Laparoscopic electrocoagulation is the oldest technique of laparoscopic sterilization. Electrocoagulation using unipolar current gained widespread popularity during the early years of laparoscopic sterilization but fell into disfavor after reports of increasing numbers of bowel burns resulting from the procedure. Although most bowel injuries were subsequently shown to be trocar injuries and not electrical burns, the majority of laparoscopists abandoned the use of unipolar electric current for sterilization.6 Today, the inherently safer bipolar electric current has essentially replaced unipolar current for tubal sterilization. Because of the widespread tubal destruction associated with electrocoagulation, this method is less affected by tubal thickness and mobility than many other methods. Thus, electrocoagulation may be preferable when the tube is edematous and thickened or cannot be easily mobilized for mechanical device placement. Conversely, the greater tubal damage associated with electrocoagulation makes tubal reversal more difficult should the patient regret her decision. Regardless of the method chosen for tubal occlusion, electrocoagulation should always be readily available during laparoscopic tubal sterilization both as a backup method of sterilization and for control of unexpected bleeding.
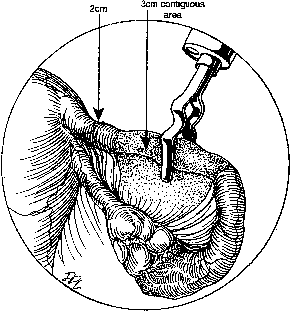
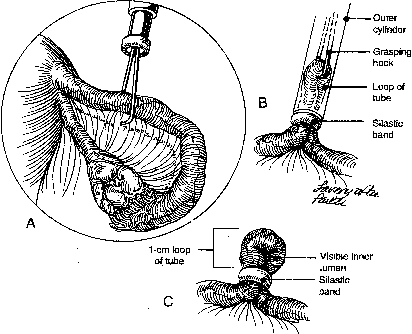
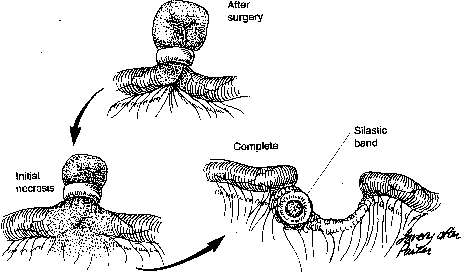
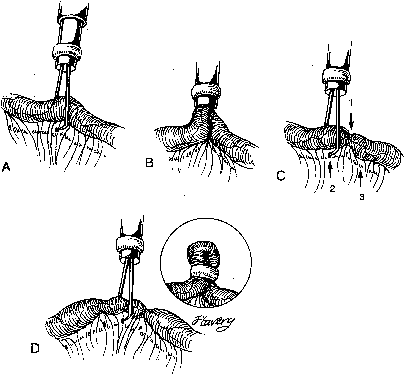
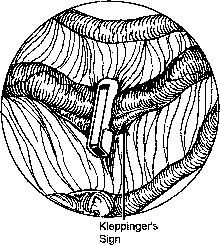
During sterilization with bipolar electrocoagulation, the fallopian tube is identified and grasped at the midisthmus region, approximately 2.5 to 3 cm from the uterotubal junction, with the bipolar forceps. The tube is tented up to ensure the forceps are not in contact with any other structure, and the current applied until coagulation is complete. Unlike the widespread coagulation seen during unipolar electrocoagulation, tissue destruction with bipolar current is confined to the area between and immediately adjacent to the bipolar paddles.7 Therefore, it is generally necessary to repeat the electrocoagulation an additional two times at immediately adjacent sites to duplicate the same amount of coagulation seen with unipolar current. Destruction of a minimum of 2 cm of tube has been suggested as adequate by some authorities,8 although others, including Kleppinger, advocate coagulation of at least 3 cm to ensure sterilization.9 Data from the CREST study also indicate that coagulation performed with three separate applications of the forceps is associated with a lower failure rate than when fewer applications are made.10
Although electrocoagulation of the tubal ampulla is more likely to produce complete occlusion, it will not achieve the same failure rate as coagulation of the isthmic portion of the tube.11 Conversely, coagulation of the tube too close to the cornu may lead to uteroperitoneal fistula formation. In 1930, Sampson12 described sprouts of endosalpinx growing out of the traumatized mucosa of the tubal stump. McCausland13 suggested that coagulation of the proximal isthmic fallopian tube tends to activate this process, which can then invade the tubal muscularis, penetrate the serosa, and result in a fistula. He named this endosalpingoblastosis. Recannulization frequently results in fistula sufficient to allow passage of sperm but usually not the ovum.14,15 This may be one explanation of the high ratio of ectopic-to-intrauterine pregnancies after failure of sterilization by electrocoagulation. The correct technique for bipolar electrocoagulation is illustrated in Figure 1.
If unipolar electrocoagulation is used, the initial site of coagulation should be chosen to allow any subsequent application to be closer to the uterus. Unipolar current returns to the ground through the path of least resistance. After the initial coagulation of the tube, the desiccated area has increased resistance, thus unipolar current applied distal to this site may flow to the end of the tube instead of through the uterus. If the distal end of the tube should touch bowel, a bowel burn could theoretically occur.
Classically, the fallopian tube was cut and divided after coagulation. A segment of tube can also be removed for histologic evaluation as part of this procedure. Some authorities now believe that division of the tube significantly increases the chance of tuboperitoneal fistula and subsequent ectopic pregnancy.16,17 The risk of a tear in the mesosalpinx with subsequent hemorrhage is also increased with this technique, and any tissue submitted to pathology is often so distorted that an accurate tissue diagnosis cannot be made. As a result, division of the fallopian tube after electrocoagulation is no longer recommended.
Although gynecologists use electrosurgery almost daily, many are unfamiliar with the physics involved. The unfortunate designation of current as “cut” and “coag” is especially confusing as “cut” current can coagulate and “coag” current can cut depending on the manner in which it is used. A more appropriate scientific designation is nonmodulated current for “cut” and modulated current for “coag” When used in a contact mode, nonmodulated (cut) current is a far more efficient desiccator of tissue than modulated (coag) current. In a similar situation, modulated current produces a rapid carbonization of the tubal surface that impends deeper electrocoagulation. Thus, nonmodulated current is the most appropriate current to use for tubal sterilization.
Electrosurgical units designed solely for tubal electrocoagulation generate only nonmodulated (cut) bipolar current while “Bovie”-type electrosurgical generators permit the selection of either modulated (coag) or nonmodulated (cut) current in the bipolar mode. Unfortunately, many surgeons and operating room nurses automatically select the less-powerful “coag” mode for tubal sterilization. The result may be a tubal lumen that remains viable despite the visual appearance of complete tubal coagulation.18
Many gynecologists also use a “blanch, swell, and collapse” visual endpoint to determine complete tubal coagulation. However, it is uncertain whether this method is completely reliable.18 Consistent, adequate coagulation is best achieved by using an ammeter to document cessation of current flow rather than depending on a visual endpoint. Alternatively, a timed coagulation period of at least 10 seconds at 25 W of nonmodulated current usually ensures complete tubal occlusion.18,19
The use of electrosurgical generators with bipolar forceps produced by different manufactures has been suggested as a cause of insufficient tubal coagulation.20 However, subsequent research would seem to indicate that complete coagulation is more dependent on selection of the proper waveform and power setting rather than generator-forceps mismatch.18,19,21
The most serious and feared complication occurring with the use of electrocoagulation is thermal injury to the bowel. The use of bipolar current eliminates the majority of risk of this complication. Care to ensure that only the fallopian tube is grasped with the forceps and that the tube is not touching other intra-abdominal structures should further reduce the risk of this complication.