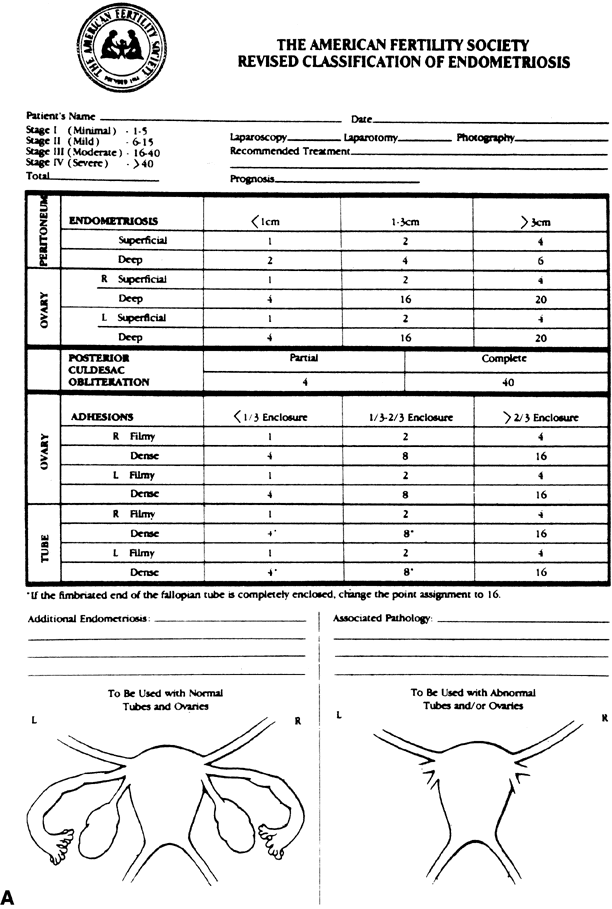
Pharmaceutical therapeutic options for endometriosis continue to expand
rapidly. Six major options are currently available: danazol, nafarelin, leuprolide, goserelin, progestins, and combined estrogen-progestin
therapy. The efficacy of these agents in the treatment of endometriosis
is attributed to the steroid responsiveness of endometriosis implants. The
majority of human endometriosis implants contain estrogen receptors, androgen
receptors, and progesterone receptors.39,40 Clinical and laboratory observations demonstrate that estrogen is critical
to the growth and function of endometriotic implants.41 Clinical observations suggesting that estrogen is critical to the growth
of endometriosis include the findings that endometriosis rarely occurs
before menarche; menopause, either surgical or natural, usually produces
an improvement in preexisting endometriosis; and new cases of endometriosis
occur very rarely after menopause unless exogenous estrogen
is administered. As discussed below, there is no evidence that medical treatment of endometriosis
in infertile women improves fecundity. A meta-analysis by Hughes
and colleagues included 7 studies comparing medical therapy to no
treatment or placebo.42 The common odds ratio for pregnancy following medical therapy was 0.85 (95% CI, 0.95 to 1.22). However, hormonal treatment is extremely effective
in reducing pelvic pain associated with endometriosis. For infertile
women with endometriosis accompanied by dyspareunia, dyschezia, dysmenorrhea, or
nonspecific pelvic pain, hormonal treatment may improve
sexual function as well as the frequency of sexual relations, thereby
increasing fecundity. In addition, hormone therapy may be of value in
the postoperative treatment of infertile women with endometriosis considering
in vitro fertilization/embryo transfer(IVF/ET).43,44 Danazol Danazol is an isoxazole derivative of ethinyl testosterone (Fig. 2). The pharmacology of danazol is best understood by examining its interaction
with intracellular steroid receptors, circulating steroid-binding
globulins, and enzymes of ovarian steroidogenesis. Danazol binds to three classes of intracellular steroid receptors: the
androgen receptor, the progestin receptor, and the estrogen receptor45 (Table 2). Danazol has high affinity for the androgen receptor, moderate affinity
for the progestin receptor, and poor affinity for the estrogen receptor.46 Danazol is both an androgen and estrogen agonist. Although danazol is
an estrogen agonist, the affinity of danazol for the estrogen receptor
is so poor that for clinical purposes danazol has no major effect on
the estrogen receptor system. In contrast, because danazol is an androgen
agonist with a very high affinity for the androgen receptor, danazol
has potent androgenic effects in vivo. Many of danazol's side
effects can be attributed to its androgenic properties. These side effects
include the following: (1) a decrease in high-density lipoprotein
cholesterol; (2) an increase in low-density lipoprotein cholesterol; (3) a
decrease in hepatic production of sex hormone-binding globulin (SHBG) and
thyroxine-binding globulin; and (4) an increase in bone density. The
effects of danazol on the intracellular progestin receptor are
complex. In some model systems, danazol can produce atypical secretory
changes in estrogen-primed endometrium, suggesting that danazol is
a progestin agonist.47 In other model systems, danazol blocks the effects of progesterone, suggesting
that danazol is a progestin antagonist. Danazol is best regarded
as a mixed progestin agonist-antagonist. Table 2. Danazol Interaction with Intracellular Androgen, Progestin, and
Estrogen Receptors and Circulating Binding Globulin
| Affinity of Danazol | Biologic Effect |
| for Receptor | of Danazol |
Class of Intracellular | | |
Receptors | | |
Androgen | High | Agonist |
Progestin | Moderate | Mixed agonist- |
| | antagonist |
Estrogen | Very poor | No effect |
Circulating Binding | | |
Globulins | | |
SHBG | High | Increases free |
| | testosterone |
CBG | Moderate | Minimally |
| | increases free |
| | cortisol |
CBG, corticosteroid-binding globulin; SHBG, sex hormone-binding globulin.
Danazol has high affinity for SHBG and can displace testosterone and dihydrotestosterone
from SHBG.48 The observation that danazol increases free and bioavailable testosterone
suggests that part of its androgenic effects may be from the displacement
of testosterone and dihydrotestosterone from SHBG. Danazol inhibits multiple enzymes of ovarian steroidogenesis, including
cholesterol side-chain cleavage enzyme, 3β-hydroxysteroid dehydrogenase-isomerase, 17-ketosteroid reductase, 17α-hydroxylase, 17,20-1yase, and
aromatase.49 Experiments using monkey models and human subjects suggest that danazol
inhibits ovarian estrogen production by a direct effect on ovarian steroidogenesis.50,51 The pharmacologic properties of danazol create an acyclic, high-androgen, low-estrogen
environment that is hostile to the growth of endometriotic
tissue. Danazol produces a high-androgen environment because it is
inherently androgenic and because it decreases the hepatic production
of SHBG and displaces testosterone from SHBG, resulting in an increase
in free testosterone. By direct actions on the ovaries and the hypothalamic-pituitary
axis, danazol produces a low-estrogen environment by
inhibiting follicular growth. Acting through hypothalamic and pituitary
androgen and progestin receptors, danazol decreases LH and FSH secretion. By
inhibiting multiple enzymes of ovarian steroidogenesis, danazol
decreases ovarian estrogen production. The low-estrogen, high-androgen
environment created by danazol is hostile to the growth of endometriotic
tissue. In addition, the acyclic endocrine environment produced
by danazol results in amenorrhea and prevents the reseeding of the
peritoneum with new implants of endometrium. The major side effects of danazol are weight gain, bloating, decreased
breast size, ache, oily skin, hirsutism, headache, deepening of the voice, hot
flashes, and muscle cramps.52 In women receiving 800 mg/day of danazol, the weight gain averages 4 kg. Approximately 80% of
women receiving danazol will report one or more
of the above side effects. Danazol can produce a large number of biochemical
abnormalities including mild increases in serum levels of creatine
phosphokinase, lactate dehydrogenase, glutamic-oxaloacetic transaminase, and
glutamic-pyruvic transaminase. These typically are minor, clinically
insignificant alterations. Danazol therapy produces marked
alterations, however, in the circulating lipid profile. At doses of 600 mg/day, danazol
produces a 50% decrease in high-density lipoprotein
cholesterol and a 40% increase in low-density lipoprotein cholesterol. These
lipid alterations resolve within 3 months of discontinuation of
danazol therapy. The clinical impact of this atherogenic lipid profile
in women of reproductive age is unclear. Seibel and co-workers and Bayer and colleagues conducted prospective, controlled, randomized
studies of the effectiveness of danazol in the treatment
of infertility associated with minimal endometriosis.8,53 Couples who had 1 year of infertility were eligible for entry into the
study after they completed a basic infertility evaluation, which included
semen analysis, basal body temperature chart, postcoital test, hysterosalpingogram, and
endometrial biopsy. Patients (n = 73) then underwent
a diagnostic laparoscopy and tubal lavage. If Kistner stage I endometriosis
was observed, the female patients were randomized to receive
danazol (n = 37) or no treatment (n = 36).54 Women who were randomized to the danazol treatment group received an initial
danazol dosage that tapered off incrementally after the first 2 months, as
follows:danazol 800 mg/day for 2 months, 600 mg/day for 2 months, and 400 mg/day
for 2 months. Patients were then observed for 12 months, beginning
either immediately after laparoscopy in the untreated
group or after completion of danazol therapy in the treated group. During
the 12 months of follow-up, 35% of the danazol-treated women and 47% of
the untreated group became pregnant; this difference was not
statistically significant (Fig. 3). In conclusion, treatment of women with mild endometriosis with danazol
did not improve fecundity. In a randomized study comparing gestrinone
with placebo, Thomas and Cooke observed similar results.55 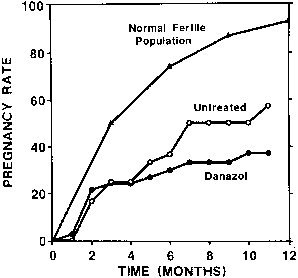 Fig. 3. Cumulative pregnancy rates in danazol-treated and untreated patients with
minimal endometriosis. The cumulative pregnancy rate in an ideal fertile
population is also shown for comparison.(Bayer SR, Seibel MM, Saffan DS et al: The efficacy of danazol treatment
for minimal endometriosis in infertile women: A prospective randomized
study. J Reprod Med 33:179, 1988) Fig. 3. Cumulative pregnancy rates in danazol-treated and untreated patients with
minimal endometriosis. The cumulative pregnancy rate in an ideal fertile
population is also shown for comparison.(Bayer SR, Seibel MM, Saffan DS et al: The efficacy of danazol treatment
for minimal endometriosis in infertile women: A prospective randomized
study. J Reprod Med 33:179, 1988)
|
Gonadotropin-Releasing Hormone Analogs: Nafarelin, Leuprolide, Goserelin Gonadotropin-releasing hormone (GnRH) is a hypothalamic decapeptide (Fig. 4) that controls the pituitary secretion of LH and FSH. In the follicular
phase of the menstrual cycle, the hypothalamus releases one pulse of
GnRH per hour into the portal circulation. The ability of GnRH to stimulate
LH and FSH secretion is critically dependent on the frequency and
amplitude of the GnRH pulse. If the GnRH pulse frequency is chronically
slow or too fast, the pituitary will not secrete LH and FSH in response
to the GnRH pulse. A continuous infusion of GnRH, through molecular
mechanisms that are poorly understood, markedly decreases pituitary
production of LH and FSH. 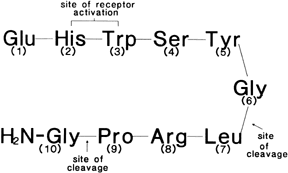 Fig. 4. Chemical structure of the hypothalamic decapeptide gonadotropin-releasing
hormone (GnRH). Amino acids 2 and 3 are important for receptor activation. Degradation
of GnRH occurs by cleavage of the decapeptide between
amino acids 5–6 and 6–7.(Friedman AJ: Leuprolide acetate: Applications in gynecology. Curr Probl
Obstet Gynecol Fertil 11:209, 1988) Fig. 4. Chemical structure of the hypothalamic decapeptide gonadotropin-releasing
hormone (GnRH). Amino acids 2 and 3 are important for receptor activation. Degradation
of GnRH occurs by cleavage of the decapeptide between
amino acids 5–6 and 6–7.(Friedman AJ: Leuprolide acetate: Applications in gynecology. Curr Probl
Obstet Gynecol Fertil 11:209, 1988)
|
Because much of the information in the GnRH signal is contained in the
pulse frequency, GnRH has a very short half-life (4 minutes). GnRH is
metabolized by endopeptidases present in the pituitary circulation and
pituitary gonadotropes, which cleave the 5–6 and 6–7 peptide
bonds. By chemically altering the sixth amino acid, GnRH analogs that
are resistant to cleavage by endopeptidases can be produced. These
GnRH analogs have a long half-life (2 to 4 hours) and are perceived by
the pituitary as a continuous infusion of GnRH. Consequently, chronic
administration of these GnRH analogs decreases LH and FSH secretion
and causes a cessation of ovarian estrogen production. GnRH analogs currently available include: leuprolide, nafarelin, and goserelin. Leuprolide
is administered as a monthly depot injection. Nafarelin
is administered in the form of a nasal spray and goserelin as a
monthly subcutaneous implant. The doses of nafarelin, leuprolide, and
goserelin necessary to reduce circulating estradiol to 15 pg/mL or 30 pg/mL
are listed in Table 3. Table 3. Dose Effects of Leuprolide, Nafarelin, and Goserelin
| | Estradiol Concentration |
Agent | Dose | Produced (pg/mL) |
Leuprolide | 3.75-mg depot | 15 |
| intramuscularly | |
| q 4 weeks | |
Goserelin | 3.6-mg implant | 15 |
| q 4 weeks | |
Nafarelin | 400 μg intranasally | 15 |
| bid | |
| 200 μg intranasally | 30 |
| bid | | In a large randomized clinical trial comparing the efficacy of danazol
and nafarelin in the treatment of endometriosis, Henzl and co-workers
observed that both drugs produced symptomatic improvement in 80% of subjects
and noted a 43% improvement in the rAFS endometriosis score56 (Fig. 5). Most of the side effects associated with nafarelin therapy (e.g., hot
flashes, headaches, mood changes, decreased libido, vaginal dryness) were
attributed to hypoestrogenism. Recent observations indicate that
GnRH analogs can produce a decrease in vertebral bone density. For example, Dawood
and colleagues reported that 6 months of GnRH analog therapy
with buserelin (1200 μg/day intranasally) resulted in a 7% decrease
in trabecular bone density.57 Six months after completing GnRH agonist therapy, the trabecular bone
density remained 4% below pretreatment values. Similar losses in trabecular
bone density during GnRH agonist therapy have been reported with
the use of nafarelin and buserelin.58,59 Interestingly, Matta and associates reported that the bone loss was completely
reversible once the GnRH analog was discontinued.59 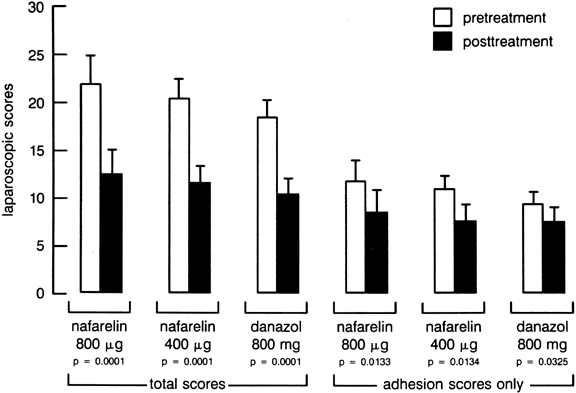 Fig. 5. Pretreatment and post-treatment American Fertility Society endometriosis
scores in women receiving 6 months of intranasal nafarelin or oral danazol
therapy.(Henzl MR, Corson SL, Moghissi K et al: Administration of nasal nafarelin
as compared with oral danazol for endometriosis: A multicenter double-blind
comparative trial. N Engl J Med 318:485, 1988) Fig. 5. Pretreatment and post-treatment American Fertility Society endometriosis
scores in women receiving 6 months of intranasal nafarelin or oral danazol
therapy.(Henzl MR, Corson SL, Moghissi K et al: Administration of nasal nafarelin
as compared with oral danazol for endometriosis: A multicenter double-blind
comparative trial. N Engl J Med 318:485, 1988)
|
In a recently reported controlled trial, Fedele and associates randomized 71 women
with rAFS stage I or II endometriosis either to receive hormonal
treatment with the GnRH agonist analog buserelin (400 μg 3 times
a day for 6 months) or to receive no treatment.60 Median follow-up was 17 months in the buserelin group and 18 months in
the control group, The 1-year actuarial pregnancy rate was similar in
both groups: buserelin, 30%; control, 37%. The 2-year actuarial pregnancy
rate was also similar in both groups: buserelin, 61%; control, 61%. This
study suggests that hormonal treatment of rAFS stage I or II endometriosis
with buserelin does not improve fecundity. Progestin-Only Regimens Numerous progestins (e.g., medroxyprogesterone acetate, norethindrone acetate, norgestrel
acetate, lynestranol) have been used as single agents
for the treatment of endometriosis. These agents produce a hypoestrogenic
hormonal environment by suppressing ovarian estrogen production
by inhibition of LH and FSH secretion. In addition, these agents have
a direct antiestrogenic effect on endometriotic tissue by binding to
intracellular progesterone and androgen receptors. Oral administration of medroxyprogesterone acetate (30 to 50 mg/day) is
effective in the treatment of endometriotic lesions.61 Side effects associated with this therapy include bloating, weight gain, depression, and
irregular uterine bleeding. Parenteral medroxyprogesterone
acetate should not be used for the treatment of endometriosis
in infertile women because it can produce prolonged amenorrhea after termination
of therapy. Despite the longstanding use of progestational agents in the treatment
of endometriosis, only three studies have reported the effects of these
medications on subsequent fertility. In one study, 9 months of treatment
with dydrogesterone resulted in a 53% cumulative pregnancy rate in 19 women.62 Another study utilizing 3 months of oral medroxyprogesterone acetate reported 12 pregnancies
in 26 patients (46%).63 In women with mild and moderate disease, the cumulative pregnancy rate
was 55%; however, neither of the above studies was controlled. Hull and associates reported a controlled comparative trial with oral medroxyprogesterone, danazol, and expectant management in women with stage
I and II endometriosis.64 On the basis of life table analysis, pregnancy rates in the three groups, respectively, were 71%, 46%, and 55%, and there were no significant
differences among the treatment groups. This study suggests that progestational
therapy is no more effective than expectant management in
the infertile patient with endometriosis. Combined Estrogen-Progestin Regimens In 1959, Kistner reported that combined estrogen-progestogen contraceptives
were effective in the treatment of endometriosis when used in a continuous “pseudopregnancy” regimen.65 Long-term administration of a combination of estrogen and a progestin
results in suppression of LH and FSH, resulting in the absence of ovarian
follicular development and decidualization of the endometrium. Pseudopregnancy
regimens produce an acyclic hormone environment but do expose
the endometriotic lesions to a significant amount of estrogen. Although
most synthetic progestins have sufficient androgenic and progestational
activity to block the effects of co-administered estrogen, in
some patients the administered estrogen actually stimulates metabolic
activity in endometriotic lesions. Therefore, the use of pseudopregnancy
for women with severe endometriosis is not recommended. One group of patients may be especially suitable candidates for pseudopregnancy
treatment. Young women with severe, incapacitating dysmenorrhea
and minimal or mild endometriosis usually report marked improvement
in their pain during “mini-pseudopregnancy” therapy. A typical
mini-pseudopregnancy regimen consists of 15 weeks of continuous
estrogen-progestin therapy (5 packages of birth control pills), followed
by a 1-week withdrawal of hormone therapy, again followed by 15 weeks
of continuous estrogen-progestin therapy, and so on. This is continued
as long as pain relief is maintained.66 In a recent randomized, controlled trial, an estrogen-progestin regimen
was compared with a GnRH analog regimen for the treatment of pelvic pain
caused by endometriosis.67 The GnRH analog was more efficacious than the estrogen-progestin regimen
in reducing dyspareunia. The estrogen-progestin and GnRH analog regimens
were equally efficacious in reducing dysmenorrhea. Because the estrogen-progestin
regimen is much less expensive than any GnRH analog
regimen, it may offer an attractive cost-benefit ratio for the treatment
of dysmenorrhea caused by endometriosis. Unfortunately, there were
no randomized-controlled trials assessing the efficacy of oral contraceptive
therapy in women with endometriosis-associated infertility. |