Protocols using pulsatile GnRH and resulting in successful ovulatory responses
and clinical pregnancies have been reported with varied doses, frequencies, and
routes of administration; these will each be discussed
later in more detail. All the clinical methods share in common the
delivery of the pulsatile GnRH via a small, portable, programmable infusion
pump. Several pumps are available in the United States, and all
store a reservoir of GnRH solution and deliver a fixed or variable volume
at specified intervals (e.g., Zyklomat, Ferring Laboratories, Ridgewood, NJ; Auto Syringe, Auto Syringe, Hookset, NH; Lutrepulse, Ortho Pharmaceutical, Raritan, NJ). These
pumps are similar to those used for insulin or tocolytic therapy, and
most deliver the pulse over a 1-minute period. Route of Administration Multiple routes of GnRH administration are available. Absorption occurs
after IV, subcutaneous (SC), intramuscular (IM), nasal, and sublingual
administration.22,23,24 Considerable debate exists over the preferred route of pulsatile GnRH
administration for ovulation induction. Several centers have reported
successful pregnancies using the SC administration of pulsatile GnRH.5,25,26,27,28 Most centers, however, have reported superior ovulation and pregnancy
rates, as well as lower spontaneous abortion rates with IV administration.29,30,31,32,33,34 The pharmacokinetic data clearly suggest the superiority of the IV route.29,35 IV administration of pulsatile GnRH results in a well-defined episodic
release of LH and FSH, whereas SC administration of identical GnRH pulses
can result in prolonged absorption with a slower sustained rise in
LH and FSH without a normal pulsatile pattern and a slower return to
baseline (Fig. 3). Advocates of SC administration stress the relative convenience and safety
of this route and save IV administration only for those subjects
who fail SC therapy. Subsequent to the higher ovulatory rates, however, the
FDA has approved only the IV route for pulsatile GnRH administration. 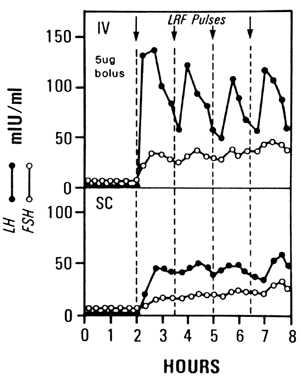 Fig. 3. LH and FSH levels in the same woman after 5-μg pulses of GnRH every 90 minutes
administered either intravenously or subcutaneously.(LRF, LH-releasing factor) (Liu JH, Yen SSC: The use of gonadotropin-releasing
hormone for the induction of ovulation. Clin Obstet Gynecol 27:975, 1984; as
modified from Reid RL, Leopold GR, Yen SSC: Induction of
ovulation and pregnancy with pulsatile luteinizing hormone-releasing
factor: Dosage and mode of delivery. Fertil Steril 36:553, 1981) Fig. 3. LH and FSH levels in the same woman after 5-μg pulses of GnRH every 90 minutes
administered either intravenously or subcutaneously.(LRF, LH-releasing factor) (Liu JH, Yen SSC: The use of gonadotropin-releasing
hormone for the induction of ovulation. Clin Obstet Gynecol 27:975, 1984; as
modified from Reid RL, Leopold GR, Yen SSC: Induction of
ovulation and pregnancy with pulsatile luteinizing hormone-releasing
factor: Dosage and mode of delivery. Fertil Steril 36:553, 1981)
|
At our institution, we favor the IV route of administration. Using sterile
technique, a 22-gauge, 1 1/4-inch Teflon catheter is inserted into
a vein in the nondominant forearm. Microbore plastic extension tubing (with
a dead space less than 1 mL) is used to connect the catheter to
the pump reservoir. Setups will vary depending on the pump and reservoir
system chosen. A three-way stopcock between the reservoir and the
extension tubing is often useful for refilling the reservoir or removing
air from the line in certain setups. In our experience, patients tolerate
the IV setup very well, and initial lines are often left in place
for an entire cycle. A few programs leave the same peripheral IV in
for several cycles,33 whereas others recommend changing the IV site every 3 to 7 days.36 Infection rates are generally low (see Complications section), and patient
acceptance has been quite high in our experience. Pulse Frequency The pulse frequency of endogenous GnRH in normal women during regular ovulatory
menstrual cycles ranges from approximately 95 minutes in the
early follicular phase to approximately 60 to 70 minutes in the periovulatory
period.37,38,39 Luteal-phase pulses are of decreasing frequency but higher amplitude. Based on Knobil's work in the monkey, original pulsatile GnRH ovulation
induction in humans was largely performed with a fixed pulse frequency.5,30 Excellent rates of ovulation (90% to 100%) and pregnancy have been reported
with the use of fixed follicular-phase pulse frequencies of every 90 minutes32 and every 60 minutes,40 with little difference in steroid response, ovulation rates, or pregnancy
rates being demonstrated between these two regimens.41 When pulse frequency was extended to 120 minutes, ovulation rates fell
to approximately 70%.42 A 60-minute follicular-phase frequency has been shown more effective than
a 120-minute frequency at inducing a spontaneous LH surge and ovulation
in women with hypothalamic amenorrhea.43 In an effort to mimic the physiologic pulsations of GnRH in the normal
menstrual cycle, some centers use a varying pulse frequency. A regimen
using a 90-minute pulse frequency in the first week of folliculogenesis, followed
by an increase in frequency to every 60 minutes in the midfollicular
phase of the induced cycle, followed by a return to slower
pulse frequency in the luteal phase, has been well described.33 In sum, the literature to date supports a follicular-phase pulse frequency
of 90 minutes or less to achieve maximum spontaneous ovulation rates, and
further studies need to be performed to determine whether there
is an added benefit to cycle-specific, nonfixed frequency regimens. Pulse Dosage Ovulation can be effectively induced over a range of GnRH doses. The optimal
dose depends somewhat on the route of administration, because the
SC route requires higher doses than the IV route. Studies have shown
that a 1-μg pulse of IV GnRH will result in peak concentrations of
GnRH within 4 minutes with levels between 200 and 260 pg/mL.44 These values correspond to the lower range of normal reported for pituitary
portal blood concentrations (40 to 2000 pg/mL) in humans.45 Martin and associates33 elegantly defined the hormonal effects of varying IV pulse dosage at optimal
pulse intervals. At a dosage of 25 ng/kg (1.25 μg/pulse for
a 50-kg patient), the mean peak estradiol level was lower than in normal
spontaneous cycles, corpus luteum function (as defined by integrated
progesterone levels) was lower than normal, and ovulatory rates were
only 80%. At 75 ng/kg (3.75 μg/pulse for a 50-kg patient), midcycle
estradiol and progesterone levels were indistinguishable from normal
cycles, and the ovulatory rate was 95%. At 100 ng/kg (5 μg/pulse for
a 50-kg patient), high ovulatory rates were maintained (93%), but peak
estradiol and integrated progesterone levels were exaggerated compared
to normal cycles (Fig. 4). 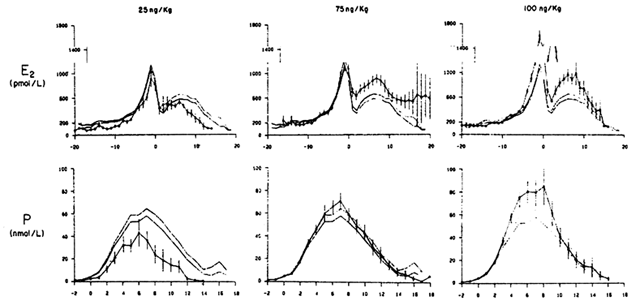 Fig. 4. Serum levels of estradiol (E2) and progesterone (P) (mean ± SEM) in women receiving varying doses
of IV pulsatile GnRH. Day 0 represents the midcycle surge, and mean ± SEM
values in 62 normal ovulatory cycles are represented by
the shaded areas. The data represent 10 cycles at 25 ng/kg, 31 cycles
at 75 ng/kg, and 25 cycles at 100 ng/kg. (Martin K, Santoro N, Hall J et al: Clinical Review 15: Management of ovulatory
disorders with pulsatile gonadotropin-releasing hormone. J Clin
Endocrinol Metab 71:1081A, 1990) Fig. 4. Serum levels of estradiol (E2) and progesterone (P) (mean ± SEM) in women receiving varying doses
of IV pulsatile GnRH. Day 0 represents the midcycle surge, and mean ± SEM
values in 62 normal ovulatory cycles are represented by
the shaded areas. The data represent 10 cycles at 25 ng/kg, 31 cycles
at 75 ng/kg, and 25 cycles at 100 ng/kg. (Martin K, Santoro N, Hall J et al: Clinical Review 15: Management of ovulatory
disorders with pulsatile gonadotropin-releasing hormone. J Clin
Endocrinol Metab 71:1081A, 1990)
|
Thus, we recommend a starting dose for IV therapy of 75 ng/kg/pulse (approximately 3.5 to 5.0 μg/pulse). For women who do not respond to this
pulse dose, the dose can be incrementally increased up to 10 to 20 μg/pulse. We
administer our pulses in a 0.9% sodium chloride solution
containing 10 μg/mL GnRH and 30 units of heparin/mL. When tailoring
optimal dosage regimens, allowances should be made for considerable
individual differences in GnRH requirements. Obese patients and those
with PCOS may require much higher doses than average. Clinical Monitoring and Luteal-Phase Support The clinical monitoring of induced cycles varies with the setting in which
therapy takes place, available clinical resources, and the comfort
level of the practitioner and patient. Many centers combine serum estradiol
and transvaginal ultrasound monitoring with urine ovulation prediction
kits for timing of intercourse. A presumptive diagnosis of ovulation
is made by clinical or ultrasonographic findings (e.g., disappearance of a dominant follicle with free pelvic fluid on ultrasound, LH
surge determined by urine monitoring, elevated progesterone, clear
rise in basal body temperature). Others take a more simple approach
and use only cycle length and basal body temperature charting as an
index of ovulation. Most patients ovulate within 10 to 20 days of initiation of IV pulsatile
GnRH therapy. Cycle variance results largely from the length of the
early follicular phase. Women with absolute GnRH deficiencies tend to
have longer follicular phases, because the pituitary must be primed for
several days before active secretion of FSH and LH. At our center, for the first treatment cycle, we prefer a combination of
ultrasound and urine LH monitoring. During subsequent cycles, usually
only LH monitoring is necessary. Serial ultrasound examinations are
performed starting about day 10, and urine LH testing is initiated when
the mean ultrasound diameter of the lead follicle is 15 mm. Sexual intercourse, or
intrauterine insemination in couples with male-factor infertility, is
timed by the urinary LH surge. In our experience, the lack
of a spontaneous LH surge is rare. This is an important advantage
of GnRH-stimulated cycles because it is the administration of human chorionic
gonadotropin (hCG) that may play a critical role in the development
of ovarian hyperstimulation syndrome in patients undergoing stimulation
with gonadotropins. However, in patients with a lead follicle
of 23 mm or greater and no spontaneous surge, ovulation may be induced
by the IM injection of 5000 units of hCG. Luteal-phase support of the corpus luteum is essential to the outcome of
a GnRH-induced cycle. Three methods are frequently used in clinical
practice, but no prospective data exist to suggest the superiority of
one method over the other. If the cycle stimulation resulted in the ovulation
of a healthy follicle, successful cycle support can be achieved
by continuing pulsatile GnRH administration at the same dose and frequency
used in the first part of the cycle.46 Other groups have reported achieving successful clinical pregnancies by
slowing the pulse frequency to every 4 hours in the luteal phase, with
no apparent shortening of the cycle.47 Similar clinical results can be achieved by discontinuing the GnRH pump
after ovulation and administering 1500 to 2000 U of exogenous hCG every 3 days
for four doses starting 2 days after presumed ovulation. This
method is more convenient than continuation of the pump, but it does
not allow for multiple monthly cycles using the same catheter, an option
that some centers offer. Pulsatile Gonadotropin-Releasing Hormone and Polycystic Ovary Syndrome Patients with PCOS or other variants of hyperandrogenic oligo-ovulation
present a special challenge for ovulation induction with GnRH. The disordered
release of endogenous GnRH and LH in this subpopulation is often
exaggerated by pulsatile GnRH therapy. Ovulatory rates in women with
PCOS treated with pulsatile GnRH alone are as low as 40% to 60% per
cycle.48 However, the addition of GnRH agonist (GnRHa) therapy to downregulate
the pituitary and reduce ambient testosterone levels before initiating
pulsatile GnRH therapy dramatically improves the outcome in these patients. Filicori
and colleagues,49 in a small study, initially showed an increase in ovulatory cycles from 38% to 90% in
PCOS patients pretreated with GnRHa. Subsequently, they
published the results of 228 GnRH-induced cycles in women with multifollicular
ovary, PCOS, and other forms of hyperandrogenic anovulation.40 Seventy-four percent of post-GnRHa cycles were ovulatory compared to 59% of 104 cycles
in the same groups of patients without GnRHa pretreatment. In
PCOS patients alone, pretreatment with GnRHa improved ovulatory
rates from 49% to 71%. In a small study, pretreatment with GnRHa was shown to be superior to pretreatment
with an estrogen-gestagen compound in terms of resultant ovulatory
GnRH-induced cycles.50 Pretreatment with GnRHa also reduces the risk of multiple pregnancy in
patients with PCOS undergoing ovulation induction with pulsatile GnRH.34 Despite GnRHa pretreatment, luteal-phase steroid secretion remains abnormal
after GnRH-induced ovulation in women with PCOS, and elevated spontaneous
abortion rates have been reported in those cycles resulting in
pregnancy.34 Continuous pulsatile GnRH therapy in women with PCOS has been suggested
to improve the endocrine milieu. In women with PCOS treated with 100 ng/kg
of IV pulsatile GnRH during consecutive cycles, first cycles were
characterized by elevated levels of LH and luteal-phase estradiol compared
to second cycles, which better approximated levels found in normal, eumenorrheic
women.51 In another study, however, a suboptimal endocrine pattern and a lower
ovulatory rate were found when a second post-GnRHa cycle occurred without
an initial repeat of analog suppression.49Table 1 summarizes selected series studying ovulation induction with GnRH in women
with PCOS.25,32,49,52,53,54,55,56,57,58 TABLE 1. Outcomes with Pulsatile GnRH Therapy in Women with PCOS in Selected
Studies
| No. of | GnRH | Frequency | | Ovulatory | Pregnancy |
Study | Cycles | Dose (μg) | (min) | Route | Cycles (%) | Rate (%) |
Saffan25 | 5 | 20 | 120 | SC | 40 | 0 |
Eshel58 | 108 | 15 | 90 | SC | 48 | 21 |
Hurwitz52 | 12 | 20–40 | 120–400 | SC | 17 | 0 |
Coelingh-Bennick53 | 42 | 10–20 | 90 | IV | 69 | 28 |
Ory546 | 1 | 90 | IV | 83 | 0 | |
Burger55 | 85 | 5–40 | 60–120 | IV | 87 | 6 |
Wilson56 | 9 | 5–40 | 90 | IV | 0 | 0 |
Surrey57 | 9 | 5 | 90 | IV | 22 | 0 |
Post-GnRHa | 7 | | | | 28 | 0 |
Filicori49 | 24 | 5 | 60 | IV | 38 | 8 |
Post-GnRHa | 21 | | | | 90 | 38 |
Jansen32 | 14 | 2.5–5 | 60–120 | IV | 50 | 7 |
GnRH = gonadotropin-releasing hormone ; GnRHa = GnRH agonist : PCOS = polycystic
ovary syndrome; SC = subcutaneous ; IV = intravenous.
(Modified from Santoro N, Elzahr D : Pulsatile gonadotropin-releasing hormone
therapy for ovulatory disorders. Clin Obstet Gynecol 27:975,1984)In sum, women with PCOS constitute a more challenging population than those
with hypothalamic amenorrhea for induction of ovulation with GnRH. For
clomiphene-resistant women, however, pulsatile GnRH still represents
a safe and relatively effective option that the clinician should
consider before pursuing gonadotropin therapy. Pretreatment with GnRHa
maximizes cycle potential in women with PCOS. |


 3–15
3–15 p21). Somat Cell Mol Genet 12: 95, 1986
p21). Somat Cell Mol Genet 12: 95, 1986