Standard Therapy CC is administered orally, starting on the 3rd to 5th day after the onset
of spontaneous or progestin-induced menses. In anovulatory women, ovulation
rates, conception rates, and pregnancy outcome are similar regardless
whether treatment begins on cycle day 2, 3, 4, or 5.18 Although the dose required to achieve ovulation correlates with body weight, there
is no reliable way to predict accurately what dose will be
required in an individual woman.19 Consequently, CC induction of ovulation amounts to an empirical incremental
titration in efforts to establish the lowest effective dose for
each individual (Fig. 2). 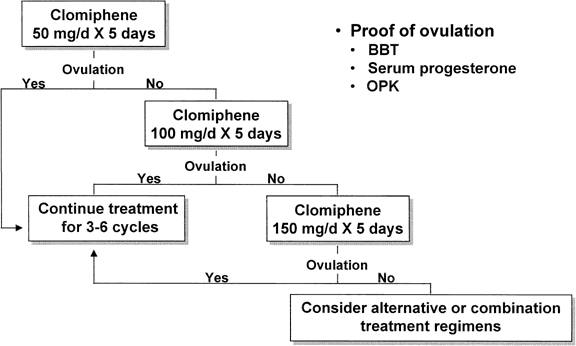 Fig. 2. Standard incremental clomiphene treatment regimen. Fig. 2. Standard incremental clomiphene treatment regimen.
|
Treatment typically begins with a single 50-mg tablet daily for 5 consecutive
days (e.g., cycle days 3 to 7 or 5 to 9), increasing by 50-mg increments
in subsequent cycles until ovulation is induced (see Fig. 2). The effective dose of CC ranges from 50 to 250 mg/day. Lower doses (e.g., 12.5 to 25 mg/day) deserve a trial in women who demonstrate exquisite
sensitivity to CC or consistently develop large ovarian cysts that
interfere with efficient cyclic treatment.20 Most women respond to treatment with 50 mg (52%) or 100 mg (22%). Higher
doses are sometimes required but also less often successful (150 mg, 12%; 200 mg, 7%; 250 mg, 5%) (Fig. 3).21 Most who fail to respond to 150 mg will ultimately require alternative
or combination treatments. Once the effective dose of CC is established, there
is no indication for further increments unless the ovulatory
response is lost. Higher doses will not improve the probability of conception, only
the risk of hyperstimulation and multiple pregnancy. 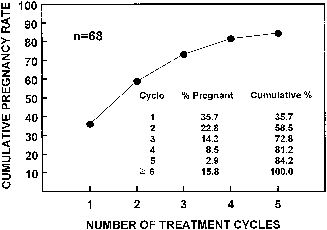 Fig. 3. Cumulative pregnancy rate during clomiphene treatment.(Adapted with permission from the American Society for Reproductive Medicine. Garcia
J, Seegar Jones G, Wentz AC: The use of clomiphene citrate. Fertil
Steril 28:711, 1977) Fig. 3. Cumulative pregnancy rate during clomiphene treatment.(Adapted with permission from the American Society for Reproductive Medicine. Garcia
J, Seegar Jones G, Wentz AC: The use of clomiphene citrate. Fertil
Steril 28:711, 1977)
|
Alternative and Combination Treatment Regimens Many women who prove resistant or refractory to standard CC treatment regimens
will ovulate in response to alternative or combination treatment
regimens. These involve longer durations of CC treatment (e.g., an 8-day
treatment regimen), the use of “insulin sensitizing” agents (e.g., metformin) or other adjuncts (e.g., exogenous human chorionic
gonadotropin [hCG], glucocorticoids), and combinations (e.g., sequential
treatment with CC and exogenous gonadotropins). A
choice among them should not be arbitrary, but based on specific elements
of the patient's history, the results of laboratory evaluation, and/or
observations in previous unsuccessful CC treatment cycles. These
regimens also should not be considered as a prerequisite for use
of more aggressive treatment strategies(e.g., exogenous gonadotropins). They
are simply useful alternatives that merit consideration, depending
on the patient's age, goals, available resources, and risk tolerance. EXTENDED COURSE CLOMIPHENE TREATMENT. Some CC-resistant anovulatory women who fail to respond to a standard 5-day
treatment regimen, even at higher dosage levels, may respond to longer
courses of CC treatment. An 8-day, high-dose treatment regimen (e.g., 200 to 250 mg/day) can be effective when shorter courses of therapy
fail.22 INSULIN SENSITIZING AGENTS. Insulin resista nce and hyperinsulinemia are now recognized as a common
feature of polycystic ovary syndrome (PCOS) and an important contributing
cause of the hyperandrogenism and chronic anovulation that characterize
the disorder.23 Most women with PCOS will respond to CC treatment, but many prove resistant
and ultimately require alternative treatment. Among these, a large
majority will have demonstrable insulin resistance. A number of recent studies have demonstrated that treatment with insulin-sensitizing
agents (e.g., metformin) alone can restore menses and cyclic
ovulation in many amenorrheic PCOS women.24,25 Some advocate metformin as primary therapy inanovulatory infertile PCOS
women with insulin resistance (1000 to 2000 mg/day in divided doses) and
add CC only in those who fail to respond. Given the greater costs
and complexity of metformin treatment and the frequency of severe gastrointestinal
side effects (e.g., nausea, vomiting, diarrhea), others
prefer to reserve metformin treatment for those who first prove resistant
to CC. In either case, many who fail to ovulate in response to either
alone will respond when the two are used in combination.25 CLOMIPHENE AND hCG. A number of clinical studies have examined the efficacy of using exogenous
hCG as a surrogate LH surge in women who fail to ovulate in response
to CC alone.26 Although this treatment can be successful, relatively few women will clearly
benefit, and the method does have distinct potential disadvantages
and consequences. The underlying premise for the use of exogenous hCG must be that CC treatment
can promote development of a preovulatory follicle that, for some
reason, cannot or does not stimulate the LH surge necessary to achieve
ovulation. Physiologically, and in practice, these circumstances are
relatively rare. In any event, they could be documented only if follicular
development were monitored with serial transvaginal ultrasound
examinations that are costly and generally unnecessary. Should these
observations be made, serial ultrasound monitoring is again required whenever
hCG treatment is anticipated to ensure that the ovulatory stimulus
is delivered at or very near the peak of follicular maturity. If
administered prematurely, before the follicle is mature enough to respond, hCG
is more likely to induce atresia than ovulation. It is difficult to determine when best to administer exogenous hCG, even
in carefully monitored cycles. Clinical studies in anovulatory infertile
women have demonstrated that on the day of the spontaneous LH surge
in successful CC-induced ovulatory cycles, mean diameter of the lead
follicle ranges between 19 and 30 mm (median diameter, 25 mm).27 Given that the average growth rate of the preovulatory follicle over the
days immediately preceding ovulation (2 mm/day), that range in peak
follicular diameter spans an interval of approximately 5 days. Normally, the
preovulatory follicle triggers its own ovulation at the peak of
maturity by generating and maintaining estrogen levels above the threshold
required to induce the LH surge. Timing of the spontaneous LH surge
is therefore always optimal, and that of hCG administration can never
be more than an educated guess. In those uncommon instances in which
hCG treatment may be effective and is necessary, it is probably best
postponed until the lead follicle reaches or exceeds 20 mm in mean
diameter. Occasionally, a couple may require both CC treatment and IUI (with partner
or donor sperm) to overcome both anovulation and a coexisting male
factor. Clearly, accurate timing of the insemination is crucial to the
success of treatment. Ideally, IUI should be performed on the morning
after the spontaneous LH surge is detected using an OPK. Use of adjunctive
hCG to stimulate ovulation is justified when efforts to detect
the LH surge prove unreliable or troublesome. In those cases, the cycle
should be monitored and hCG administered as described above, with IUI
performed approximately 36 hours thereafter. To be effective, IUI must
be timed to coincide with the anticipated time of ovulation. Normally, ovulation
occurs between 24 and 48 hours after initiation of the LH
surge.28 An OPK detects the LH surge only when urinary LH concentration exceeds
the test threshold, necessarily sometime well after initiation of the
surge; IUI is therefore best performed 12 to 24 hours later. In contrast, when
hCG is used as a surrogate, the “surge” begins at
the time of injection with ovulation then expected 24 to 48 hours later. For
convenience in scheduling, hCG is generally best administered
in the evening hours (i.e., 10:00 p.m.) and IUI performed 2 days later (i.e., 10:00 a.m.). CLOMIPHENE AND GLUCOCORTICOIDS. In some CC-resistant PCOS women, addition of glucocorticoids (e.g., dexamethasone 0.5 mg
or prednisone 5 mg hs) to the CC treatment regimen will
achieve ovulation when CC alone has failed.29 Adjunctive glucocorticoid treatment is perhaps most useful in those having
serum DHEA-S levels greater than 200 μg/dL, but can also be empirical, continued
if successful and promptly discontinued when it is
not.30 Given the potential side effects and risks of chronic glucocorticoid administration, continued
treatment should be limited to those who respond
and extended durations of treatment avoided. CLOMIPHENE AND GONADOTROPINS. CC-resistant anovulatory women who ultimately require exogenous gonadotropins
to achieve ovulation might benefit from a trial of sequential
CC/gonadotropin therapy using either traditional menotropins (hMG) or
newer preparations of purified recombinant FSH (rFSH).31 Those in whom CC stimulates significant but only transient follicular
growth are the logical candidates. In a typical cycle, CC treatment (e.g., 50 to 100 mg/day, cycle days 5 to 9) is immediately followed by low
dose rFSH (e.g., 75 IU/day, cycle days 9 to 12). Monitoring(e.g., transvaginal
ultrasound, serum estradiol determinations) begins on cycle
day 13, and treatment is individualized thereafter, in the same way
as with traditional gonadotropin therapy. Cycle fecundity with this approach
is similar to that achieved with gonadotropins alone, but the dose
and duration of treatment and the associated costs of monitoring are
significantly reduced. OVARIAN “DRILLING” A contemporary version of the classical ovarian wedge resection is another
treatment option in CC-resistant, hyperandrogenic, anovulatory women (e.g., PCOS). The
technique involves laparoscopic cautery, diathermy, or
laser vaporization of the ovaries at multiple sites, the objective
being to decrease circulating and intraovarian androgen levels by reducing
the volume of ovarian stroma. Serum testosterone concentrations
typically fall by approximately 40% to 50%, at least for a period of
months.32 Data from a number of published series indicate that 70% to 90% of properly
selected candidates will ovulate after drilling, and 50% to 80% of
those will conceive.33,34 If not spontaneous ovulation, the procedure may restore sensitivity to
CC treatment.33 Postoperative adhesions that may compromise fertility are a concern and
must be considered. Consequently, ovarian drilling is perhaps best reserved
for CC-resistant women in whom costs or logistic considerations
effectively preclude alternative treatments (e.g., exogenous gonadotropins). |