Definitions
Infertility is defined as the inability of a couple practicing frequent intercourse and not using contraception to fail to conceive a child within 1 year. This definition is based on investigations by Tietze and colleagues1 who reported in 1950 that 90% of 1727 couples followed for 1 year became pregnant. The probability of conception depends on the length of exposure, coital frequency, and the age of the couple. In normal, young couples the chances of conception after 1 month of unprotected intercourse is 25%; 70% by 6 months, and 90% by 1 year. Only an additional 5% will conceive after waiting an additional 6 to 12 months.2 Once fertility therapy is initiated, couples must be counseled that a given period of time is required to test the adequacy for any given treatment regimen.
A thorough evaluation of the infertile couple often reveals one or more causes for failure to conceive. Motivated couples that comply with therapeutic guidelines can expect a 50% to 60% chance of conception. A spontaneous, treatment-independent cumulative pregnancy rate of about 30% to 40% exists in couples in which no identifiable cause for the infertility can be determined.
Age Factors
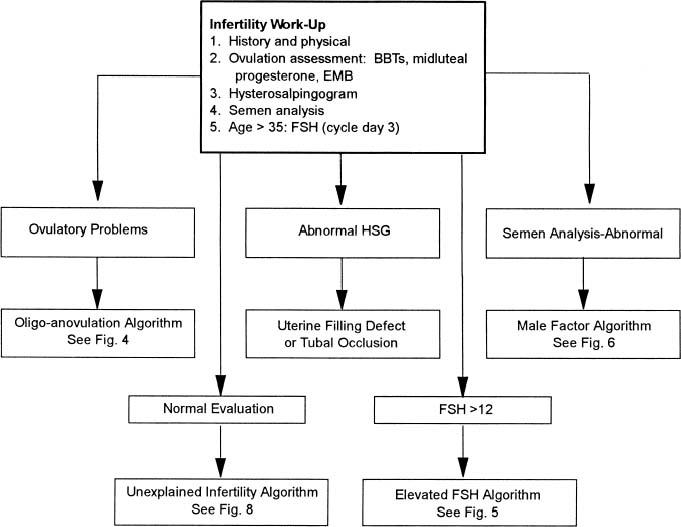
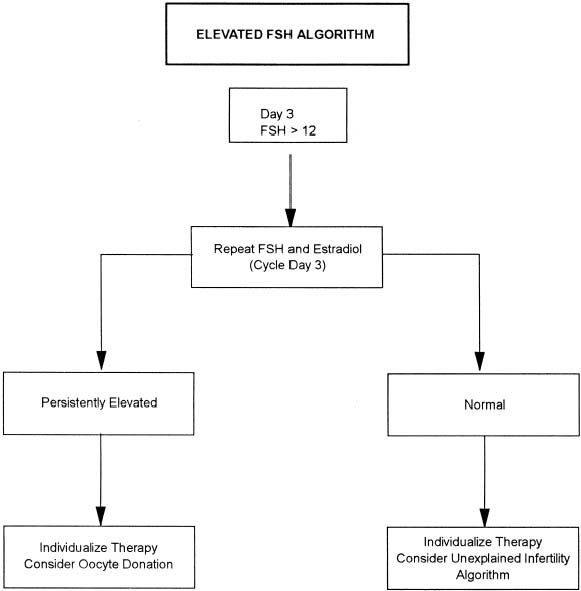
With more women pursuing careers and delaying childbearing, infertility is becoming an increasing problem in our society. The peak rate of conception occurs in both men and women in the mid-20s. A consistent decline in fecundity after 30 to 35 years of age has been demonstrated, with an incidence of involuntary infertility in women at the age of 40 ranging from approximately 33% to 64%. Reduction in age-related fertility is predominantly the result of the decline in oocyte quality, enhanced follicular atresia, and an increased rate of chromosomal abnormalities in fertilized oocytes and resulting embryos. These ovarian effects are associated with a rise in basal follicle-stimulating hormone levels, indicative of reduced ovarian reserve. With decreasing frequency of intercourse, the per-cycle probability of conception also declines. Thus, in the couple over 30 who meets the definition of either primary or secondary infertility, the work-up should be initiated and completed as soon as possible (Table 1).
Age Group | Conceiving in 12 months(%) |
20–24 | 86 |
25–29 | 78 |
30–34 | 63 |
35–39 | 52 |
Adapted from Hendershot GE, Mosher WD, Pratt WF: Infertility and age: An unresolved issue. Fam Plann Perspect 14:287, 1982.
Causes of Infertility
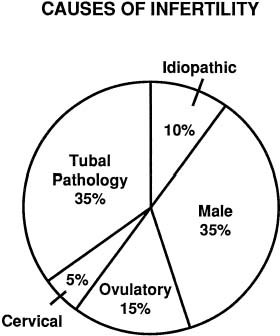
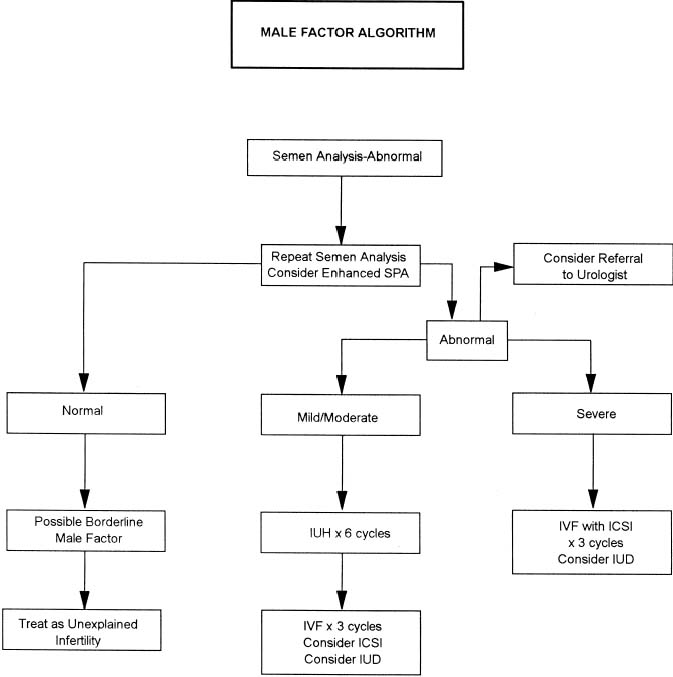
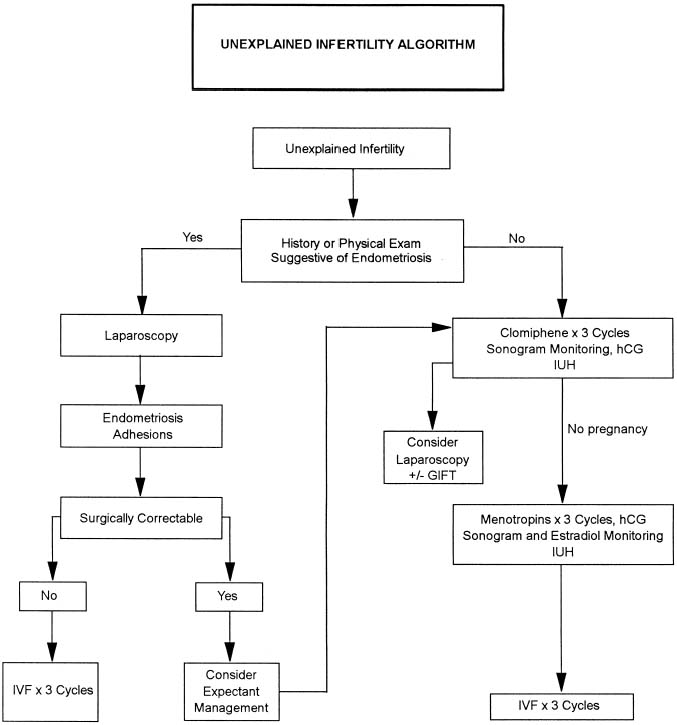
The exact incidence of the various etiologic factors for infertility appears to vary with the population studied. In the broadest of terms, 15% to 20% of the causes of infertility are the result of ovulatory dysfunction; 30% to 40% are caused by pelvic factors such as endometriosis, adhesions, or tubal disease; 30% to 40% are because of male factors such as oligospermia, increased semen viscosity, decreased sperm motility, or decreased semen volume; less than 5% are because of abnormal sperm-cervical mucous penetration or anti-sperm antibodies. In approximately 10% to 15% of couples no direct cause of their infertility can be found, but on further evaluation and treatment, occasionally factors such as poor sperm penetration, abnormal-appearing oocytes, etc., are elucidated. This group is referred to as unexplained infertility (Fig. 1).3
The etiology of infertility varies among races and economic strata. A recent study indicated that while the leading diagnosis of infertility in Caucasian patients is ovarian in origin (46.5%) followed by male factor (24.5%), the leading diagnosis of infertility in the African American population was tubal (41%) followed by surgical sterilization (25.6%). Also, patients without insurance were more likely to be surgically sterilized than those patients with insurance regardless of race or marital status.4
Given the growing incidence of infertility, it should become routine practice for physicians and educators to educate females about their lifestyle choices and how they may affect their reproductive capacity. For instance, early education that STDs may decrease future fertility may motivate some women to practice more abstinence or use condoms and spermicide. Additionally, anorexia, bulimia, and obesity result in ovulatory dysfunction and infertility. The first-line treatment for these diseases and their associated infertility is weight adjustment. Furthermore, couples should realize the effects of aging on reproduction. This knowledge will empower couples to make more informed decisions regarding childbearing. Finally, caffeine and smoking have all been associated with decreased fertility.5 Cigarette smoking in particular has been associated with an adverse affect on ovarian function and also on implantation. Furthermore, smoking may impair the uterotubal motility, thus increasing the risk for ectopic pregnancy in smokers.6 It is the role of all primary care physicians to educate patients on these risks from a young age, so that they may make responsible decisions. A more thorough list of causes of infertility is listed in Table 2.
TABLE 2. Causes of Infertility
Male | Female |
Disturbed spermatogenesis | Congenital anomalies |
|
Acute/chronic illness | Vaginal |
Exposure | Uterine |
Chemicals | Fallopian tubes |
Recreational Drugs | Sexual dysfunction |
Heat | Endocrine disorders |
Radiation | Ovary |
Genital disorders | Adrenal |
Genital injuries | Thyroid |
Endocrine disorders | Pituitary |
Varicocele | Hypothalamus |
Insemination disturbances | Sequelae of pelvic infections and inflammation |
Genital anomalies | Pelvic adhesions |
Genital trauma | Endometriosis |
Genital surgery | Tubal occlusion/phimosis |
Pelvic surgery | IUD complications |
Sexual dysfunction | Postsurgical |
Spinal cord injuries | Oophorectomy/cystectomy |
Veneral diseases | Myomectomy |
Abnormal seminal fluid/cervical mucus | Conization of cervix |
interaction | Pregnancy complications |
Infections | Abortion |
Immunologic | Cesarean section |
Intrinsic spermatozoal defects | Postpartum infections |
Unknown | Ectopic pregnancy |
Abnormal sperm/egg interaction | Immunologic |
Infection | Serum/cervical mucus antisperm antibodies |
Immunologic | Inadequate cervical secretions |
Intrinsic spermatozoal defects | Drug effects |
Unknown | Postsurgical |
Unexplained (?) | Unexplained (?) |
IUD, intrauterine device.
Evaluation of the Couple as a Unit
Infertility should be regarded as a two-patient disorder. Male and female partners must be thoroughly evaluated, counseled, and included in the therapeutic decision-making processes. Exclusion of the male partner may lead to feelings of isolation in the female and to disinterest and lack of cooperation of the male partner. A questionnaire is often helpful prior to the first visit and should include questions regarding prior conceptions, contraception, and coital frequency and techniques. This document serves as a basis for review and in-depth questioning and does not replace the history. Both partners should be screened for the use of drugs or alcohol that may affect fertility. Studies in males with heavy marijuana use show reduced testosterone levels, decreased sperm counts, and impotency.7 Alcohol is well known to affect libido and potency as well. Decreases in gonadotropin levels and ovulation are noted in females with frequent drug or alcohol ingestion. Cigarette smoking has also been implicated in subfertility. Early menopause, reduced spermatogenesis and decreased steroid production have been noted in individuals who use cigarettes. Life-tablele analysis studies demonstrate a longer period of time to conception for smokers as compared to nonsmokers.
Quality and Availability of Services
The ideal environment for care of the infertile couple would involve almost total dedication of a clinical setting to attend to the needs of the infertile couple. Physicians and ancillary staff should be specifically oriented to respond appropriately to the sometimes unrealistic expectations and profound emotional stresses experienced by couples with infertility problems. The nurse-clinician often provides the crucial link between the couple and the medical staff, thus making harmonious an evaluation that is often viewed by the couple as constituting personal invasion and physical trauma.
Infertility services may be offered either through clinic-like settings associated with large institutions or via the services of private practitioners. Clinic services may be viewed as involving too many physicians and fellows in training. A rotational schedule of physicians for ovulation induction or assisted reproductive technology (ART) may be seen as precluding the development of an appropriate relationship with the responsible physician. Also, participation in randomized clinical trials, the backbone of scientific clinical research, may be seen as cold and impersonal. On the other hand, private care can be fragmented when the infertile patient is seen amidst 20 to 30 obstetrical and gynecologic cases. In such an environment, infertility assumes a relatively unimportant position and patient experiences are often negative. Great care and an appropriate commitment to the infertile couple are vital to success in both settings.
Because of the unique needs of the infertile patients, the coordination of medical care must be well organized in either setting. Achievement of this goal can be extremely difficult. The seemingly endless frustrations and patient demands are influenced to some degree by the increasing specialization of fertility services, as well as the incessant exposure of consumers to multiple negative and positive opinions via the media or through episodic contact with various medical services. Nurses and coordinators of fertility services must be more sensitive and knowledgeable than usual. They must continually be involved in education designed to broaden their appreciation for the medical and emotional aspects of fertility disorders.