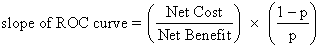Effective treatment of immunities to sperm rests on the accuracy of diagnosis. Four
approaches have been used to treat couples with antisperm
antibodies. Condom “therapy” is mentioned only to emphasize
its ineffectiveness, in the presence of more active treatment approaches.109 Its value was never clearly documented in the past, and the psychological
burden of using contraception when desiring pregnancy is great. The
remaining approaches have included corticosteroids, IUI, and IVF, and
gamete intrafallopian transfer (GIFT). However, the risk: benefit ratio
in the use of corticosteroids is still not well known for either men
or women. Preliminary evidence suggests that this treatment approach
is relatively ineffectual. IUI, when carefully timed to follicular maturation
in superovulated and hormonally and sonographically monitored
cycles, results in an increased chance of pregnancy, but success rates
at best are no greater than 35% within six treatment cycles. Although
technically intense and expensive, if IUI fails, IVF offers the greatest
likelihood of achieving fertilization, in the presence of both autoantibodies
to sperm in men and circulating antisperm antibodies in
women. Early evidence indicates that GIFT may also be effective in treating
couples in whom the man has developed antisperm immunity.110 These results with the newer assisted reproductive techniques suggest that
immunities to sperm impair the ability of spermatozoa to reach the
site of fertilization within the fallopian tubes to a far greater extent
than their ability to penetrate eggs, should the gametes meet in the
presence of these antibodies. In 1976, Shulman reported the successful use of corticosteroids to treat
a man with autoimmunity to sperm.111 Although various degrees of success have subsequently been described, most
of these earlier reports suffer from their inability to document
the change in antibody binding on the sperm themselves (Table 11).111a,112,112a,113,114,115,116 Success of treatment was often not judged on the basis of an observed
quantitative change in the status of autoimmunity but rather on the rate
of pregnancy following treatment. However, in retrospectively analyzing
the pregnancy outcome of 108 women whose mates were found to have
autoimmunity to sperm but were not treated during a 2-year period, spontaneous
conception rates varied with the proportion of sperm antibody
coated.115 When more than 50% of sperm were bound with immunoglobulins, 22% of women
conceived, whereas 45% conceived when less than 50% of sperm in the
ejaculate were immunoglobulin coated. The results were even more distinct
in couples whose sole cause of infertility was the man's autoimmunity
to sperm. Here, pregnancy rates were 15.6% if the majority (more
than 50%) of sperm were antibody coated and rose to 63% if less
than 50% of sperm were bound. Given these well-documented spontaneous
conception rates, depending on the extent of autoimmunity to sperm, the
use of pregnancy as a validation of treatment is misleading, without
adequate placebo controls. TABLE 11. Pregnancy Rates After Corticosteroid Treatment of Men with Immunity
to Spermatozoa
| | Number of Pregnancies (%) |
Investigators and Citations | Treatment | Number of Men Treated |
DeAlmeida M, Jouannet P116 | Dexamethasone, 2 or 3 mg/ | 3/14 (21%) |
| day × 9–13 weeks, then 7 | |
| weeks taper | |
Hendry W et al111a | Methylprednisone, 96 mg/day | 14/45 (35%) |
| × 7 days starting on CD* | |
| 21 of wife's menstrual | |
| cycle | |
Hargreave T, Elton RA112a | Methylprednisone, 96 mg/day | 5/13 (38%) |
| × 7 days starting on CD 21 | |
| of wife's menstrual cycle | |
Shulman S, Shulman J112 | Methylprednisone, 96 mg/day | 31/71 (44%) |
| × 7 days starting on CD 21 | |
| of wife's menstrual cycle | |
Hendry W et al113 | Prednisolone, 20 or 40 mg | 25/76 (33%) |
| twice a day on CD 1 of | |
| wife's menstrual cycle; | |
| then 5 mg/day on CD 11 | |
| and 12 for 9–12 cycles | |
Alexander NJ, Sampson | Prednisolone, 20 mg 3 times/ | 7/19 (37%) |
JH, Fulgham DL114 | day | |
Ayvaliotis B et al115 | No treatment over 2 years | (21.8%)† |
| retrospective period of | (43.4%)‡ |
| observation | |
* CD = cycle day
† Greater than 50% of sperm antibody coated as determined by direct
immunobead binding.
‡ Less than 50% of sperm antibody coated.
A single study has documented a variable suppression of antisperm antibodies
within seminal plasma in some men treated with corticosteroids.116 A single study has also reported changes in the amount of antibody bound
to sperm.117 Whether the degree of suppression of autoimmunity in these cases would
be sufficient to increase the number of antibody-free sperm in the ejaculate
to a clinically significant level remains unproven. Given the
side-effects of corticosteroids, such as mood changes, leg muscle cramps, hypertension, reactivation of ulcers, alterations of glucose tolerance, and
rare but severe aseptic hip joint necrosis, one must currently
remain conservative about their use pending further well-controlled
studies. A rationale for IUI is to place within the uterine cavity a large population
of living sperm that were excluded after coitus because of the presence
of antisperm antibodies either on their surface or within cervical
mucus. in theory, this would increase the likelihood that sperm might
enter the fallopian tubes and reach the egg. Using careful sonographic
and hormonal monitoring of follicular maturation, insemination can
be timed to within a few hours of the expected ovulation. The accuracy
of timing is theoretically important in that antibody-bound spermatozoa
have a shortened survival time within the female reproductive tract. Pregnancy
rates have varied in different clinical series over the
range of 20% to 40%. Conception occurred rapidly within four to six cycles, as
in the case of women without antisperm antibodies,118,119 and appeared to be higher in gonadotropin-stimulated cycles (Table 12).83,120 These studies have been difficult to interpret, unfortunately, because
the diagnosis of immune-mediated infertility is often unclear. Hence, the
association of circulating antisperm antibodies in sera of women, in
conjunction with impaired sperm survival within cervical mucus, is
not necessarily proof of a cause-and-effect relationship. The clinical
data do suggest, however, that this approach should be attempted before
IVF. In addition, given the relatively low conception rates, these
results suggest that antibody-coated sperm may not always reach the fallopian
tubes after IUI, despite their placement high within the uterine
fundus. Alternatively, they may be unable to fertilize, despite successfully
meeting the egg at the site of fertilization, because of the
presence of antisperm antibodies in tubal secretions. TABLE 12. Conception Rates Per Cycle Following IUI in Women with Humoral
Antisperm Antibodies and Impaired Postcoital Tests*
| Number of Pregnant/Number of Inseminated (% Pregnant) |
| | | Human Menopausal |
Cycle Number | Natural Cycle | Clomiphene Citrate | Gonadotropins |
1 | 4/67 | 2/25 | 5/28 |
| (6%) | (8%) | (18%) |
2 | 5/62 | 4/23 | 1/20 |
| (8%) | (17%) | (5%) |
3 | 0/47 | 0/14 | 3/15 |
| | | (20%) |
4 | 0/38 | 0/9 | 1/11 |
| | | (9%) |
5 | 0/24 | 0/7 | 1/8 |
| | | (12%) |
6–10 | 0/47 | | 0/20 |
* Fewer than 5 motile sperm per high-power field
(Adapted from Margalioth EJ, Sauter E, Bronson RA et al: Intrauterine insemination
as treatment for antisperm antibodies in the female. Fertil
Sterile 50:444, 1988. Reproduced with permission of the publisher, The
American Fertility Society.)If IUI fails, IVF currently appears to offer the best chance of conception
in couples with documented immunities to sperm. Antisperm antibodies
within follicular fluid can be removed by washing the cumulus oocycte
complex, and any residual immunoglobulins that may remain within the
cumulus oophorus that surrounds the egg do not usually appear to interfere
with sperm penetration. IVF in women with antisperm antibodies
has been anecdotally reported to be successful at rates comparable to
those in their absence.121,122,123 However, a more critical examination of the results of IVF in women with
immunities to sperm has now revealed a diminished fertilization rate, embryonic
cleavage rate, and ultimate pregnancy rate.124 This study used bovine serum albumin (BSA) in the culture medium in lieu
of the patient's serum if significant levels of antisperm antibodies
were present. Fertilization and embryo cleavage rates, as well
as pregnancy rates, were compared with those in women in the absence of
antibodies, but also in the presence of BSA. In the immune infertile
group, 44% of eggs (251 of 569) were fertilized in 50 cycles, versus 74.2% and 75.1% for
the maternal serum and BSA controls, respectively. The
percent of high-quality embryos was 49% for the immunologic infertile
group versus 78% and 74% for the controls. Ten clinical pregnancies
occurred in the former group versus 18 and 19 in the latter. Although
promising in that they illustrate that significant pregnancy rates
can be achieved in these couples despite the presence of antisperm antibodies, these
results suggest that either these antibodies cannot be
eliminated from the egg by washing, hence impairing fertilization, or
that they may be egg cytotoxic. In men with autoimmunity to sperm, the diminished number of antibody-coated
sperm within cervical mucus after coitus markedly lowers the chances
that the gametes will meet. IVF circumvents this problem of sperm
transport and ensures the meeting of spermatozoa and egg. In contrast
to women with immunities to sperm. however. in whom follicular fluid containing
antisperm antibodies can be washed from the egg, immunoglobulins
in the ejaculates from men with autoimmunity to sperm remain bound
to the sperm surface after their recovery from seminal fluid.125 Although antibodies present on the sperm tail do not appear significantly
to prevent fertilization in vitro, sperm head-directed antibodies
have the potential to alter the spermatozoan egg-penetrating ability, as
previously described in both the hemizona assay and the zona-free hamster
egg penetration test. Fortunately, these effects only become apparent
when more than 75% of the sperm population used in IVF are coated
with immunoglobulins (Table 13).126,127 If more than one quarter of sperm recovered in semen for IVF are free
of antisperm antibodies, the likelihood that fertilization rates will
not be impaired is high. Results also depend on the sperm antigen to which
these antibodies are directed. In other words, should the antibodies
be directed against antigens that have no role in fertilization, the
process of penetration by the spermatozoon would not be impaired. This
has clearly been documented after the generation of antisperm monoclonal
antibodies, under experimental conditions.128,129 Unfortunately, no clinical test can predict this outcome before an actual
attempt at IVF. As a technique to minimize the amount of antibody
binding on sperm after ejaculation, it appears to be desirable clinical
practice to produce the semen specimen directly on hospital premises, requesting
ejaculation directly into a washing buffer.130 This appears beneficial in terms of both maximizing sperm recovery and
minimizing the amount of antibody coating sperm. Finally, the number
of spermatozoa placed in culture with eggs for IVF can also be increased, in
an attempt to increase the likelihood of fertilization.125,131 Because the percent of eggs fertilized by antibody-coated sperm may be
diminished, the use of hormonal stimulus protocols leading to the formation
of a greater number of mature follicles yielding fertilizable eggs
would also increase the likelihood that some of these eggs would be
fertilized. In those instances in which spermatozoa failed to attach
to and penetrate the zona pellucida, preliminary studies have suggested
that fertilization may be successful by partial zona dissection132 or subzonal microinjection133 of antibody-labeled sperm into the perivitelline space surrounding the
egg. However, it should be emphasized that the effectiveness and safety
of these procedures still need to be clearly documented.134,135 TABLE 13. Effect of Antisperm Antibodies on In Vitro Fertilization Rates
in 32 Men with Autoimmunity to Spermatozoa
| Percent Spermatozoa Coated with Immunoglobulin* |
| >80% IgA & IgG | >80% IgA | >80% IgG | <80% IgA & IgG |
Percent Eggs Fertilized | 25% (102) | 30% (10) | 89% (29) | 73% (75) |
(No. of Eggs Inseminated) | | | | |
*As determined by direct immunobead binding.
(Adapted from DeAlmeida M, Gazagne I, Jeulin C et al: In vitro processing
of sperm with auto-antibodies and in vitro fertilization results. Hum
Reprod 4:49, 1989 and Clarke GN, Lopata A, McBain JC et al: Effect
of sperm antibodies in males in human in vitro fertilization (IVF). Am
J Reprod Immunol 8:62, 1985) |
 4)
4) 256
256