In the conventional in vitro fertilization (IVF) procedure, oocytes are coincubated with motile sperm to achieve fertilization. This conventional method usually produces a fertilization rate averaging between 50% and 70%. In the case of infertility caused by a male factor, fertilization can be severely compromised, if it occurs at all. Improving fertilization for male infertility was probably the first application of micromanipulation in ART.
Human oocytes, similar to oocytes from most mammalian species, are covered by a transparent glycoprotein coat called the zona pellucida (ZP). In order to gain access to the oocyte plasma membrane (oolemma) for fertilization, a sperm has to be able to negotiate its way through this barrier. Microassisted fertilization techniques have been designed to circumvent this ZP barrier. The first technique reported for human IVF, known as zona drilling, involved “driling” a hole in the ZP by applying an acidic solution (e.g., Tyrode’s solution with pH adjusted to 2 to 3) to a localized area with a micropipette.8 Partial zona dissection (PZD) refers to a micromanipulation procedure whereby the ZP is sliced open by a microneedle.9 Zona opening can also be accomplished by laser ablation.10 An alternative to zona opening is to place sperm directly next to the oolemma in the perivitalline space using a micropipette, which is named SUZI (subzonal insemination).11 The efficacy of these assisted fertilization techniques is compromised by two problems. First, not all sperm can effectively interact with the oolemma for the subsequent fertilization events to occur, even after they have bypassed the ZP barrier. Second, the block to polyspermic fertilization is not effective at the oolemma level in the human. Thus, multiple sperm could enter the oocyte resulting in polyploidy. Consequently, these techniques have produced relatively low and inconsistent fertilization results as treatment for male infertility. However, these techniques have the advantages of being relatively atraumatic to the oocyte and allowing, to some extent, natural selection of the fertilizing sperm.
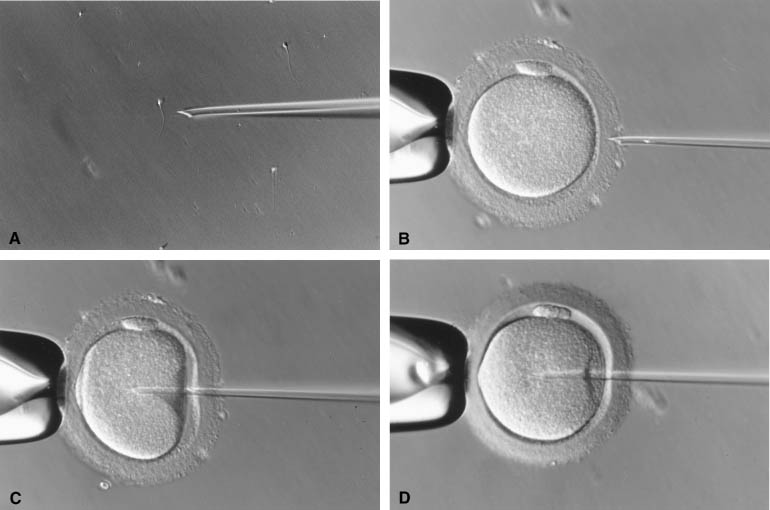
The injection of a single sperm into the cytoplasm of the oocyte, or intracytoplasmic sperm injection (ICSI), provided a satisfactory solution to the problems of the assisted fertilization techniques developed earlier.12 In this procedure, a single sperm is first immobilized by touching the sperm tail with an injection pipette (inner diameter = 5 to 7 μm). The injection pipette picks up the immobilized sperm, pierces the ZP and oolemma, and delivers the sperm inside the oocyte cytoplasm (Fig. 1). In 1976 using hamsters as a model, Uehara and Yanagimachi13 were probably the first to report the injection of sperm into oocyte cytoplasm (ooplasm). It was later attempted on rabbit14 and human oocytes,15 although the first successful human pregnancy was not reported until 1992 by the Free University of Brussels’ group in Belgium.12 During the natural fertilization process, the human sperm undergoes acrosome reaction on binding to ZP sperm receptor. The acrosome reaction enables the sperm to penetrate the ZP and to come into contact with the oolemma. The sperm cell membrane fuses with the oolemma and the sperm cell is incorporated into the ooplasm. The fusion activates the oocyte. Only after activation can an oocyte proceed to complete meiosis with the extrusion of the second polar body and allow sperm nucleus decondensation.16 In comparison with this natural process, ICSI does not require the sperm to complete the acrosome reaction. Fusion between sperm and oocyte membranes does not take place. Instead, the dislodging of the ooplasm by the ICSI pipette serves as a trigger for oocyte activation.17,18,19 The breakage of cell membrane on the sperm tail during immobilization facilitates the release of a putative oocyte-activation factor from the sperm that is also essential for activating the oocyte.20,21
Because ICSI is much more effective in fertilizing oocytes than other assisted fertilization techniques, it has become the ART of choice for male infertility.5,22,23 Indications for ICSI encompass most types of male infertility. Satisfactory fertilization can be achieved by ICSI when any of the following is diagnosed: low sperm count or motility, poor sperm morphology, presence of anti-sperm antibodies in the male (auto-) or female (allo-), or prior experience of failed or suboptimal fertilization with conventional IVF. In case of azoospermia or necrospermia, viable sperm can be extracted surgically from the epididymis or testis under many circumstances, and such surgically collected sperm can effectively fertilize oocytes with the aid of ICSI.24,25,26 Cryopreservation does not significantly reduce the fertilizing ability of surgically collected sperm,27,28,29 which negates having to schedule the urology surgery and oocyte retrieval on the same day. Although immature spermatozoa have been successfully used for fertilization by ICSI,30 treatment outcome after using spermatids has been disappointing, largely because of the difficulty in correctly identifying round spermatids from somatic cells.31,32
The applications of ICSI have expanded beyond treating male infertility. ICSI can be used when suboptimal fertilization is anticipated because of the adverse effects of some in vitro conditions. For example, mammalian oocytes matured in vitro tend to develop a hardened ZP (resistance to proteinase digestion) that may hinder fertilization.33,34 Optimal fertilization of in vitro matured oocytes has been obtained by ICSI.35,36,37 Similarly, cryopreservation also causes zona hardening38,39 and fertilization of the cryopreserved human oocytes can be optimized by the use of ICSI procedure.40,41 In the conventional IVF method, oocytes are coincubated with a suspension of 50,000 to 100,000 sperm for fertilization. In comparison, oocytes are exposed to individual sperm during ICSI. Thus, IVF by ICSI can greatly reduce the likelihood of transmitting microorganisms from the male to the female partner. Successful attempts have been reported for using ICSI as a procreation method to prevent transmission of human immune deficiency viruses between couples with discordant serotypes.42,43 However, this practice has been cautioned against by the findings from rhesus monkey studies in which foreign DNA attached to sperm were introduced to oocytes via ICSI and the DNA was subsequently incorporated as functional genes into the embryo genome.44,45 Taken together, these studies suggest that ICSI may minimize, but does not eliminate, the chance of transmitting infectious diseases between partners. The procedure may facilitate the introduction of foreign DNA and other pathogens to the oocytes, potentially leading to greater health complications. In some ART centers, the conventional IVF method has been replaced by ICSI not only for male infertility but also for infertility without an identified cause (unexplained infertility) in order to ensure that fertilization occurs. This is because fertilization usually fails to occur in 2% to 5% of IVF attempts even though all semen parameters are within normal ranges. The use of ICSI may prevent such complete failures.46 However, fertilization failure may still occur even when ICSI is used.47 Therefore, taking into account the added expenses and the potential risks of the procedure, it remains debatable whether ICSI should be used exclusively in place of the conventional IVF method for all patients.48
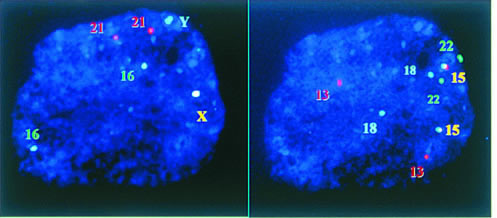
As ICSI becomes an established ART procedure, many concerns have been raised over its potential detrimental effects on the resultant embryos and children. One of the main concerns arises from the fact that ICSI bypasses the natural selection of sperm for fertilization. Sperm with defects that would be eliminated by natural selection are now allowed to reproduce with the aid of ICSI and may pass the defects to the next generation. Submicroscopic deletions (microdeletions) in the long arm of the Y chromosome have been found in approximately one fifth of men with azoospermia or severe oligospermia who would be prime candidates for ICSI.49,50,51 These microdeletions are probably de novo events and may be transmitted to the male offspring.52 In some men the microdeletions are caused by mitotic errors postfertilization, resulting in mosaicism in the affected individual. For these men, microdeletions may not be transmitted to all male offspring.53 Furthermore, because a direct cause relation has not been established between these chromosomal anomalies and fertility, it is uncertain whether the transmission of Y chromosome microdeletions will necessarily render all the male offspring infertile. Sperm from infertile men have a higher prevalence of aneuploidy than those from fertile controls.54,55 This may explain, at least partially, the small increase in the incidence of chromosomal anomalies in ICSI-generated embryos.56,57,58
Apart from facilitating the transmission of genetic defects, the process of sperm injection may inevitably cause physical damages to the oocyte and interfere with subsequent embryo development. In comparison with embryos from conventional IVF method, ICSI-generated embryos have been found to be less likely to attain the blastocyst stage in vitro and more likely to develop fragments.59,60,61 However, such differences were not observed in other studies.62,63,64 The discrepancy may be partially the result of technical differences or operator variations, which are known to influence fertilization and embryo development.65,66 Because the position of the second meiotic spindle is variable and is not always beneath the first polar body,67,68 the insertion of ICSI pipette could damage the meiotic spindle even if the area adjacent to the first polar body is avoided. Wang and coworkers69 reported a polarizing optical system that allows the visualization of the meiotic spindle without destroying the oocyte. Under the guidance of this system, damage to the spindle by the ICSI pipette can be avoided, thereby reducing the incidence of chromosomal anomalies or other cellular damages in ICSI-generated embryos. To prepare for the ICSI procedure, motile sperm are usually placed in a high-viscosity medium containing 10% (w/v) of a synthetic polymer, polyvinylpyrrolidone (PVP). A small amount of PVP is inevitably injected into the oocyte together with the sperm. To date, there is no evidence that PVP has any detrimental effect on oocytes or embryos. Nevertheless, the replacement of PVP with a natural material, hyaluronic acid, has been reported for preparing sperm for ICSI but its benefit remains to be confirmed.70
Despite the observed and potential detrimental effects on the embryos at the genetic and cellular levels, ICSI has not been associated with increased incidence of birth defects.71,72,73,74 Recently, public concerns over the safety of ART were raised again by a report showing a higher incidence of birth defects in IVF babies.75 In that report, however, the incidence of birth defects was not further increased by ICSI. It is possible that the damage done by ICSI, if any, is restricted to early stages of embryo development. Embryos that survive this early stage do not have further disadvantages compared to embryos resulting from conventional IVF during their subsequent development. The limited information available suggests that ICSI children may have a small delay in mental development,76,77 although it is unknown if this mental impairment is caused by the ICSI procedure or by factors inherent to the patients who require ICSI in the first place. Obviously further studies of the long-term effects of ICSI on the offspring involving multiple centers with well-controlled study designs are needed to minimize confounding variables, such as operator/technical variations and population variations. Until conclusive data become available, patients should be counseled carefully before ICSI is offered as an ART treatment.