab 73: 590, 1991 57. Barbieri RL: Hyperandrogenism, insulin resistance, and acanthosis nigricans: 10 years
of progress. J Reprod Med 39: 327, 1994 58. Flack J, Sowers J: Epidemiologic and clinical aspects of insulin resistance and hyperinsulinemia. Am J Med 91: 11, 1991 59. Zimmerman S, Phillips RA, Wikenfeld C et al: Polycystic ovary syndrome: Lack of hypertension despite insulin resistance. J Clin Endocrinol Metab 75: 508, 1992 60. Wild RA, Painter PC, Coulson PB et al: Lipoprotein lipid concentrations and cardiovascular risk in women with
polycystic ovary syndrome. J Clin Endocrinol Metab 61: 945, 1985 61. Graf M, Brown V, Richards C et al: The independent effects of hyperandrogenemia, hyperinsulinemia, and obesity
in lipid and lipoprotein profiles in women. Clin Endocrinol 33: 119, 1990 62. Wild RA, Bartholomew MS: The influence of body weight on lipoprotein lipids in patients with polycystic
ovary syndrome. Am J Obstet Gynecol 159: 423, 1988 63. Wild RA, Alaupovic P, Parker IJ: Lipid and apolipoprotein abnormalities in hirsute women: I, the association
with insulin resistance. Am J Obstet Gynecol 166: 1191, 1992 64. Barth JH, Ng LL, Wojnarowska F, Dawber RP: Acanthosis nigricans, insulin resistance, and cutaneous virilism. Br J Dermatol 118: 613, 1988 65. Jackson RL, Dockerty MB: The Stein-Leventhal syndrome: Analysis of 43 cases with special reference
to association with endometrial carcinoma. Am J Obstet Gynecol 73: 161, 1950 66. Coulam CB, Annegers JF, Kranz JS: Chronic anovulation syndrome and associated neoplasia. Obstet Gynecol 61: 403, 1983 67. Dahlgren E, Friberg LG, Johansson S et al: Endometrial carcinoma: Ovarian dysfunction--a risk factor in young women. Eur J Obstet Gynecol 41: 143, 1991 68. Secreto G, Toniolo P, Berrino F et al: Increased androgenic activity and breast cancer risk in premenopausal women. Cancer Res 44: 5902, 1984 69. Secreto G, Toniolo P, Berrino F et al: Serum and urinary androgens and risk of breast cancer in postmenopausal
women. Cancer Res 51: 2572, 1991 70. Bruning PF, Bonfrer JMG, van Noord PAH et al: Insulin resistance and breast cancer risk. Int J Cancer 52: 511, 1992 71. Welborn TA, Wearne K: Coronary heart disease incidence and cardiovascular mortality in Brusselton
with reference to glucose and insulin concentrations. Diabetes Care 2: 154, 1979 72. Dahlgren E, Johansson S, Lindstedt G et al: Women with polycystic ovary syndrome wedge resected in 1956 to 1965: A
long-term follow-up focusing on natural history and circulating hormones. Fertil Steril 57: 505, 1992 73. Dahlgren E, Janson PO, Johansson S et al: Polycystic ovary syndrome and risk for myocardial infarction. Acta Obstet Gynecol Scand 71: 599, 1992 74. Talbott E, Guzick D, Clerici A et al: Coronary heart disease risk factors in women with polycystic ovary syndrome. Arterioscler Thromb Vasc Bio 15: 821, 1995 75. Harris MI, Hadden WC, Knowler WC, Bennett PH: Prevalence of diabetes and impaired glucose tolerance and plasma glucose
levels in U.S. population aged 20 – 74 yr. Diabetes 36: 523, 1987 76. Martin BC, Warram JH, Krolewski AS et al: Role of glucose and insulin resistance in development of type 2 diabetes
mellitus: Results of a 25 year follow-up study. Lancet 340: 925, 1992 77. Defronzo RA, Tobin JD, Andres R: Glucose clamp technique: A method for quantifying insulin secretion and
resistance. Am J Physiol 239: E214, 1979 78. Bergman RN, Prager R, Aage V, Olefsky JM: Equivalence of the insulin sensitivity index in man derived by the minimal
model method and the euglycemic glucose clamp. J Clin Invest 79: 790, 1987 79. Parra A, Ramirez A, Espinosa de los Monteros A: Fasting glucose/insulin ratio: An index to differentiate normal from hyperinsulinemic
women with polycystic ovary syndrome. Rev Invest Clin 46: 363, 1994 80. Laakso M: How good a marker is insulin level for insulin resistance? Am J Epidemiol 137: 959, 1993 81. Guzick DS, Wing R, Smith D et al: Endocrine consequences of weight loss in obese, hyperandrogenic, anovulatory
women. Fertil Steril 61: 598, 1994 82. Bates GW, Whitworth NS: Effect of body weight reduction of plasma androgens in obese, infertile
women. Fertil Steril 38: 406, 1982 83. Pasquali R, Antenucci D, Casimirri F et al: Clinical and hormonal characteristics of obese amenorrheic hyperandrogenic
women before and after weight loss. J Clin Endocrinol Metab 68: 173, 1989 84. Kiddy DS, Hamilton-Fairley D, Bush A et al: Improvement in endocrine and ovarian function during dietary treatment
of obese women with polycystic ovary syndrome. Clin Endocrinol 36: 105, 1992 85. Andersen P, Selifeflot, Abdelnoor M et al: Increased insulin sensitivity and fibrinolytic capacity after dietary intervention
in obese women with polycystic ovary syndrome. Metabolism 44: 611, 1995 86. Jaatinen TA, Antilla L, Erkkola R et al: Hormonal responses to physical exercise in patients with polycystic ovarian
syndrome. Fertil Steril 60: 262, 1993 87. Nestler JE, Singh R, Matt DW et al: Suppression of serum insulin level by diazoxide does not alter serum testosterone
or sex hormone-binding globulin levels in healthy, normal nonobese
women. Am J Obstet Gynecol 163: 1243, 1990 88. Prelevic GM, Wurzburger MI, Balint-Peric L, Nesic JS: Inhibitory effect of Sandostatin on secretion of luteinizing hormone and
ovarian steroids in polycystic ovary syndrome. Lancet 336: 900, 1990 89. Velasquez EM, Mendoza S, Hamer T et al: Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin
resistance, hyperandrogenemia, and systolic blood pressure, while
facilitating normal menses and pregnancy. Metabolism 43: 647, 1994 90. Dunaif A, Scott D, Finegood D et al: The insulin-sensitizing agent troglitazone improves metabolic and reproductive
abnormalities in the polycystic ovary syndrome. J Clin Endocrinol Metab 81: 3299, 1996 91. Korytkowski MT, Mokan M, Horwitz MJ, Berga SL: Metabolic effects of oral contraceptives in women with polycystic ovary
syndrome. J Clin Endocrinol Metab 80: 3327, 1995 92. Fahmy K, Abdel-Razik M, Shaaraway M et al: Effect of long-acting progestagen-only injectable contraceptives on carbohydrate
metabolism and its hormonal profile. Contraception 44: 419, 1991 93. Ramsay LE, Yeo WW, Jackson PR: Influence of diuretics, calcium antagonists, and alpha-blockers on insulin
sensitivity and glucose tolerance in hypertensive patients. J Cardiovasc Physiol 20 (suppl): S49, 1992 94. Shoupe D, Lobo RA: The influence of androgens on insulin resistance. Fertil Steril 41: 385, 1984 95. Diamanti-Kandarakis E, Mitrakou A, Hennes MM et al: Insulin sensitivity and antiandrogenic therapy in women with polycystic
ovary syndrome. Metabolism 44: 525, 1995 96. Elkind-Hirsch KE, Sherman LD, Malinak R: Hormone replacement therapy alters insulin sensitivity in young women with
premature ovarian failure. J Clin Endocrinol Metab 76: 472, 1993 |