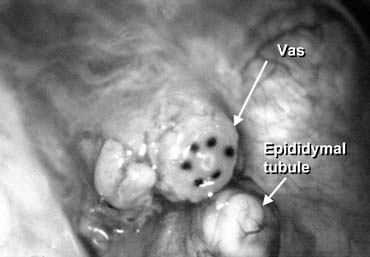
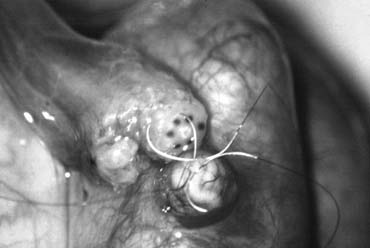
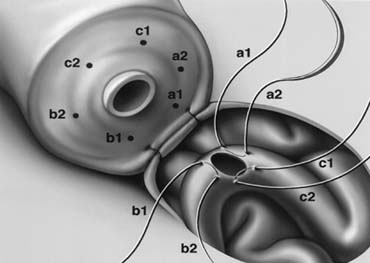
Varicocele is by far the most commonly performed operation for the treatment
of male infertility. Dilation of venous pampiniform plexus is secondary
to defective or absence of valves in the internal spermatic veins. This
is especially common on the left side, where the internal spermatic
vein drains directly into the renal vein. Dilatation of the venous
structures results in impaired counter current heat exchange and
increased testicular temperature. Testicular temperature is elevated by
almost 2 °C in men with varicoceles compared with controls.77 Both spermatogenesis and Leydig cell function for hormonal production
are impaired at this elevated temperature. In addition, poor venous drainage
may lead also to hypoxia and increased testicular pressure that
may be detrimental to normal testicular function. Finally, elevated spermatic
venous catecholamines, possibly from adrenal reflux, may contribute
to the negative impacts of varicoceles on testicular function. Varicocele is found in approximately 15% of the general population, 35% of
men with primary infertility, and in 70% to 81% of
men with secondary infertility.23,78 Animal and human studies have demonstrated that varicocele is associated
with a progressive and duration-dependent decline in testicular
function.17,18,20,22,23,78,79,80,81 A careful physical examination is the key to diagnose varicoceles. Before
the examination, the patient should be relaxed. If the examination
room is cold, a heating pad can be used to keep the scrotum warm and relaxed
to allow a proper examination. In grade I varicocele, an impulse
can be palpated in the scrotum during a Valsalva maneuver. A grade II
varicocele is large enough for tortuous and dilated veins to be palpated
without a Valsalva maneuver. Grade III varicocele is visible through
the scrotal skin. Color Doppler ultrasound can be used to confirm
the diagnosis. Typically, when more than two to three veins of 3 mm or
greater in size were found, with enlargement on standing and reflux on
Valsalva maneuver, the diagnosis is made. Varicoceles that are impalpable
on a good physical examination are considered subclinical and probably do not warrant treatment.81 Repair of varicocele will halt any further damage to testicular function79 and, in a large percentage of men, result in improved spermatogenesis,82 as well as enhanced Leydig cell function.26 Table 2 summarizes the pros and cons of various methods of varicocele repair. Retroperitoneal Operations Retroperitoneal repair of varicocele involves incision at the level of
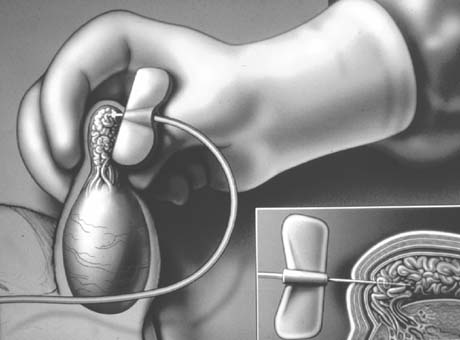
the internal inguinal ring (Fig. 40A), splitting of the external and internal oblique muscles (see Fig. 40B), and exposure of the internal spermatic artery and vein retroperitoneally
near the ureter (see Fig. 40C). This approach has the advantage of isolating the internal spermatic
veins proximally, near the point of drainage into the left renal
vein. At this level, only one or two large veins are present and, in addition, the
testicular artery has not yet branched and is often distinctly
separate from the internal spermatic veins. Retroperitoneal approaches
involve ligation of the fewest number of veins. This approach is
still a commonly employed method for the repair of varicocele, especially
in children. 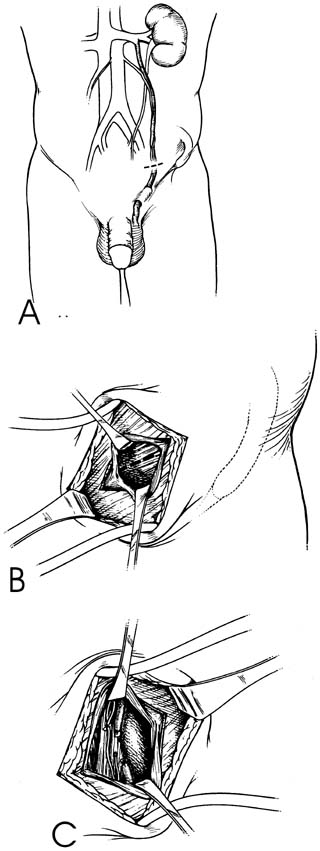 Fig. 40. Retroperitoneal varicocelectomy. Fig. 40. Retroperitoneal varicocelectomy.
|
A disadvantage of a retroperitoneal approach is the high incidence of varicocele
recurrence, especially in children and adolescents, when the
testicular artery is intentionally preserved. Recurrence rates after
retroperitoneal varicocelectomy are in the range of 15%.83,84 Failure is usually caused by preservation of the periartertial plexus
of fine veins (venae comitantes) along with the artery. These
veins have been shown to communicate with larger internal spermatic
veins. If left intact they may dilate with time and cause recurrence. Less
commonly, failure is caused by the presence of parallel inguinal
or retroperitoneal collaterals, which may exit the testis and bypass the
ligated retroperitoneal veins rejoining the internal spermatic vein
proximal to the site of ligation.85,86 Dilated cremasteric veins, another cause of varicocele recurrence,87 cannot be identified with a retroperitoneal approach. Positive identification
and preservation of the .5- to 1.5-mm testicular
artery via the retroperitoneal approach is difficult, especially in children
in whom the artery is small. Because at this level the internal
spermatic vessels cannot be delivered into the wound, the operation
involves working in a deep hole to dissect and ligate the vessels in situ in the retroperitoneum. In addition, the difficulty in positively identifying
and preserving lymphatics using this approach results in postoperative
hydrocele formation in 7% to 33% of retroperitoneal
operations.88 The incidence of recurrence appears to be higher in children, with rates
reported between 15% and 45% in adolescents.89,90,91,92,93,94 Kass95 reports that recurrence can be markedly reduced in children and adolescents
by intentional ligation of the testicular artery. This assures ligation
of the periarterial network of fine veins. Although reversal of
testicular growth failure has been documented with intentional testicular
artery ligation at the time of retroperitoneal repair in children, the
effect of artery ligation on subsequent spermatogenesis is uncertain. In
adults bilateral artery ligation has been documented to occasionally
cause azoospermia and testicular atrophy. At the least, it is
inarguable that testicular artery ligation will not enhance testicular
function. Laparoscopic Varicocelectomy Laparoscopic repair is, in essence, a reproperitoneal approach and many
of the advantages and disadvantages are similar to those of the open
retroperitoneal approach.96,97,98,99,100,101 Using the laparoscope, the internal spermatic vessels and vas deferens
can be clearly visualized through the laparoscope as they course through
the internal inguinal ring. The magnification provided by the laparoscope
allows visualization of the testicular artery. With experience, the
lymphatics may be visualized and preserved as well. With laparoscopic
varicocelectomy, the internal spermatic veins are ligated at the
same level as the retroperitoneal (Palomo) approach described. Laparoscopic
varicocelectomy should allow preservation of the testicular
artery in a majority of cases, as well as preservation of lymphatics. The
incidence of varicocele recurrence would be expected to be
similar to that associated with the open retroperitoneal operations. As
in open retroperitoneal operation, these recurrences would be caused
by collaterals joining the internal spermatic vein near its entrance
to the renal vein or entering the renal vein separately. Currently, reported series of laparoscopic varicocelectomy are too small
and the follow-up interval too short to determine the incidence
of recurrence and complications. The potential complications of laparoscopic
varicocelectomy (injury to bowel, vessels or viscera, air
embolism, peritonitis) are significantly more serious than those
associated with the open techniques. Furthermore laparoscopic varicocelectomy
requires a general anesthetic. The microsurgical techniques
described next can be performed using local or regional anesthesia and
employ an incision of 2.5 to 3 cm for unilateral repair. This is equal
to or less than the sum of incisions used for a laparoscopic approach. Furthermore, postoperative pain and recovery from the laparoscopic
technique are the same as those associated with subinguinal varicocelectomy.97 Finally, laparoscopic varicocelectomy takes at least twice as long to
perform and is less cost-effective than open varicocelectomy. Only
in the hands of an experienced laparoscopist, the approach may be
considered a reasonable alternative for the repair of bilateral varicoceles.99 Microsurgical Inguinal and Subinguinal Operations: The Preferred Approaches Inguinal and subinguinal varicocelectomy are currently the most popular
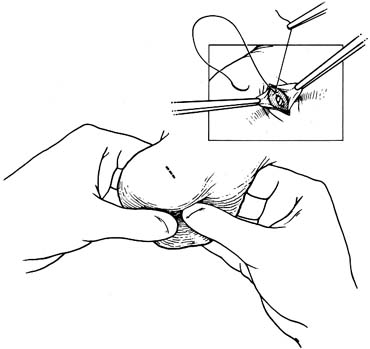
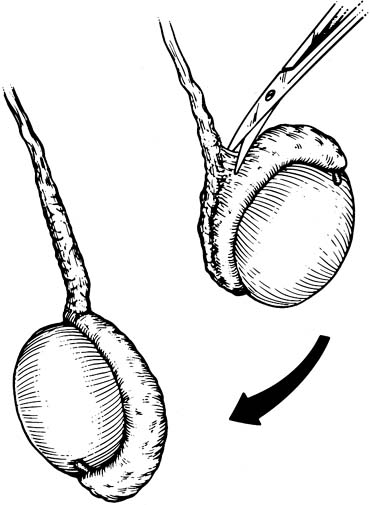
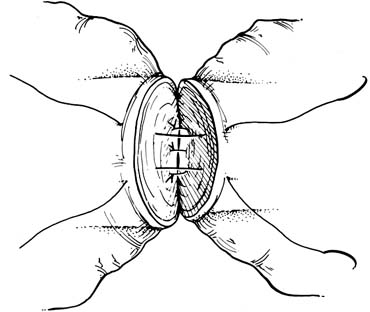
approaches (Fig. 41). They have the advantage of allowing the spermatic cord structures
to be pulled up and out of the wound so that the testicular artery, lymphatics, and
small periarterial veins may be more easily identified. In
addition, an inguinal or subinguinal approach allows access to external
spermatic and even gubernacular veins, which may bypass the spermatic
cord and result in recurrence if not ligated. Lastly, an inguinal
or subinguinal approach allows access to the testis for biopsy or
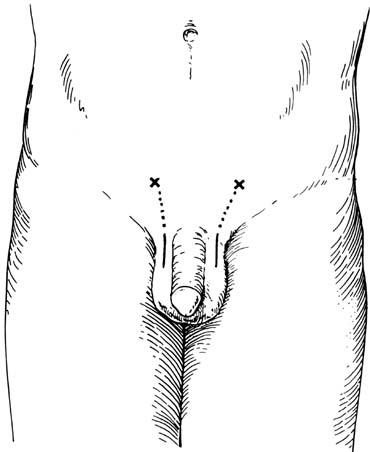
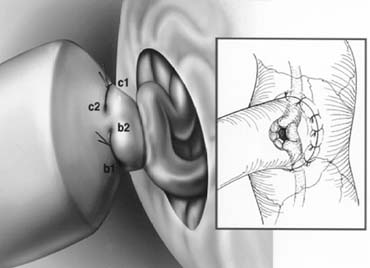
examination of the epididymis for obstruction. 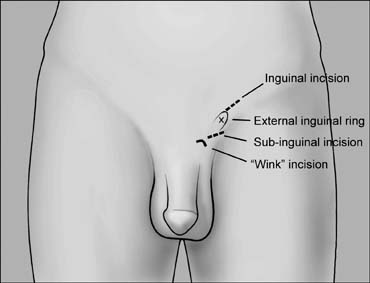 Fig. 41. Inguinal incision beginning at the external inguinal ring (X) and
extending 3 cm laterally along the skin lines. Subinguinal incision
just below the ring. Fig. 41. Inguinal incision beginning at the external inguinal ring (X) and
extending 3 cm laterally along the skin lines. Subinguinal incision
just below the ring.
|
Traditional approaches to inguinal varicocelectomy involve a 5- to 10-cm
incision made over the inguinal canal, opening of the
external oblique aponeurosis, and encirclement and delivery of the spermatic
cord. The cord is then dissected and all the internal spermatic
veins are ligated.92 The vas deferens and its vessels are preserved. An attempt is made to
identify and preserve the testicular artery and, if possible, the lymphatics. In
addition, the cord is elevated, and any external spermatic
veins that are running parallel to the spermatic cord or perforating the
floor of the inguinal canal are identified and ligated. Compared with
retroperitoneal operations, conventional nonmagnified inguinal approaches
lower the incidence of varicocele recurrence but do not alter the
incidence of either hydrocele formation or testicular artery injury. Conventional
inguinal operations are associated with an incidence of
postoperative hydrocele formation varying from 3% to 15% with
an average incidence of 7%.88 Analysis of the hydrocele fluid has clearly indicated that hydrocele formation
after varicocelectomy is caused by ligation of the lymphatics.88 The incidence of testicular artery injury during nonmagnified inguinal
varicocelectomy is unknown. Case reports, however, suggest that this
complication may be more common than realized. It can result in testicular
atrophy and, if the operation is performed bilaterally, azoospermia
may ensue in a previously oligospermic man. Furthermore, Starzl and
his transplant group102 reported a 14% incidence of testicular atrophy and 70% incidence
of hydrocele formation when the spermatic cord was divided and
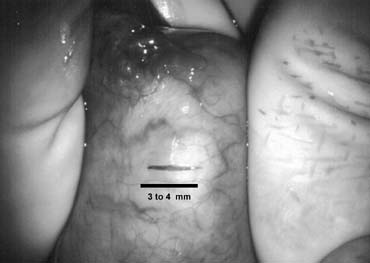
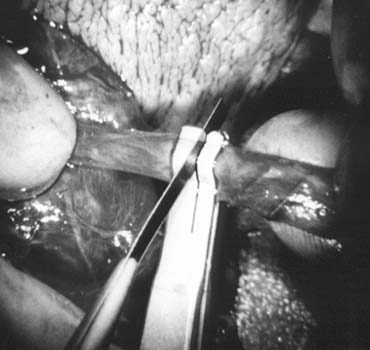
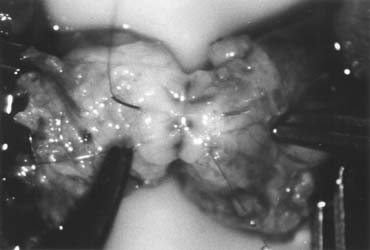
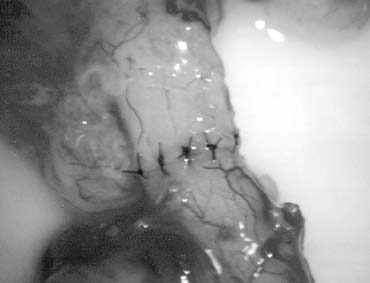
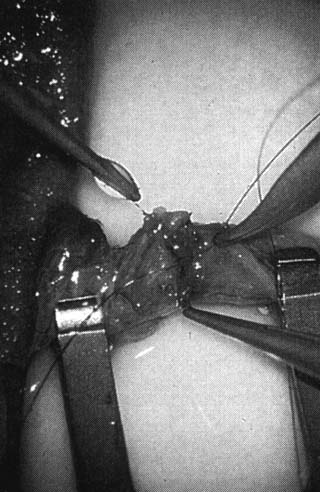
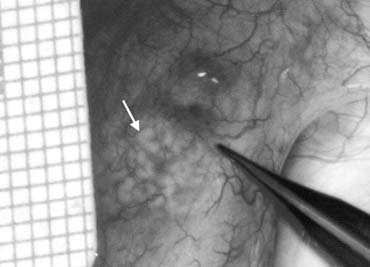
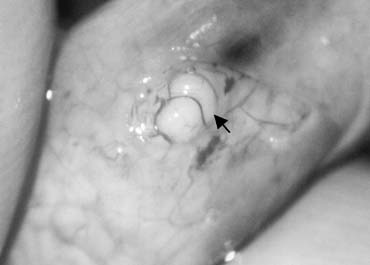
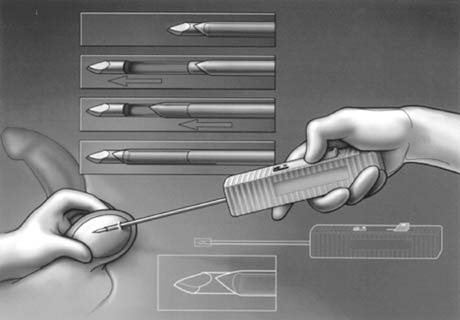
only the vas and vasal vessels preserved. The introduction of microsurgical technique to varicocelectomy (Fig. 42) has resulted in a substantial reduction in the incidence of hydrocele
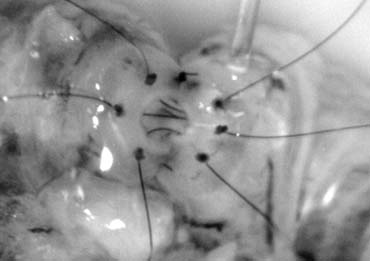
formation.5,103 This is because the lymphatics can be more easily identified and preserved (Fig. 43). Furthermore, the use of magnification enhances the ability to identify
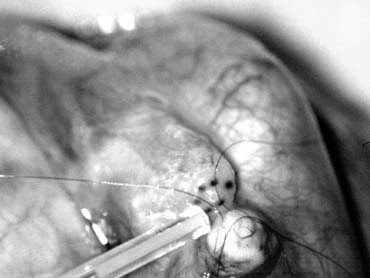
and preserve the .5- to 1.5-mm testicular artery (Fig. 44), thus avoiding the complications of atrophy or azoospermia. 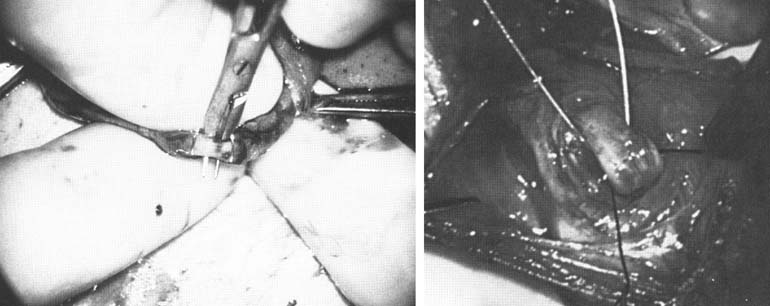 Fig. 42. Internal spermatic veins are cleaned and ligated with either hemoclips
or double 4-0 silks, one black and one white, passed beneath them
before ligation and division. Fig. 42. Internal spermatic veins are cleaned and ligated with either hemoclips
or double 4-0 silks, one black and one white, passed beneath them
before ligation and division.
|
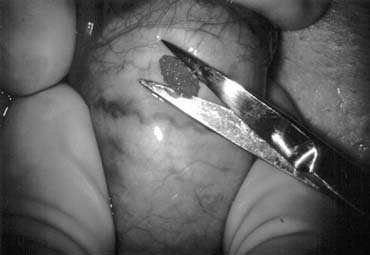
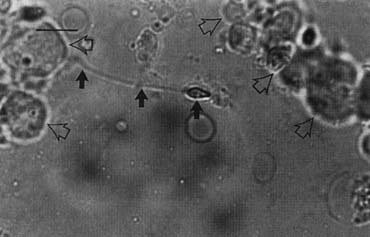
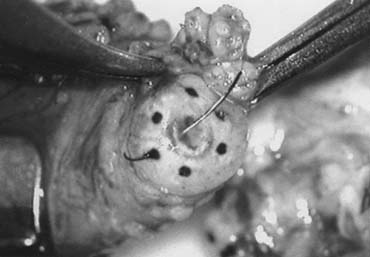
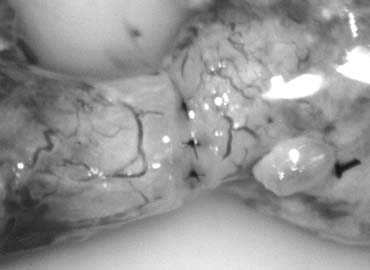
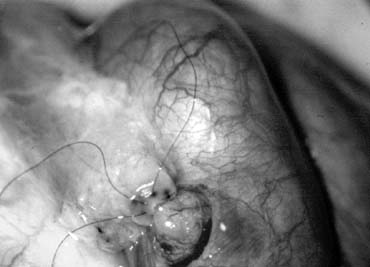
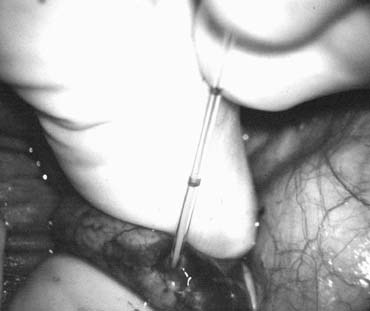
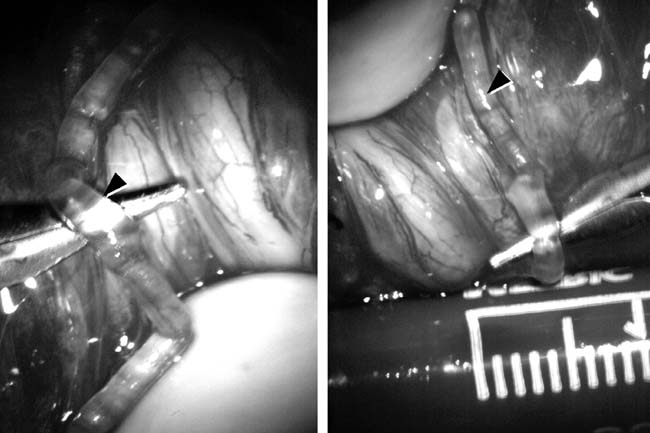 Fig. 43. Lymphatics (arrowheads) are clearly identified and preserved. Fig. 43. Lymphatics (arrowheads) are clearly identified and preserved.
|
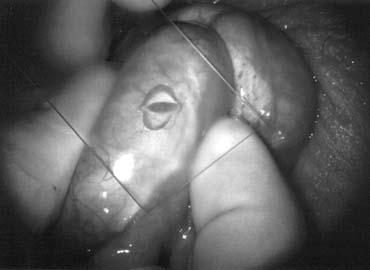
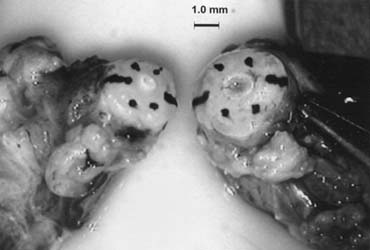
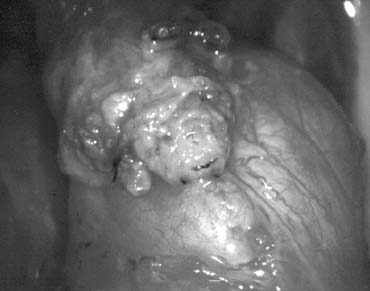
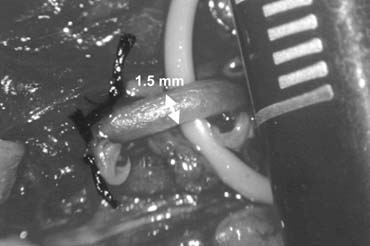 Fig. 44. The testicular artery is identified and tagged with a vessel loop. Fig. 44. The testicular artery is identified and tagged with a vessel loop.
|
Advocates of nonmicrosurgical techniques contend that the deferential (vasal) artery
and, if preserved, the cremateric artery, will
provide adequate blood supply to the testes to prevent atrophy. However
anatomic studies have shown that the diameter of the testicular artery
is greater than the diameter of the deferential artery and cremasteric
artery combined. The testicular artery is the main blood supply to
the testes. At the very least, it is inarguable that ligation of the
testicular artery is unlikely to enhance testicular function. Radiographic Occlusion Techniques Intraoperative venography has been used to visualize the venous collaterals
that, if left unligated, may result in varicocele recurrence.85,90,104,105 Intraoperative venography does reduce the incidence of varicocele recurrence, but
the two-dimensional view afforded often does not enable
the surgeon to identify the location of all collaterals. Percutaneous procedures for varicoceles include the traditional retrograde
occlusion106,107,108 and the more recently described anterograde technique.109,110,111 In the retrograde technique, which can be performed under a local anesthetic, the
right femoral vein is punctured to insert an angiocatheter
to gain access to the internal spermatic vein via the inferior vena cava
and the left renal vein. On confirming the anatomy and the presence
of reflux in the testicular vein, it is occluded in a retrograde fashion (against
the natural direction of the internal spermatic venous
return). Percutaneous occlusion is a suitable treatment option for persistent/recurrent
varicoceles postsurgical repair.112 The use of imaging techniques to identify the cause of varicocele recurrence
allows accurate venous occlusion while eliminating the need for
a difficult dissection of the fibrous adhesions from previous surgery. In
expensive sclerosing agents are commonly used for retrograde occlusion. Newer
embolization techniques, using more expensive materials such
as detachable coils113,114 and occlusive balloons,108 have been described. Complications, including contrast reaction, flank pain, migration of embolizing
materials, infection, thrombophlebitis, arterial puncture, and
hydroceles, occur at a significant rate (9% to 30%).86,112–119,120–122 In addition, the radiographic techniques take between 1 and 3 hours to
perform compared with 25 to 45 minutes required for surgical repair. Venographic
placement of a balloon or coil in the internal spermatic vein
is successfully accomplished in 75% to 90% of attempts;123,124,125 therefore, a significant number of men undergoing attempted radiographic
occlusion will ultimately require a surgical approach. Unsuccessful
cases are commonly seen in right-side varicoceles because of venous
anatomical variations and difficulties in gaining proper venous
access. Hence percutaneous retrograde varicocele occlusion is best used
for isolated left-side varicoceles. The recurrence rate after balloon occlusion was originally 11% and
more recently is reportedly as low as 4%.86,112–119,120–124 Failure to successfully cannulate small collaterals and external spermatic
veins results in recurrence. Percutaneous anterograde varicocele occlusion by injection of sclerosing
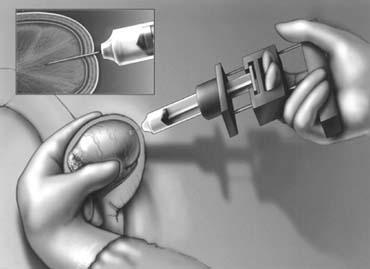
agents into an isolated vein from the pampiniform plexus in the scrotum
after confirming its drainage fluroscopically (Fig. 45) has been described.109,110,111 As with the retrograde procedure, anterograde occlusion can be performed
under local anesthesia. Furthermore, the anterograde technique is associated
with a lower operating time (10 to 15 minutes). Although
the complication rate is only 3% to 8%, testicular
atrophy posttreatment, likely secondary to unidentified arterial injury, has
been reported in 1% of cases.109 While the persistence/recurrence rate for large varicoceles can be
as high as 25%, the overall figure in short-term follow-up
is 5% to 9%. Long-term follow-up, however, is
not available and the consequence of escape of the sclerosing
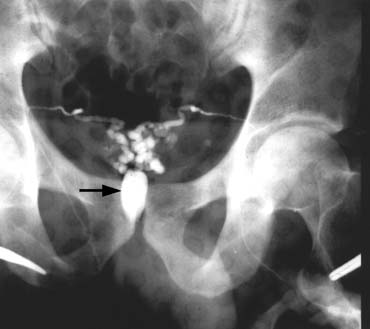
agent into the renal vein and vena cava is unknown. 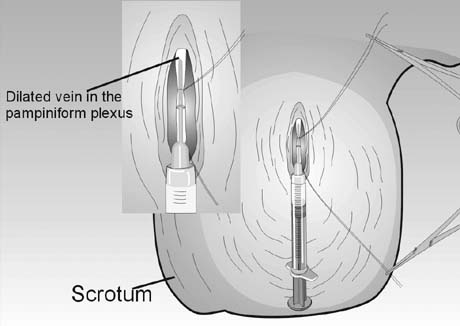 Fig. 45. Percutaneous anterograde varicocele occlusion. A dilated vein from the
pampiniform plexus of the spermatic cord is dissected and cannulated for
injection of sclerosing agent for occlusion. Fig. 45. Percutaneous anterograde varicocele occlusion. A dilated vein from the
pampiniform plexus of the spermatic cord is dissected and cannulated for
injection of sclerosing agent for occlusion.
|
Complications of Varicocelectomy Hydrocele formation is the most common complication reported after nonmicroscopic
varicocelectomy. The incidence of this complication varies
from 3% to 33%, with an average incidence of approximately 7%. Analysis
of the protein concentration of hydrocele fluid
indicates that hydrocele formation after varicocelectomy is caused by
lymphatic obstruction.88 At least half of postvaricocelectomy hydroceles grow to a size large enough
to warrant surgical excision caused by the discomfort and growth
of the hydrocele to a large size. The effect of hydrocele formation on
sperm function and fertility is uncertain. It is known that men with
varicocele have significantly elevated intratesticular temperatures,77,126,127 and this appears to be an important pathophysiological phenomenon mediating
the adverse effects of varicocele on fertility. The development
of a large hydrocele creates an abnormal insulating layer that surrounds
the testis. This may impair the efficiency of the countercurrent heat
exchange mechanism and therefore obviate some of the benefits of varicocelectomy. Use of magnification to identify and preserve lymphatics can virtually
eliminate the risk of hydrocele formation after varicocelectomy.103,128,129 Also, radiographic balloon or coil occlusion techniques eliminate the
risk of hydrocele formation. Testicular Artery Injury The diameter of the testicular artery in humans is .5 to 1.5 mm (see Fig. 44). Microdissections of the human spermatic cord have revealed that
the testicular artery is closely adherent to a large internal spermatic
vein in 40% of men. In another 20% of men, the testicular
artery is surrounded by a network of tiny veins.130 During the course of cord dissection for varicocelectomy, the artery may
go into spasm and even in its unconstricted state is often difficult
to positively identify and preserve. Injury or ligation of the testicular
artery carries with it the risk of testicular atrophy and/or
impaired spermatogenesis. Starzl's transplant group102 reported a 14% incidence of frank testicular atrophy when the testicular
artery was purposely ligated. The actual incidence of testicular
artery ligation during varicocelectomy is unknown, but some studies
suggest it is common.131 Animal studies indicate that the risk of testicular atrophy after testicular
artery ligation varies from 20% to 100%.30,132 In humans, atrophy after artery ligation is probably less likely because
of the contribution of the cremasteric as well as vasal arterial supply. In
children, the potential for neovascularization and compensatory
hypertrophy of the vasal and cremasteric vessels is probably greater
than in adults, making atrophy after testicular artery ligation less
likely. Use of magnifying loupes, or preferably an operating microscope
and/or a fine-tipped Doppler probe, facilitates identification
and preservation of the testicular artery and therefore minimizes
the risk of testicular injury.133 Radiographic balloon or coil occlusion techniques also eliminate this
risk. Varicocele Recurrence The incidence of varicocele recurrence after surgical repair varies from .6% to 45%. Recurrence is more common after repair of
pediatric varicoceles. Radiographic studies of recurrent varicoceles visualize
periarterial, parallel inguinal or mid-retroperitoneal
collaterals, or, more rarely, transcrotal collaterals.120 Retroperitoneal operations miss parallel inguinal collaterals. Nonmagnified
inguinal operations have a lower incidence of varicocele recurrence
than retroperitoneal operations but fail to address the issue of scrotal
collaterals or small veins surrounding the testicular artery. The
microsurgical approach with delivery of the testis lowers the incidence
of varicocele recurrence to 1% to 2%, compared with 9% to 16% using nonmagnified inguinal techniques.103,128,129 Results Varicocelectomy results in significant improvement in semen analysis in 60% to 80% of men. Reported pregnancy rates after varicocelectomy
vary from 20% to 60%. A randomized controlled
trial of surgery versus no surgery in infertile men with varicoceles
revealed a pregnancy rate of 44% at 1 year in the surgery group
versus 10% in the control group.134 In our series of 1500 microsurgical operations, 43% of couples
were pregnant at 1 year128 and 69% at 2 years when couples with female factors were excluded. Microsurgical
varicocelectomy results in return of sperm to the ejaculate
in 50% of azoospermic men with palpable varicoceles.5,25 The results of varicocelectomy are also related to the size of the varicocele. Repair
of large varicoceles results in a significantly greater
improvement in semen quality than repair of small varicoceles80,135(Fig. 46). In addition, large varicoceles are associated with greater preoperative
impairment in semen quality than small varicoceles, and consequently
overall pregnancy rates are similar regardless of varicocele size. In
the presence of small (grade I) varicoceles along with
larger (grade II and III) contralateral ones, greater improvement
in semen parameters can be expected if repair is performed bilaterally
than if only the larger side is repaired.136 Some evidence suggests that the younger the patient is at the time of
varicocele repair, the greater the improvement after repair and the more
likely the testis is to recover from varicocele induced injury.21 Varicocele recurrence, testicular artery ligation, or postvaricocelectomy
hydrocele formation are often associated with poor postoperative results. In
infertile men with low-serum testosterone levels, microsurgical varicocelectomy alone results in substantial improvement
in serum testosterone levels.26 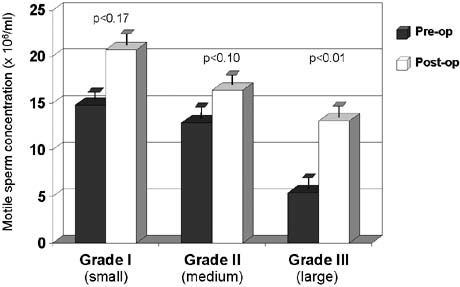 Fig. 46. Relationship between varicocele size and response to repair. Fig. 46. Relationship between varicocele size and response to repair.
|
Summary Varicocele is an extremely common entity, present in 15% of the
male population. Varicoceles are found in approximately 35% of
men with primary infertility but 75% to 81% of men with
secondary infertility. Mounting evidence clearly demonstrates that varicocele
causes progressive duration-dependent injury to the testis. Larger
varicoceles appear to cause more damage than small varicoceles
and, conversely, repair of large varicoceles results in greater improvement
of semen quality. Varicocelectomy can halt the progressive
duration-dependent decline in semen quality found in men with varicoceles. The
earlier the age at which varicocele is repaired, the more
likely is recovery of spermatogenic function. Variococelectomy can
also improve Leydig cell function resulting in increased testosterone levels.26 The most common complications after varicocelectomy are hydrocele formation, testicular
artery injury, and varicocele persistence or recurrence. The
incidence of these complications can be reduced by using microsurgical
techniques, with inguinal or subinguinal operations, and exposure
of the external spermatic and scrotal veins. Use of these advanced
techniques of varicocelectomy provide a safe effective approach to elimination
of varicocele, preservation of testicular function, and, in
a substantial number of men, an increase in semen quality and likelihood
of pregnancy. |