The morphogenic sequence of ovarian development is divided into two basic
periods: prefollicular and follicular. The prefollicular period precedes
differentiation of primary isolated ovarian follicles and includes
mitotic proliferation of oocytes and their entrance into the meiotic
prophase. The follicular period is related to the interruption of meiosis
I, after termination of the prophase, and to the differentiation
of isolated ovarian follicles with a distinct granulosa layer. The first
phase does not require the presence of two X chromosomes (as observed
also in 45,X fetuses).43 The prefollicular phase of ovarian development has two stages: the embryonal
ovary and the early fetal ovary. Embryonal Ovary The embryonal ovary40 is present in embryos of 18- to 40-mm CRL and is characterized by mitotic
proliferation of germ cells (oogonia) within the genital ridges. Simultaneously, the
blastema of the indifferent gonad differentiates into
epithelial cords and interstitium (Fig. 10). The interstitium contains desmogenic fibroblasts, the cords that form
the rete adjacent to the mesonephros, and medullary cords, which are
incompletely separated from the surface epithelium. Medullary cords contain
undifferentiated supportive cells and oogonia. The PGCs located
in contact with and under the surface epithelium undergo an intensive
mitotic proliferation, resulting in the formation of cortical cords composed
almost exclusively of oogonia. Embryonal ovaries are characterized
by intensive mitotic proliferation of oogonia, which do not interact
with other cells. The ovarian cords result from differentiation of
the connective tissue. By definition, no meiotic activity occurs in the
embryonal ovary. The mitotic wave of oogonia exhausts their entire
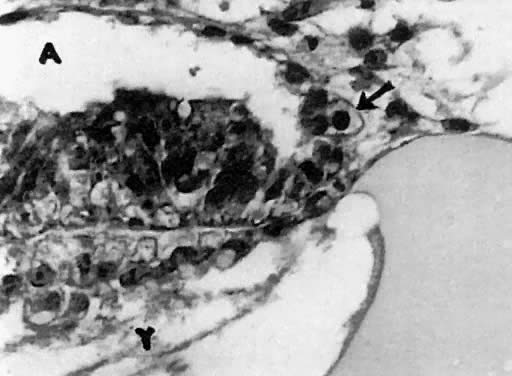
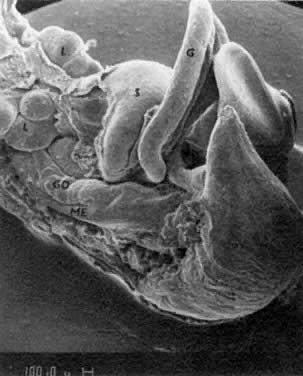
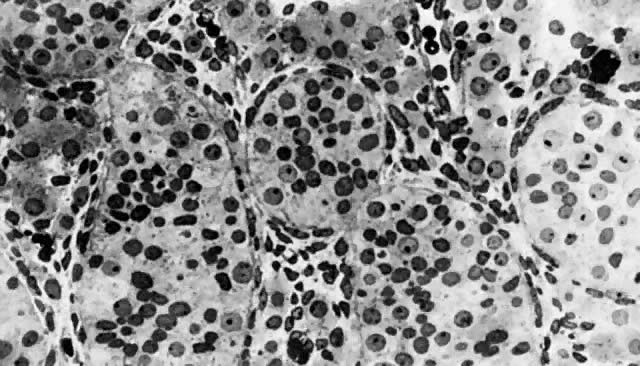
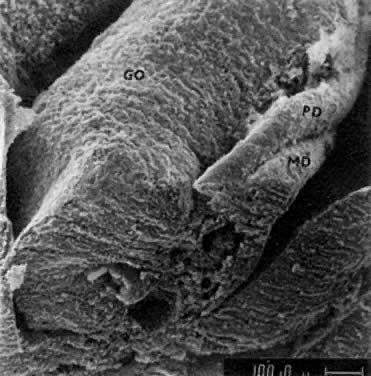
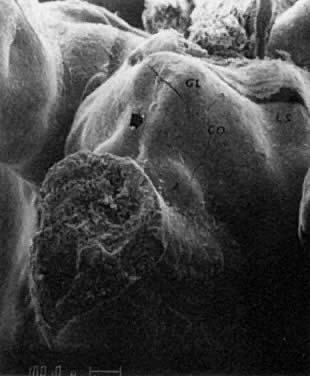
mitotic capacity during fetal development. 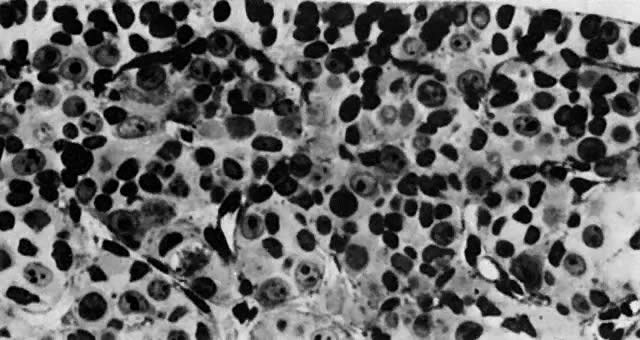 Fig. 10. Embryonal ovary, cortical portion (fetus of 40-mm crown-rump length). Epithelial
component, consisting of surface epithelium incompletely separated
from irregular epithelial cellular groups (cortical cords), is
formed by oogonia and primitive granulosa cells. Epithelial cords are
separated by thin connective tissue septa. Fig. 10. Embryonal ovary, cortical portion (fetus of 40-mm crown-rump length). Epithelial
component, consisting of surface epithelium incompletely separated
from irregular epithelial cellular groups (cortical cords), is
formed by oogonia and primitive granulosa cells. Epithelial cords are
separated by thin connective tissue septa.
|
Early Fetal Ovary At the beginning of meiosis I, meiotic oocytes appear in the medullary
cords and the deepest portions of the cortical cords in fetuses of about 40 mm
CRL (end of week 10). Oocytes entering the meiotic phase form
clones and are interconnected by cytoplasmic bridges. All interconnected
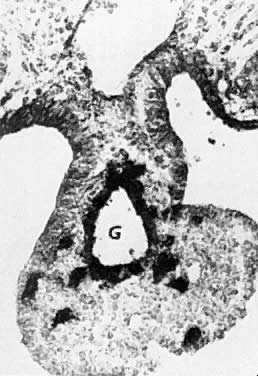
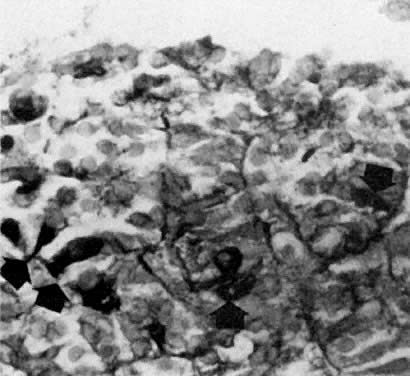
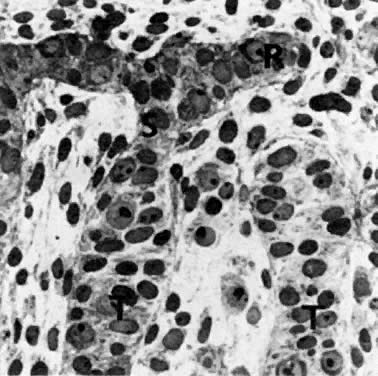
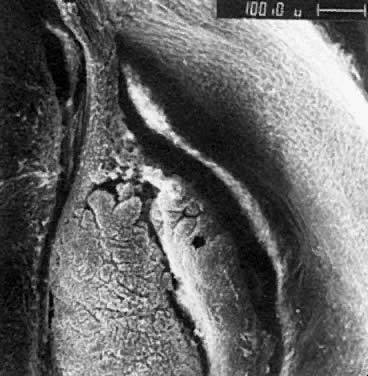
oocytes are at the same stage of meiotic prophase (Fig. 11). Leptotene, zygotene, and pachytene oocytes are present by week 11; diplotene
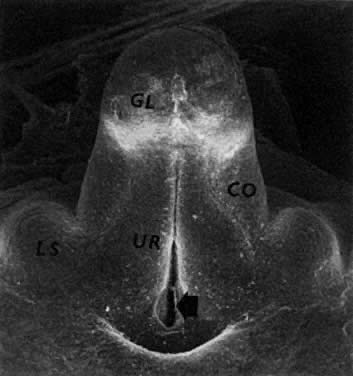
oocytes appear a week later. 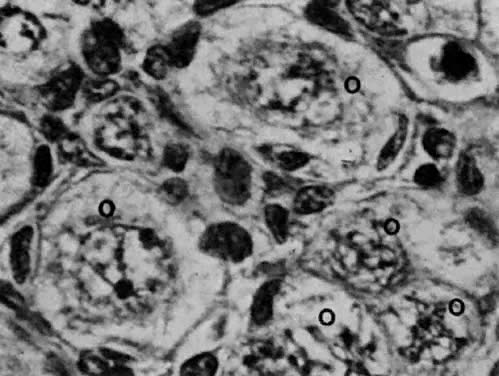 Fig. 11. Early fetal ovary. Oocytes (O) located within the epithelial groups at different stages of meiotic prophase. Fig. 11. Early fetal ovary. Oocytes (O) located within the epithelial groups at different stages of meiotic prophase.
|
Simultaneously, as the “oldest” oocytes enter meiosis, the “young” oogonia underneath the surface epithelium continue
mitotic proliferation. In early fetal ovaries, there are no primary isolated
follicles with a complete granulosa layer and connective tissue
envelope. In fetuses affected by X-chromosome monosomy, normal ovarian development
proceeds until this early fetal stage. The second phase—the follicular
phase—of ovarian development is related to the interruption
of meiosis I after the prophase has been completed, and to the differentiation
of isolated primary follicles. During the phase of follicular
differentiation, the stages of late fetal ovary and perinatal ovary
are distinguished. Late Fetal Ovary After the oocytes reach the diplotene stage of the first meiotic prophase, connective
tissue invades groups of meiotic oocytes, and at the same
time granulosa cells—steroidogenic progenitors of coelomic origin—spread
over the surface of the oocyte and contribute an epithelial
granulosa layer around the oocyte (Fig. 12). At this stage, the granulosa cells interact with the oocyte and interrupt
the meiosis. The nuclei of oocytes return to an interphase state. If
the proliferation of granulosa cells is inadequate, oocytes come
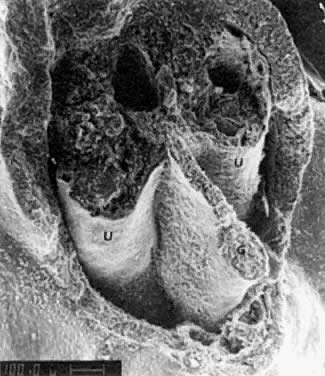
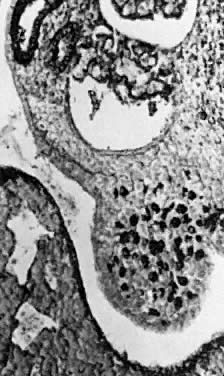
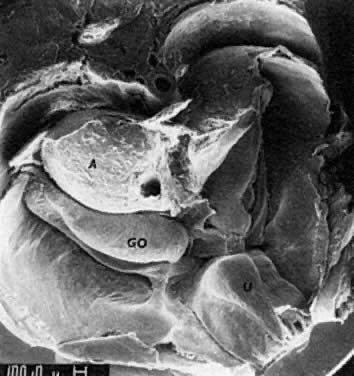
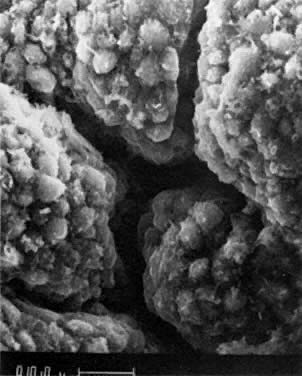
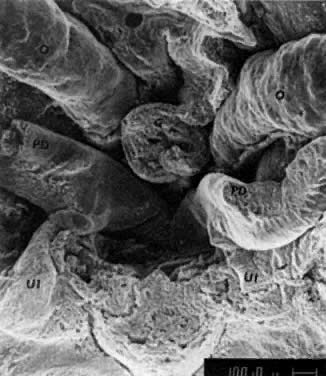
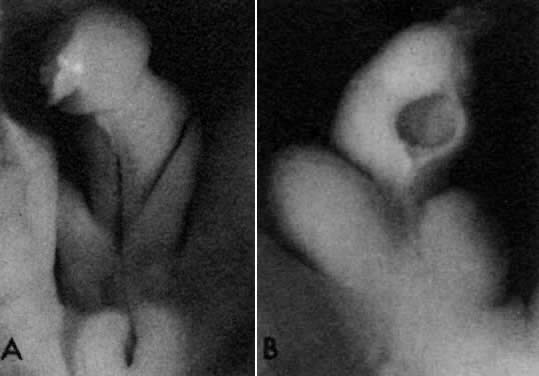
into direct contact with the connective tissue and undergo degeneration (atresia). 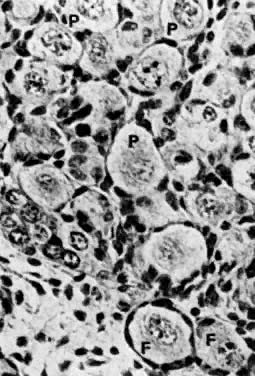 Fig. 12. Late fetal ovary. Groups of oocytes undergoing meiotic prophase are incompletely
separated by primitive granulosa cells. For such formations, the
term primordial follicles(P) is used. Completely separated follicles with granulosa cells and oocytes
with nuclei at an interphase state, after meiotic prophase has been
completed and arrested, are known as primary follicles (F). Fig. 12. Late fetal ovary. Groups of oocytes undergoing meiotic prophase are incompletely
separated by primitive granulosa cells. For such formations, the
term primordial follicles(P) is used. Completely separated follicles with granulosa cells and oocytes
with nuclei at an interphase state, after meiotic prophase has been
completed and arrested, are known as primary follicles (F).
|
Although the primary isolated follicles differentiate in the deepest portions
of the medullary and cortical oocytes (the “oldest” oocytes), the
superficially located oogonia continue mitotic division, differentiate
into oocytes, and enter the meiotic prophase. There are
no steroid-producing cells around primary ovarian follicles. The interval between the end of the first meiotic prophase and metaphase
lasts until ovulation of the follicle, occurring throughout the reproductive
years (i.e., 12 to 50 years of age). The formation of complete follicles requires
the presence of at least two X chromosomes. In late fetal ovaries, the
number of oocytes44 is estimated to be 6 million; at birth, about 2 million oocytes are present, incorporated
into follicles. Both ovaries of a 20-year-old woman
are thought to contain 400,000 oocytes. Of these, only about 400 are
ovulated during the entire reproductive period of a woman. The formation of follicles within late fetal ovaries in the presence of
two X chromosomes represents a rescue operation, breaking meiosis I and
saving some oocytes before termination of meiosis and consequent degeneration
for a maximum of 50 to 60 years. In normal fetal ovaries, only 30% of
the oocytes that are formed reach the stage of primary follicles. Perinatal Ovary The growth and cavitation of the follicles are characteristic aspects of
the perinatal ovary. All stages of folliculogenesis are present in perinatal
ovaries during the second postnatal month: primary resting follicles
with flat granulosa cells, primary growing follicles with cylindrical
granulosa cells, compact growing follicles with a multilayered
granulosa, and vesicular (cavitated) follicles. Epithelioid thecal cells
containing the 3β-hydroxysteroid dehydrogenase first appear in
perinatal ovaries around follicles with a multilayered granulosa at
the stage of beginning cavitation beneath the cumulus—oocyte complex. Follicular
cavitation occurs in newborns after separation from the
placenta. Drafting of oocytes begins in the perinatal ovaries. As the production
of hypophyseal gonadotropins is discontinued, shortly after birth, the
perinatal ovary changes during the first postnatal year into the inactive
or prepubertal ovary of a child. During childhood, follicular drafting
continues, and the drafted follicles (in the absence of gonadotropins) develop
to the stage of cavitated follicles. There are, however, no
thecal cells around the growing follicle. The absence of thecal cells
is a characteristic feature of the ovaries of a child. During puberty, the
ovary gradually begins to produce steroids under the influence
of FSH and LH. In addition to drafting, in the adult ovaries oocytes
grow and are selected, and some are ovulated. Ovulated follicles change
into the corpora lutea. As the follicles become exhausted, the adult
ovary changes into a menopausal ovary completely deprived of follicles (at
the age of 50 to 60 years). Differences Between Male and Female Gonadal Development The most important difference between males and females is that there is
lifelong proliferation of spermatogonia, but the proliferation of oogonia
is limited to a short prenatal period. The analysis of morphogenesis
suggests that the early differentiation of PGCs, which are not incorporated
into germ layers, inhibits almost all their genes related to
differentiation. The basic programming of germ cells is related to their
migration into gonads and to a programmed number of mitoses followed
by meioses. If there is one germ cell in the eight-cell inner cell
mass of the blastocyst, and if the population of germ cells reaching
the genital ridges is about 1,000, there are about 11 mitotic generations
of PGCs. Within the ovaries, if the population of oocytes is around 5 million, there
are an additional 12 to 14 generations of oogonia. The
entire mitotic capacity of the oogonia is exhausted by this mitotic
division; thereafter, germ cells undergo terminal differentiation into
oocytes, which enter meiosis. The prophase of meiosis I prenatally
creates a unique combination of genes from the paternal and maternal chromosomal
sets. The formation of ovarian follicles, which is related to the presence of
two X chromosomes, is based on interaction between granulosa cells and
meiotic oocytes. Meiosis in oocytes is interrupted after the prophase
has been completed, and some of the oocytes are saved for a period of
no longer than 55 years. Therefore, the formation of ovarian follicles
represents a rescue operation, saving mitotic oocytes. In testicular development, the mitotic programming of male germ cells is
modified by the cell-to-cell interaction of germ cells with the progenitors
of gonadal supportive cells. This interaction between male germ
cells and undifferentiated supportive cells changes the mitotic program
of male germ cells, extending their mitotic proliferation for the
man's entire life span and preventing them from undergoing terminal
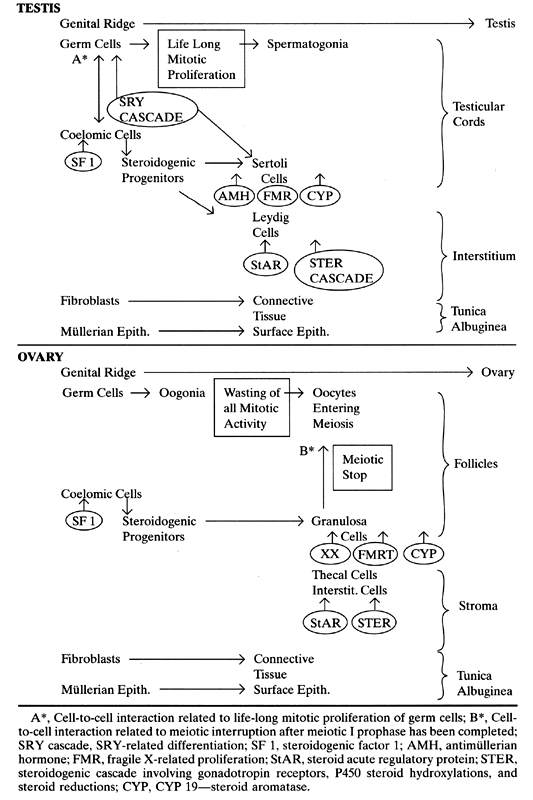
differentiation and meiosis before puberty. Proposed gene activations and a comparison of testicular and ovarian development
are summarized in Table 1. TABLE 1. Genes and Gonadal Development
 |