The Subunit Genes
hCG is the first hormone known to be elaborated by the conceptus.4 It is a glycoprotein heterodimer consisting of two dissimilar subunits, α and β , that are joined noncovalently. The single α subunit gene resides on the long arm of chromosome 6. The β subunit is encoded by a cluster of six transcriptionally active genes located on chromosome 19 (Fig. 1).11,20 Three of the hCG-β genes are expressed at significant levels during pregnancy: gene 5 predominates, whereas genes 3 and 8 are transcribed at lower levels. There is very low expression of genes 1, 2, and 7. All six genes share extensive (> 80%) sequence homology with the LH-β gene, which is located in the same region on chromosome 19 and from which the the hCG-β genes probably evolved.20,21
Structure
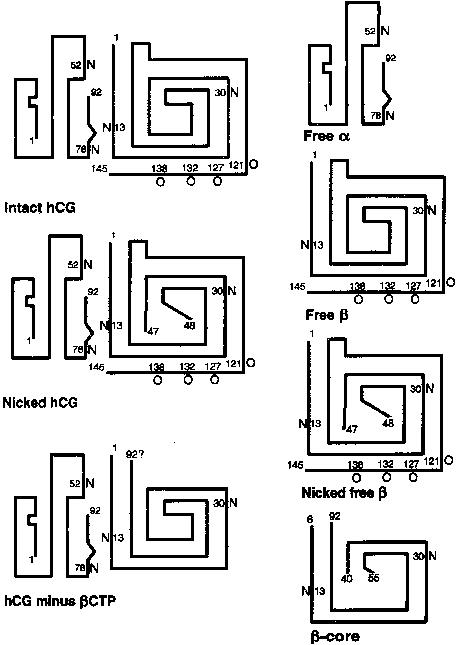
The α subunit is a 92-amino-acid polypeptide that is common to the pituitary gonadotropins and thyrotropin. The α subunit contains two asparagine-linked (N-linked) oligosaccharide side chains attached at amino acid residues 52 and 78 (Fig. 2). The trophoblast secretes two forms of the free α subunit, regular free α , which is the same as the α subunit of hCG, and a large free α subunit, which is hyperglycosylated with larger, more complex N-linked oligosaccharides that prevent its combination with the β subunit.22 Most of the free α subunit in maternal serum is large free α , although current immunoassays cannot discriminate between the two forms. The biological significance of these posttranslational modifications remains to be determined.
The 145-amino-acid hCG-β subunit is unique and distinguishes hCG from the other glycoprotein hormones. The major difference between LH-β (121 amino acids in length) and hCG-β is a 24-amino-acid C-terminal extension in hCG-β that results from a one base pair deletion and a two base pair insertion that removes a termination codon and produces an extended open reading frame. The C-terminal peptide of hCG-β contains four serine-linked (O-linked) oligosaccharide chains (see Fig. 2). The C-terminal peptide of hCG-β does not have a major impact on the biological activity of the hormone in vitro, but it markedly increases the half-life of holo-hCG in vivo.23 The β subunit also contains two N-linked oligosaccharides at amino acid positions 13 and 30.
The Role of Carbohydrates in the Function of hCG
hCG contains approximately 30% carbohydrate by weight. The carbohydrate chains usually terminate with sialic acid, the removal of which substantially reduces the half-life of hCG in the circulation by enhancing clearance of the hormone by the asialoglycoprotein receptor. There is considerable heterogeneity in glycosylation of hCG during normal pregnancy. Carbohydrate heterogeneity is also marked in hCG produced by the trophoblast of gestational trophoblast disease (moles and choriocarcinomas).
Removal of N-linked oligosaccharides on the α or β subunit, or both, has little effect on the binding of hCG to the LH/hCG receptor; however, the deletion of certain of these N-linked oligosaccharides affects intracellular assembly of the subunits and secretion of hCG.24 Additionally, the N-linked oligosaccharide at residue 52 of the α subunit is critical to signal transduction of the hCG hormone by stimulating cAMP accumulation and steroidogenesis in target cells.25,26,27
Synthesis and Secretion During Pregnancy
hCG is produced by trophectoderm in the preimplantation embryo.4 hCG can be detected in maternal serum 8 to 10 days after ovulation in a fertile cycle, which coincides with trophoblast formation and implantation of the blastocyst into the uterine wall (Fig. 3).20 As the trophoblast organizes into chorionic villi during the first trimester, hCG production is localized to the syncytiotrophoblast. Although hCG-β gene transcription can be detected in cytotrophoblast cells, synthesis of hCG-β and intact hCG (both subunits in their dimeric form) is restricted to the villous syncytiotrophoblast after 6 weeks of gestation.20 The factors responsible for coupling trophoblastic differentiation with the synthesis of hCG have not been identified.
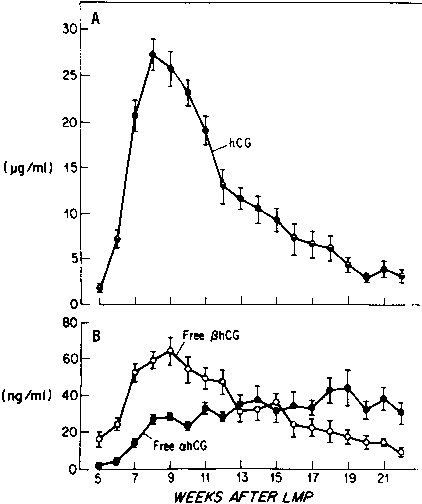
Most of the hCG produced by the syncytiotrophoblast is released into the maternal circulation. Maternal serum hCG levels increase progressively and reach a peak at 8 to 10 weeks' gestation. Initially, hCG concentrations double every 1.4 days; by the 5th week of pregnancy, the levels double more slowly (every 2.3 days).28,29 After 10 weeks of pregnancy, placental hCG secretion declines to comparatively low levels, which are maintained until term. hCG concentrations in maternal blood are generally higher in multiple gestation pregnancies, reflecting a greater trophoblast mass.30 Higher hCG levels are also found in pregnancies complicated by chromosomal abnormalities, which permits clinicians to measure maternal serum hCG levels as one component of multiple-marker screening.31,32 There is, however, no correlation between hCG levels in early pregnancy and fetal sex or birth weight.
Regulation of hCG Synthesis
Several models have been advanced to account for the biphasic pattern of placental hCG production during pregnancy, including differentiation-modulated gene expression, the sequential action of stimulating and inhibiting factors, and autocrine control by hCG itself. Boime and others have suggested that the formation of syncytia was linked to hCG production.20,33 Observations consistent with this idea include the finding that isolated cytotrophoblast cells increase hCG secretion as they spontaneously fuse to form syncytial structures.34 However, the mononucleated trophectoderm of the blastocyst produces hCG before implantation, and the formation of syncytia and hCG expression can be dissociated in vitro.2 Therefore, the formation of a syncytium is not an absolute prerequisite for hCG expression, and the factors responsible for coupling trophoblast differentiation with synthesis of hCG remain obscure.20
hCG secretion may also be under the control of tropic factors. A number of substances have been reported to increase hCG production by trophoblast cells or placental explants, including (placental) GnRH,35 activin,1 epidermal growth factor,15 colony-stimulating factor-1 (CSF-I),36 retinoic acid,14 agents that stimulate adenylate cyclase,15,37,38 and the cytokines interleukin-1 and interleukin-6.39,40 These factors act by increasing hCG subunit gene transcription or by increasing the stability of the hCG subunit mRNAs, and possibly by enhancing secretory mechanisms. As described above, cAMP, CRE-binding proteins, and other trans-acting factors control α subunit transcription by interacting with CREs, JREs, and upstream response elements in a combinatorial fashion.11,13,14,15,16 The hCG-β genes are also transcriptionally regulated by cAMP, but respond more slowly.41 The cAMP response elements in the hCG-β gene promoters are different from those of the hCG-α subunit gene. The activation of cAMP-dependent protein kinase, however, appears to be essential for the stimulation of both α and β subunit genes' cAMP-responsive enhancer elements.15 For example, the trans-acting factor CREB, which binds to the CREs, is phosphorylated in response to cAMP-dependent protein kinase activation. Glucocorticoids can also modify the response to cAMP, increasing the cAMP-stimulated hCG production in normal trophoblast cells.8 Another potential stimulator of trophoblast cAMP accumulation, which would lead to increased transcription of the α and β subunit genes, is hCG itself. hCG is known to activate placental adenylyl cyclase, and trophoblast cells express LH/CG receptors.42,43,44 Thus, a positive feedback might exist whereby low levels of hCG augment its own production in an autocrine fashion.43,44 High levels of hCG, however, suppress trophoblast LH/CG receptor expression. This concentration-dependent effect of hCG on trophoblast cells may account, in part, for the initially rapid rise in hCG production through positive feedback effects of low levels of hCG on trophoblast cells in early pregnancy and the subsequent reduction in hCG expression after hCG levels rise to peak concentrations.
The placenta produces GnRH and possesses GnRH receptors, but these receptors have a lower affinity for GnRH than do pituitary GnRH receptors.35 Placental tissue responds to GnRH in vitro through a calcium-mediated second messenger system with increased hCG secretion.45 The immunodetection of GnRH in villous cytotrophoblast cells, the correspondence of the peak of placental hCG secretion with the greatest abundance of villous cytotrophoblast cells relative to syncytiotrophoblast, and the greater responsiveness to GnRH by first trimester placental tissue than by term placenta, are consistent with the idea that cytotrophoblast-derived GnRH governs syncytiotrophoblast hCG secretion.35 However, administration of GnRH to pregnant women does not increase serum hCG levels.46 The failure of exogenous GnRH to affect hCG levels in vivo may be attributed to the fact that placental GnRH receptors have a lower affinity for this releasing factor compared with pituitary GnRH receptors and would not be activated at the concentrations of GnRH administered or because the trophoblast GnRH response systems are saturated by endogenous hormone.
Placental hCG secretion is also subject to negative regulation. Specific transcription factors including OCT-4 and Jun suppress transcription of the α and β subunit genes,47,48 and protein and steroid hormones as well as other substances (e.g., peroxisome proliferators) reduce hCG secretion.49 In fact, the ligand-dependent nuclear receptor peroxisome proliferator-activated receptor-gamma is expressed in trophoblast cell lines and has opposing, ligand-specific effects on placental differentiation, which likely affects the expression of the α and β subunit genes.49 Petraglia and colleagues proposed that inhibin and activin, also produced by the placenta, contribute to the biphasic pattern of hCG secretion.50 In vitro studies carried out by these investigators indicate that inhibin and follistatin block GnRH stimulation of hCG secretion, whereas activin stimulates hCG secretion and may promote trophoblast differentiation. Others have reported that inhibin only suppresses hCG secretion by term placental tissue, but not in the first trimester.51 Progesterone reduces hCG release by placental explants, and it has been suggested that the decline in hCG secretion after the 10th week of gestation is coupled with increasing placental progesterone biosynthesis.52,53 A 7- to 10-kd protein of decidual origin has also been reported to inhibit hCG secretion.54
Integration of Factors Controlling hCG Secretion
Based on the existing literature, it is possible to construct a model in which stimulators working in a paracrine or autocrine mode such as GnRH, activin, and hCG itself increase hCG synthesis, whereas inhibin and progesterone suppress it. Early in pregnancy, placental GnRH and positive feedback effects of hCG on trophoblast function may cause increasing hCG secretion; as hCG levels peak and placental progesterone secretion rises, hCG secretion would be reduced. This concept, however, is based exclusively on in vitro studies and does not fully account for the differential patterns of hCG-α and hCG-β gene expression during pregnancy. Furthermore, only a fraction of newly synthesized hCG is stored in secretory granules and is responsive to secretagogues, whereas most hCG is released constitutively. Thus, the magnitude of induced hCG release is much less than GnRH-induced LH secretion by the pituitary gland, and construction of an integrated model for control of hCG synthesis/secretion remains elusive.20
Circulation of hCG Subunits in Maternal Blood
Free hCG-α and hCG-β subunits are present in maternal blood during pregnancy (see Fig. 3).55,56,57 These free subunits are secreted from trophoblast cells; they are not derived from the dissociation of holo-hCG. Over the course of pregnancy, placental hCG-α production is more than fivefold that of holo-hCG and hCG-α . Significant quantities of the hCG-α subunit are present throughout pregnancy, whereas concentrations of holo-hCG and free hCG-β subunit fall to relatively low levels during the second trimester. These observations suggest that hCG-β subunit synthesis limits holo-hCG formation.
When the hCG-α subunit does not combine intracellularly with hCG-β , it undergoes posttranslational modifications leading to a different pattern of N-glycosylation (more branched structures) and increased sialic acid content, which distinguishes secreted free hCG-α (large free α ) from the α subunit of holo-hCG (regular α subunit).58,59 The pattern of glycosylation of large free hCG-α subunit changes with increased incorporation of more highly branched fucosylated oligosaccharides as gestation progresses.60,61 Although large free α will not combine with the β subunit to form holo-hCG, the biological significance of these glycosylation patterns remains to be determined.
Free hCG-β , as a percentage of total immunoreactive hCG, is high in very early pregnancy, accounting for as much as 50% of total immunoreactive hCG during the first few weeks of pregnancy, and 4% to 10% of total immunoreactive hCG between 4 and 6 weeks of gestation. However, from 8 weeks of pregnancy to term, free hCG-β is 1% or less of the total hCG-β immunoreactivity in serum.55,56,57
Metabolism and Clearance of hCG and Its Subunits
The clearance of holo-hCG from the circulation has two components: a fast component (approximately 7 hours) and a slow component (approximately 39 hours).62 The half-life of holo-hCG is considerably longer than that of LH, primarily because of the glycosylated C-terminal peptide of the β subunit. The metabolic clearance rate of holo-hCG, or volume of plasma cleared of hCG per unit of time, is approximately 4.4 L/day. Renal clearance, a major pathway of removal of hCG from maternal circulation, is unchanged during pregnancy. Approximately 30% of the cleared hormone appears more or less intact in the urine. The remainder is removed through nonrenal mechanisms. Because the rate of holo-hCG clearance does not change significantly during pregnancy, steady-state hCG levels in maternal blood are directly related to hCG synthesis and secretion.
The half-lives of free hCG-α β and hCG-β are short compared with that of holo-hCG.62 The fast and slow components for hCG-α and hCG-β subunits have been estimated to be 13 and 94 minutes and 43 and 239 minutes, respectively. Thus, the metabolic clearance rate for hCG-α is about threefold greater than that for hCG-β , but the free subunits are eliminated at a rate that is 10- to 30- fold faster than that of holo-hCG. Although the metabolic clearance rates of the free subunits are much more rapid than the clearance rate of holo-hCG, less than 1% of the free subunits are excreted in urine; thus, rapid renal excretion does not account for their shorter half-lives.
The hCG-β subunit is subject to proteolysis. Nicking of the hCG-β subunit between residues 47 and 48 or less commonly, between residues 43 and 44 or 44 and 45, markedly reduces biological activity (see Fig. 2).22 Nicked hCG molecules typically account for 10% to 20% of the hCG molecules present in maternal serum and urine. Further catabolism of the nicked β subunit in the maternal kidney produces the β core fragment. A unique form of the β subunit, consisting of hCG-β residues 6 to 40 with disulfide bond links to residues 55 to 92, the β core fragment accounts for as much as 90% of immunoreactive hCG-β in urine during pregnancy.63,64 Although the β core fragment is a catabolite of hCG that is generated largely in the kidney, there is a possibility that some of this material originates from the placenta.65 In fact, placental macrophages may degrade hCG into nicked hCG and the β core fragment, thereby preventing intact hCG from entering the fetal circulation and deranging the developing fetal endocrine system.
hCG Actions: The Conceptus' Signal to the Ovary
The primary role of hCG in the maternal organism is to serve as a signal to the ovary to maintain the corpus luteum, which would regress if it were not rescued by hCG. hCG acts on the luteal cell LH/CG receptor, which activates adenylyl cyclase and possibly other signal transduction mechanisms that lead to maintenance of the steroid machinery. It appears that exponentially increasing amounts of hCG are required to prolong the functional lifespan of the corpus luteum, which explains why the corpus luteum survives early pregnancy but regresses during unfertilized menstrual cycles, despite the fact that LH and hCG have similar actions on the corpus luteum.66 Thus, administration of antisera to hCG in early gestation causes pregnancy termination in primate models, and immunization against hCG epitopes may be an effective contraceptive vaccine.67 Other roles for hCG have been suggested based on the discovery of LH/CG receptors in the fallopian tube, endometrium, and myometrium. Although hCG produced by the primate blastocyst appears to modulate decidualization of the maternal endometrium prior to implantation, the presence of LH/CG receptors in human endometrial cells in vitro is disputed, and treatment of these cells with hCG does not promote decidualization.68,69 Thus, the signal transduction pathway by which hCG may affect decidualization remains to be elucidated. In the myometrium, hCG was reported to reduce the amplitude but increase the frequency of contractions of human myometrial strips collected during the proliferative phase of the cycle, providing evidence that the hCG receptors detected in myometrium are functional.70
There is abundant clinical evidence that hCG is a weak thyrotropin (TSH) agonist.71 The hormone, particularly certain glycosylated isoforms, binds to TSH receptors and stimulates thyroid adenylyl cyclase.71,72 Hence, one of the factors contributing to increased maternal thyroxine secretion during pregnancy may be hCG, which may cause hyperemesis gravidarum during pregnancy and clinical hyperthyroidism during molar pregnancies.71
Actions of hCG on Extraembryonic and Fetal Tissues
As noted above, hCG may be an autocrine/paracrine regulator of trophoblast function in a concentration-dependent fashion.42,43,44 In addition to the previously described effects of hCG on its own expression, there are several reports of hCG stimulation of placental progesterone synthesis.74 LH/ CG receptors have also been detected in fetal membranes and umbilical cord, but the physiologic functions of the receptors in these locations are unknown.
hCG derived from the trophoblast or possibly produced by fetal kidneys or other fetal tissues may regulate fetal testicular testosterone secretion, which is needed to drive differentiation of the internal and external genitalia.75 hCG may also stimulate dehydroepiandrosterone production by the fetal adrenal gland.
A Biological Role for Free hCG Subunits?
Although it is generally believed that the free hCG subunits do not have important biological functions, experiments have linked the α subunit to growth of nontrophoblastic tumors.76 This is of particular interest, given the not uncommon expression of the α subunit gene by malignant cells. Neutralization of α subunits by antisera or suppression of hCG-α synthesis by anti-sense nucleic acid sequences reduces growth of lung tumor cells. The mechanisms by which free α subunit affects tumor cell growth have not been elucidated, and it is not known whether similar actions of free α subunit could influence proliferation of normal or malignant trophoblast cells.
Free hCG-α has been shown to function synergistically with progesterone to stimulate the differentiation of cultured human endometrial cells to decidualized cells77 and to be a potent stimulator of decidual prolactin secretion in vitro.78 Given the proximity of the trophoblast to the decidua and the substantial levels of free α subunit produced, it is reasonable to propose that free hCG-α could play a key role in controlling decidual formation and prolactin release in vivo.
Standardization of hCG Assays and Assay Specificity
hCG does not exist in biological fluids in a single molecular form (see Fig. 2). There is a spectrum of glycosylation patterns, and some of the circulating immunoreactive hCG undergoes proteolytic cleavage, including nicking.22,79 The different molecular forms of hCG and its subunits bind to antibodies with different affinities. Most commercial hCG assays employ multiple antibodies to different sites on holo-hCG, free β subunit (nicked or non-nicked), and the β core fragment. In sandwich assays, one antibody is used to capture hCG at a specific site, whereas a second labeled antibody is used to detect or quantitate captured molecules. This technique improves the specificity of hCG assays, but the clinician must recognize that different kits use a wide variety of antibody combinations. Thus, discordant results can be obtained when two different immunoassays are carried out on a single specimen because the different antibodies detect various molecular forms of hCG that may be present in the sample with different efficiencies.
Because there is no single molecular form of hCG, assays must be calibrated to reference preparations of the hormone. Two different reference preparations have been widely employed for hCG assay standardization: the Third International Standard, formerly known as the First International Reference Preparation (IRP) of hCG for Immunoassay (1974), and the Second International Standard of hCG for Bioassay (1964).79,80 These materials are not equivalent, having been characterized for immunoassays and bioassays, respectively. Both standards were made from the same large preparation of completely pure hCG, and 1 mg of the Second International Standard corresponds to 5.7 IU of the Third International Standard, whereas 1 mg of the Third International Standard equals 12 IU.80 Therefore, immunoassays standardized to the Third International Standard give values in international units that are approximately two times the value of immunoassays standardized to the Second International Standard.81 The hCG levels given in this chapter are based on the Third International Standard.
Although any of the hCG assays are excellent for the detection of normal pregnancy, the development of highly purified hCG immunoassays and standards (i.e., intact nick-free hCG, nicked hCG, or hCG subunits) may be more important for abnormal pregnancies.22 In some cases, such as gestational trophoblastic disease or Down syndrome pregnancies, only nicked hCG or only free β subunit is present in the circulation. Therefore, clinicians must be aware of what an assay is detecting, and immunoassays should be labeled appropriately.
Pregnancy Tests
The detection of hCG in serum or urine serves as the basis of contemporary pregnancy tests. Current serum hCG radioimmunoassays have a sensitivity as low as 2 to 10 IU/L and are able to detect pregnancy when implantation occurs, or approximately 8 to 10 days after ovulation. Urine hCG assays generally detect hCG concentrations as low as 25 to 50 IU/L, and results may be positive as early as 3 to 4 days after implantation or 10 to 14 days after ovulation. Ninety-eight percent are positive within 7 days after implantation.82
Monitoring Early Pregnancy
Quantitative hCG assays can provide an estimate of gestational age, and sequential monitoring of maternal serum hCG provides a biochemical index of growth of the conceptus.82 During the first 6 weeks of normal pregnancies, hCG titers in maternal serum double (100% increase) every 1.3 to 2 days. This pattern of hCG increase in maternal serum is abnormal in pregnancies that subsequently fail or when implantation of the embryo occurs at an ectopic site.28,83 By determining maternal hCG levels and assessing the uterus and adnexa by transvaginal ultrasonography, one can possibly distinguish between intrauterine and ectopic pregnancies once hCG titers have reached certain threshold values.83,84 The discriminatory zone is defined as the hCG level above which a gestational sac can be identified by ultrasound.84 At most institutions where transvaginal ultrasound is used, the discriminatory zone ranges from 1000 to 1500 IU/L. This level is achieved in a normal pregnancy approximately 1 week after the missed menses or 5 weeks after the last menstrual period. In a recent decision analysis, the most efficient method of diagnosing ectopic pregnancy (e.g., no ectopic pregnancy diagnoses missed, fewest intrauterine pregnancies interrupted) was determined to be transvaginal ultrasound followed by quantitative serum hCG measurements when ultrasound is nondiagnostic.84 In Table 1, a summary of events in early pregnancy observed by ultrasound is presented, together with the corresponding hCG levels.
|
| hCG [ IU/I] | |
Author | Event | Days | (Range) |
Cacciatore et al: Br J Obstet Gynaecol 97:899, 1990 | Gestational sac 1–3 mm | 31 | 730 (432–935) |
| Yolk sac apparent | 36 | 4103 (1120–7280) |
| Heart action seen | 41 | 12,050 (9280–22,950) |
Bernaschek et al: Am J Obstet Gynecol 158:608, 1988 | Gestational sac seen |
| > 600 |
Nyberg et al: Radiology 167:619, 1988 | Gestational sac seen |
|
|
| 20% of cases |
| < 1000 |
| 80% of cases |
| 1000–2000 |
| 100% of cases |
| > 1000 |
Fossum et al: Fertil Steril 49:788, 1988 | Gestational sac seen | 34.8 ± 2.2 | 1398 ± 155 |
| Fetal pole seen | 40.3 ± 3.4 | 5113 ± 298 |
| Fetal heart seen | 46.9 ± 6 | 17,208 ± 3772 |
Daya et al: Can Med Assoc J 144:441, 1991 | Yolk sac first seen | 36 | 1900 |
| Yolk sac always seen | 40 | 5800 |
| Heart action first seen | 41 | 9200 |
| Heart action always seen | 46 | 24,000 |
The presence of hCG in the blood of a woman who is or has been pregnant reflects the presence of viable trophoblast tissue or the kinetics of hCG clearance, respectively. The time for clearance depends in part on the level of hCG. For example, hCG can be found in maternal serum or urine up to 24 days after normal delivery. However, because of the high levels of hCG in the maternal circulation during the first 13 weeks of pregnancy, hCG may be detected in maternal blood or urine for as many as 60 days after pregnancy termination in the first trimester.85
Prenatal Genetic Screening
Measurements of serum hCG and its subunits in conjunction with alpha-fetoprotein and plasma unconjugated estriol have been employed in the prenatal diagnosis of chromosomal abnormalities in so-called multiple marker screening (MMS).86 In pregnancies in which the fetus has trisomy 21, maternal serum hCG levels are frequently elevated and α -fetoprotein and unconjugated estriol levels are frequently reduced at or after 15 weeks of pregnancy. In pregnancies in which the fetus has trisomy 18, the maternal serum level of all three markers is frequently lower than expected.87 Combined measurements of hCG, α -fetoprotein, and unconjugated estriol are able to detect approximately 65% of fetuses with Down syndrome, with a false-positive rate of approximately 5%.79,86 The usefulness of these maternal serum markers may be a result of altered growth of the extraembryonic tissues when chromosomal abnormalities are present.
Because the maternal serum holo-hCG and free hCG-β levels are markedly elevated when the fetus has trisomy 21, hCG is considered the most sensitive individual marker for fetal Down syndrome.88 Higher than usual maternal serum proportions of nicked hCG and free β subunit, and β core fragment in maternal urine, also are associated with Down syndrome pregnancies, but there does not appear to be any benefit to using free hCG-β versus holo-hCG in MMS.89,90,91 Integration of other hCG molecules, such as urinary hyperglycosylated hCG, novel maternal serum markers (i.e., inhibin, pregnancy-associated plasma protein A [ PAPP-A] ), and ultrasound findings, with traditional MMS analytes is under investigation.92,93
Gestational Trophoblastic Disease and Nontrophoblastic Tumors
hCG measurements are used to diagnose and monitor therapy of gestational trophoblastic disease as well as numerous hCG-secreting nontrophoblastic neoplasms (Table 2). Molar pregnancies are associated with extremely high levels of hCG that may be greater than 1,000,000 IU/L. These concentrations are usually much greater than those found in women with choriocarcinomas. Trophoblastic disease samples typically contain unduly high proportions of nicked hCG and free β subunit, and in many cases, nicked hCG persists longer than non-nicked hCG after successful treatment.94 Thus, serial monitoring of hCG with appropriate immunoassay kits can provide an index of success of tumor extirpation and chemotherapy.
Table 2. Immunoreactive Human Chorionic Gonadotropin in Sera of Patients
with Cancer
Tumar Type/Site | No. | Positive (%) |
Islet cell | 104 | 39.4 |
Gynecologic | 2010 | 28.9 |
Ovary | 633 | 28.6 |
Endometrium | 348 | 16.7 |
Cervical | 976 | 33.8 |
Vulva/vagina | 37 | 27 |
Carcinoid | 41 | 26.8 |
Gas trointe stinal | 2165 | 18 |
Oropharynx | 298 | 14.8 |
Esophagus | 124 | 17.7 |
Gastric | 232 | 20.7 |
Small intenstine | 24 | 16.7 |
Colon/rectum | 693 | 9.8 |
Liver | 281 | 22.1 |
Gallbladder | 26 | 30.7 |
Pancreas | 200 | 20 |
Pulmonary | 1365 | 17.4 |
Breast | 3031 | 16.8 |
Melanoma | 244 | 13.9 |
Genitourinary | 658 | 11.8 |
Kidneys | 119 | 6.7 |
Bladder | 176 | 23.3 |
Prostate | 363 | 8 |
Sarcoma | 136 | 11.8 |
Hematopoietic | 544 | 6.1 |
Lymphoma | 339 | 5.3 |
Miscellaneous | 576 | 10.4 |
Total | 11,213 | 18.0 |
Among testicular tumors, approximately 5% of seminomas and 66% of nonseminoma germ cell tumors secrete immunoreactive hCG.95 hCG is detectable in blood in 10% to 16% of subjects with other nontrophoblastic malignancies, including a high percentage of patients with pancreatic islet cell tumors, epithelial ovarian tumors, carcinoids, breast and gastrointestinal cancers, and nonovarian gynecologic malignancies.95,96 The level of hCG found in these instances is usually modest, being in the range of 10 to 340 lU/L.
hCG in Normal Nonpregnant Subjects
Immunoreactive hCG has been found in low concentrations in normal men and women.96,97 This hCG is probably produced by the pituitary gland, and its secretion may be under GnRH regulation.98,99 Normal men are reported to have serum immunoreactive hCG levels of 0.02 to 0.8 lU/L, and normal premenopausal women are reported to have levels between 0.02 and 0.2 lU/L. Postmenopausal women have higher levels, ranging from less than 0.02 to 2.8 lU/L. Therefore, the presence of low levels of immunoreactive hCG in an apparently healthy nonpregnant subject does not necessarily indicate the presence of an occultmalignancy.