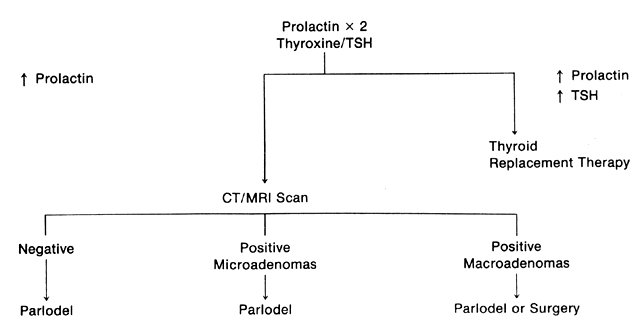
Historically, patients with hyperprolactinemia were not treated unless
they had large pituitary tumors. Generally, the patients underwent transsphenoidal
hypophysectomy if visual impairment was noted or fertility
was desired. The longitudinal studies by Weiss and associates71 at the University of Southern California showed that adenomas grow very
slowly when untreated, and in fact only 10% of the lesions expand. In
addition, 32% of the time a progressive decrease in prolactin level
was demonstrated. Such observations might negate the need for treatment
of patients with all but macroadenomas. However, four reports have appeared
in the literature that associate hyperprolactinemia with profound
bone demineralization.72,73,74,75 Patients who are both hypoestrogenic and hyperprolactinemic demineralize
bone more rapidly than patients who are hypoestrogenic. Klibanski and
Greenspan76a have shown that treatment of these patients with bromocriptine and restoration
of normal prolactin levels causes a reversal of the demineralization
process. Therefore, until the issue of bone demineralization is
clarified, one could argue that all patients with hyperprolactinemia
should be maintained in an estrogenic state to prevent bone loss. In view of the association of bone demineralization with hyperprolactinemia, it
would seem prudent to treat patients with functional hyperprolactinemia (i.e., patients who do not have pituitary tumors demonstrated on radiographic
surveillance) with pharmacologic agents that will render them estrogenic. This
could be accomplished by administering cyclic overlapping estrogen-progestogen
therapy, oral contraceptive agents, or dopamine agonists. Concern had been expressed in the past about treating patients with functional
hyperprolactinemia or prolactinomas with estrogens because it
was believed that these compounds stimulated prolactin secretion and because
of the clinical observation that pituitary tumors had been shown
to grow during pregnancy.76b Review of the literature shows that the stimulation of prolactin secretion
by both estrogens and progestogens occurs primarily in lower animal
models and has not been demonstrated in humans. In addition, most microadenomas
show little or no growth during pregnancy, and most patients
with macroadenomas have an uncomplicated pregnancy, labor, and delivery. A
poll of reproductive endocrinologists directly involved in prolactin
research showed that most would treat patients with functional
hyperprolactinemia or microadenomas with replacement estrogen or oral
contraceptive agents.77 Further, oral contraceptive agent treatment of patients with microadenomas
produced no long-term change in tumor size. In addition, although
birth control pills have been shown to induce a small increase in prolactin
secretion, this effect is not thought to be clinically significant. The primary mode of therapy for the treatment of functional hyperprolactinemia
involves the use of dopamine agonists. Bromocriptine (Parlodel) is
the only compound currently approved for the treatment of hyperprolactinemia
in the United States.78 This type of drug is an ergot derivative closely related to LSD. The ergots
were originally isolated from a fungus (Claviceps purpurea) that grew on rye. Ingestion of such grain resulted in St. Anthony's
fire and the cessation of lactation. Modification of the molecule
has attenuated some of the undesirable ergot side effects, but many patients
experience nausea and vomiting, nasal stuffiness, lethargy, and
dysphoria when taking bromocriptine. A few patients are hypersensitive
to ergots and have hypotensive episodes. When ergots are administered
in extremely high doses for the treatment of Parkinson's disease, tachyarrhythmias (often
fatal) have been reported, as has the induction
of psychosis. The dose range for the treatment of hyperprolactinemia
with bromocriptine is 5 to 10 mg/day. However, subtherapeutic doses
have often been shown to suppress prolactin levels and shrink pituitary
tumors.79 The medicine should be administered at bedtime to take advantage of the
blockade of the sleep-associated surge in prolactin. It has also been
suggested that patients who have side effects such as nausea with oral
bromocriptine might benefit from vaginal delivery of this drug.80 Serum Parlodel levels have been shown to rise more slowly than those achieved
with oral delivery, but serum levels are maintained for a longer
duration.81 Also, bromocriptine has been shown to have no deleterious effect on sperm
function when tested in vivo or in vitro.82,83,84 Patients with functional hyperprolactinemia are often treated for 12 to 14 weeks, followed
by discontinuation of the medication. In general, spontaneous
cures are rare, and patients require long-term therapy. After
pregnancy, patients often have a lower basal prolactin level, and
at times spontaneous cures occur. Several new forms of bromocriptine have been introduced into the European
market. Parlodel SRO, a long-acting oral bromocriptine, suppresses
the prolactin level with a single oral dose for more than 24 hours.85 After 1 month's therapy, 63% of patients had normal prolactin levels
and 43% had return of menstruation. Likewise, Parlodel LAR is a long-acting
repeatable injectable form of bromocriptine.86 The polymer matrix has a total mass degradation of less than 3 months
and is being used every 28 days for prolactin suppression. These drugs
are not available on the current US market. Another drug currently marketed in the United States is pergolide mesylate (Permax). It
may be used for the suppression of prolactin secretion, although
it is not approved by the Food and Drug Administration for
that purpose.87 Pergolide is used in Canada and Europe for the treatment of hyperprolactinemia.88 It is administered once a day and is considerably more potent than bromocriptine. Its
side effects appear to be no different than those reported
with bromocriptine, and it is as effective in suppressing prolactin
levels and in shrinking pituitary tumors. Several other compounds are being investigated for the treatment of hyperprolactinemia
and prolactinomas.88,89 CV205-502, a nonergoline dopamine agonist (Fig. 4), is many times more potent than bromocriptine and suppresses prolactin
secretion to between 60 and 80 μg/day.90 It also is longer-acting and is administered at bedtime. The drug has
fewer side effects than bromocriptine, the most serious of which seems
to be transient headache. It is suspected that this drug will be marketed
within several years and should add considerably to the armamentarium
of the clinician in treating the patient with hyperprolactinemic
syndromes. 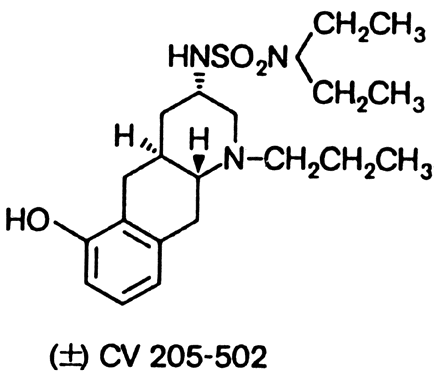 Fig. 4. Structure of the nonergoline dopamine agonist CV205-502. Fig. 4. Structure of the nonergoline dopamine agonist CV205-502.
|
The patient with radiographic evidence of microadenoma may be treated in
an identical manner to the patient with functional hyperprolactinemia. In
general, bromocriptine is the treatment of choice on an indefinite
basis to maintain bone stability. Considering the slow growth of these
tumors, follow-up CT or MRI scanning is not recommended unless the
patient were to break through bromocriptine suppression and develop symptoms
of an expanding central nervous system lesion. Historically, transsphenoidal
hypophysectomy was used to treat patients with microadenomas
of the pituitary. Recent studies have shown a 50% failure rate in
terms of normalization of prolactin levels in patients undergoing such
surgery. It is now recommended that surgery not be used as the primary
mode of therapy in patients with microadenomas of the pituitary gland. The
patient who fails to show suppression of prolactin levels or
shrinkage of tumor with bromocriptine therapy possibly houses a non-endocrine-secreting
tumor or one that produces another trophic hormone.91,92 Patients with macroadenomas of the pituitary gland require lifelong therapy. The
vast majority of these patients will promptly respond to bromocriptine
therapy by lowering prolactin secretion and tumor reduction. These
patients should be followed at intervals with either CT or MRI
scanning until the tumor becomes nondetachable or stable. Subsequently, if
continuous therapy is maintained and the patient has no sign of
galactorrhea, headache, or menstrual dysfunction, she can be followed
with visual field examinations yearly and serum prolactin evaluation every 6 months. Surgery
should be reserved for patients refractory to bromocriptine
therapy. About 70% of the patients undergoing transsphenoidal
hypophysectomy with macroadenomas are not cured with surgery in terms
of resolution of the pituitary tumor or restoration of the euprolactinemic
state.93 Radiation treatment, either with cobalt or with Bragg peak proton therapy, can
be used for refractory macroadenomas. Kjellberg and Kliman94 cured 678 patients with all types of adenomas (0.7% recurrence rate) using
this type of therapy; however, some of these patients were rendered
hypopituitary (10% to 20%) and must receive replacement therapy with
thyroid, corticoid, and often posterior pituitary hormones. Several investigators have recommended that bromocriptine therapy be used
in persons with unexplained infertility. Ben-David and Chrambach95 have isolated an isoform of prolactin that may interfere with ovulation. It
was suggested that when patients were rendered euprolactinemic in
terms of native prolactin that the isoB form often remained elevated, requiring
higher does of bromocriptine to produce suppression. Once
isoB-prolactin returned to normal, a high conception rate was reported. Harrison
and co-workers96 isolated a group of “prolactin spikers” with high anxiety
scales. These women become pregnant when treated with bromocriptine as
compared with controls. DeVane and Guzick97 treated patients with euprolactinemic galactorrhea; they attained a significant
pregnancy rate when compared with patients treated with vitamin
B6 or clomiphene. On the other hand, rendering a patient hyperprolactinemic
with metoclopramide 4 to 5 days before ovulation does not appear to
disrupt the process, and in a double-blind randomized placebo control
study, investigators could not show a significant difference between
patients treated with bromocriptine versus placebo in terms of conception
rates.98,99 Therefore, it is recommended that patients with unexplained infertility
not be treated with dopamine agonist therapy empirically. Although of historical importance, the choice of contraception in patients
with hyperprolactinemia does not differ from that of the population
at large, with the exception of patients with macroadenomas. These persons
should not be treated with oral contraceptive agents, although
prospective studies have not clearly shown an association between the
use of birth control pills and tumor growth. Until this issue is resolved, it
is probably best to have these patients treated by reproductive
endocrinologists under protocol. Another issue of historical importance is whether patients with hyperprolactinemia
and prolactinoma should breastfeed. We recommend that patients
with functional hyperprolactinemia or microadenomas be allowed to
breastfeed because they are not at risk for exacerbating their condition. Patients
with macroadenomas may also breastfeed, although they should
be made aware that this process may stimulate growth of their tumor. In
general, breastfeeding should be restricted to women who showed
no sign of tumor on bromocriptine therapy before pregnancy. The patient who presents with persistently elevated prolactin levels who
is treated with bromocriptine can expect a pregnancy rate in excess
of 80% within three or four cycles. There is no increased incidence of
multiple births or malformations in patients treated with bromocriptine, although
the drug should be discontinued as soon as a positive pregnancy
test is obtained. Some reproductive endocrinologists treat patients
with macroadenomas with bromocriptine throughout pregnancy. Long-term
follow-up studies carried out in Japan, France, and Switzerland have
shown no increased defects in motor or sensory development in children
exposed to dopamine agonist therapy.100,101 One issue that cannot be answered at this time is the natural history of
pituitary tumors in the face of estrogen replacement therapy. It will
take a generation for this question to be answered, using sophisticated
radiographic techniques and serum prolactin monitoring to evaluate
the growth pattern of microadenomas and macroadenomas in patients who
do or do not receive estrogen replacement therapy. |