The transdermal contraceptive patch is 20 cm2, roughly the size of a matchbook.7 The patch consists of a matrix in three layers: an outer polyester protective layer that is light tan, a middle layer containing an adhesive and the contraceptive steroids, and an inner clear polyester liner that is removed prior to application to the skin. The transdermal patch contains a total of 6.0 mg of norelgestromin and 0.75 mg of ethinyl estradiol. Norelgestromin, also known as 17-deacetylnorgestimate, is the active metabolite of norgestimate, which is the progestin contained in certain oral contraceptives. Ethinyl estradiol is the estrogen component of most oral contraceptives. The transdermal contraceptive patch is designed to deliver 150 μg of norelgestromin and 20 μg of ethinyl estradiol daily for a 7-day period of time. After 7 days, the patch is removed and a new one is applied to another skin site. Three consecutive 7-day patches are applied in a typical cycle followed by a 7-day patch-free period to allow withdrawal bleeding. Application sites that have been determined to be therapeutically equivalent in clinical trials include the buttocks, lower abdomen, upper outer arm, and upper torso except for the breasts.8
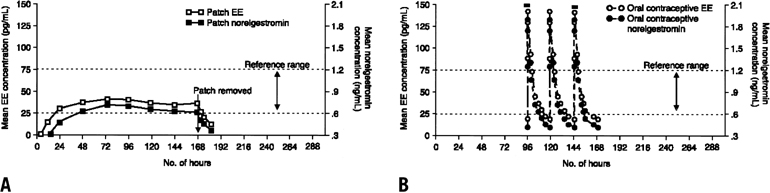
Figure 1 contrasts the contraceptive steroid absorption characteristics of the transdermal patch and an oral contraceptive. As shown in the left half of the figure, both of the contraceptive steroids reach the reference range within 24 to 48 hours after skin application. The reference range represents the range of serum concentrations of both steroids in which ovulation is usually suppressed. After removal of the transdermal patch after 7 days of use, serum steroid levels fall to nondetectable levels within 24 hours. In contrast, the right half of the figure demonstrates the typical dose-response curves seen with oral administration of a contraceptive. As shown, the transdermal contraceptive system maintains relatively steady-state serum concentrations of steroids without the characteristic peaks and troughs of an oral contraceptive. Although the usual duration of use of each patch is seven days, its ability to maintain serum concentrations of the steroids in the desirable reference range has also been evaluated for more extended use. In this study, serum concentrations remained in the reference range when a patch was left on for more than 7 days. The data indicated that the efficacy of the transdermal contraceptive patch would be maintained even if a scheduled change was missed for as long as 2 full days.
|