The anatomy of the breast and the hypothalamic-pituitary axis for release of prolactin and the production of breast milk may be found elsewhere in this text. These same structures are integral to the mammary-hypothalamic-pituitary-ovarian axis that mediates lactational infertility. The current understanding of the physiology of this feedback system is derived from studies of the different systems under conditions of lactation and nonlactation. Because of the difficulties in studying of these phenomena, as previously outlined, this section summarizes current understanding and presents also a summary of studies that confirm the underlying conclusions presented here.
Summary
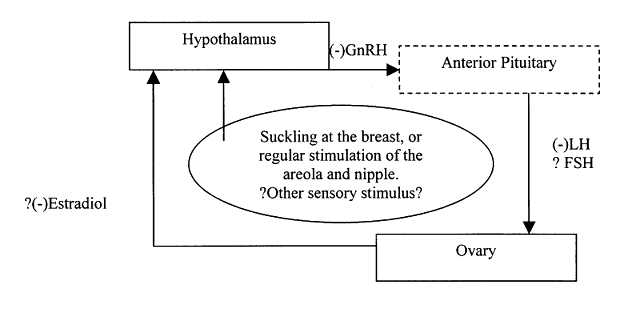
Suckling at the breast, especially with active stimulation of the nipple and areola and the structures that underlie them, stimulates a modification in gonadotropin-releasing hormone (GnRH) release from the hypothalamus, which in turn, disorganizes the pulsatility and levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). The result of the disruption of the levels of LH, and the sometimes reduced levels of FSH, is suppression of the development and release of a viable follicle and ovum. If there is some follicular development and estradiol levels rise, there is a paradoxical resuppression of fertility, rather than the stimulation of ovulation seen in nonbreastfeeding women.5 Oxytocin release from the posterior pituitary, which stimulates the let-down reflex, allowing milk to flow through the capillary-like mammary tubules, has not as yet been linked to suppression of fertility; however, its establishment is necessary for successful lactation, and, as such, is a necessary part of the lactational infertility.6 Prolactin plays little role in this feedback system, and maternal nutritional status is much less important than the pattern of infant breastfeeding. Although much remains to be elucidated, the basic parameters of this feedback system may be seen in Figure 1.
|
Discussion of Research
A recent symposium attempted to bring together scientists to discuss the current understanding and gaps in the knowledge concerning the physiologic basis of lactational infertility. Based on exploration of published and unpublished literature, three stages were identified7: early postpartum lactational amenorrhea, continued lactational amenorrhea, and menses return.
The early postpartum period, in this context, is the 6 to 8 weeks postpartum during which the inhibitory mechanisms of pregnancy continue to produce an impact, with diminished pituitary response to the hypothalamic release of GnRH. This is possibly due to reduced activity of the GnRH pulse generator; however, in the first month postpartum there is no LH response to a GnRH bolus. Studies of LH activity during this same period have yielded variable findings, indicating that there may be individual or behavior-based variation. Another study shows that the LH pulse frequency is not significantly different from that found in the early follicular phase; the peak levels, however, are significantly reduced.8 By the second postpartum month, LH response to a GnRH bolus may be seen, indicating a shift in the underlying mechanisms.9 This would imply decreased end-organ receptivity, in this case the pituitary. Opiates do not seem to mediate this shift in humans as they do in some nonhuman animal models.10 The timing of this shift may be dictated by intensity and pattern of feeding or by individual physiologic differences.
Those factors that influence the duration of continuing lactational amenorrhea, in relation to the duration of lactation, are not fully understood. At the same time, ongoing diminished GnRH and pituitary responsiveness continue in relationship to the intensity of the suckling stimulus. However, quantifying this phenomenon is difficult. Other mechanisms are thought to contribute, creating a complex of sometimes-conflicting feedback loops. These include enhanced or paradoxical negative feedback of ovarian secretions on the hypothalamic-pituitary axis; failure of positive feedback actions, lack of stimulation of the hypothalamus; decreased number or function of GnRH receptors; altered biologic activity of the hormones of ovulation; and a variable role of prolactin. It was concluded that there is a possibility of redundancy in these mechanisms.6
Attempts to elucidate the role of prolactin have found little direct involvement in the suppression of fertility. One important study included 20 women, with 24-hour blood sampling every 10 minutes, at either 4 or 8 weeks postpartum, at either time of introduction of supplements, at first menses while continuing breastfeeding, and in the follicular phase of the first cycle after weaning. Results included that the pattern of prolactin levels was responsive to breastfeeding pattern, but that there was no relationship between the plasma concentrations, day or night, and the duration of amenorrhea. There was, however, a strong and statistically significant correlation between the timing of introduction of supplements and the duration of amenorrhea.11 Another study of 10 women in Chile found that women who returned to fertility earlier exhibited a smaller prolactin response to suckling as early as the first month postpartum than was found among those with a delayed return of fertility.12 Although prolactin levels clearly are responsive to suckling, and there is an association of prolactin response and earlier return of menses, the pattern of prolactin release is not predictive of fertility return for the individual.
Another long-held hypothesis was that maternal fat stores dictated the duration of lactational infertility. To investigate the extent to which better maternal nutrition is associated with a reduction in the duration of lactational amenorrhea, data on 339 mother-infant pairs were analyzed.13 Maternal triceps skinfold was negatively associated with the length of amenorrhea; once controlled for infant feeding pattern, the effect was small; only a 0.5-month difference was seen when the 25th and 75th percentiles were compared. Improved maternal nutritional status by supplementation was not associated with change in length of amenorrhea when infant supplemental feeding was controlled for. This suggests that infant, not maternal, supplementation influences the duration of lactational amenorrhea, and that maternal nutritional status has modest influence. In a recent review of the physiology of lactation, Neville raises the possibility that another hormone associated with nutritional status, such as the appetite-suppressing hormone leptin, may play a role.14 Clearly, although the impact of nutritional status on lactational amenorrhea is less significant than earlier believed, the factors that mediate this association remain to be elucidated.
The return of menses during lactation is highly variable, among individuals and among cultural groupings.15,16 There are many behavioral and physiologic parameters that may have some impact on the timing of the return of fertility during lactation. Clearly, the sucking stimulus is a major variable and accounts for much of the variation seen. Frequency, with recovery time during the interval between feeds, intensity of the child's suckling, and hence breast stimulation, and the sensitivity of the nipple and areola of the breast, may all play a role. There appears to be increased pituitary responsiveness to GnRH over time; however, the presumed mediation by β-endorphins or opiates, has not been substantiated in humans. Whether it is dictated by individual variation in neuroendocrine response to the stimuli, variation in bioactivity of specific hormones and end organ, including ovarian, response to the changes remains to be assessed.
Fertility does not necessarily return immediately with the return of regular vaginal bleeds. The first cycles during breastfeeding frequently are associated with abnormal ovulatory activity and luteal phase defects. Studies have found, on average, a gradual return to normality over the first three cycles. An analysis of survey data found that, in areas where breastfeeding is practiced physiologically, that is, frequently day and night with little to no supplementation given, the continuation of breastfeeding after menses return is associated with significant continuing delay in fertility. In this study, for each addition month of breastfeeding after menses return there is about 7.4% reduction in risk of conception.17 This is thought to be primarily due to ongoing luteal phase defects.18
Differentiation of the first hormonally induced bleed from continued lochia or perceived end-of puerperium bleeding episodes may present an issue for some women, especially those interested in charting their cycles or predicting the next bleed. A multicenter multinational study of the return of a hormonally induced bleed, retrospectively defined as a bleed followed by another bleed within 21 to 70 days. This study found that duration of lochia varied significantly and concluded that among intensively breastfeeding women, about 11% experienced such a bleed in the first 2 months postpartum.19
The suckling stimulus is key throughout the duration of the feedback; however, the specific attributes of the suckling that contribute to the suppression of fertility are not readily elucidated. Clearly, frequency of the suckling episodes is vital. In the very early postpartum stage, suckling of 10 to 12 times a day appears the minimum number necessary to establish full lactation and fertility suppression. During the second stage, frequency may be reduced, but increasing intervals between feeds and initiation of or increase in supplementation are associated with hastened return of both ovulation and return of menses.17,20 After menses, or regular bleeds, recurs, frequent suckling continues to be associated with decreased fertility. Other parameters of the suck stimulus have been harder to study. In an effort to assess the impact of sucking intensity, a pressure transducer was attached to the nipple of exclusively breastfeeding women (n = 62) at 2 and 5 months postpartum. Although the efficiency and duration of the suckling, per se, were not correlated with duration of amenorrhea, the time at the breast in nonsuckling pauses was positively associated with a delay in menses return.21 This may indicate that sensory stimulation other than suckling alone may have a vital role in the feedback to the hypothalamus. Possible areas for further exploration include the importance of olfaction in early mother-infant bonding22 and cosleeping. These areas remain to be studied in relation to the duration of lactational infertility, and the physiologic impact of behaviors and sensory stimuli.