Diagnostic Ultrasonography in Gynecology
Authors
INTRODUCTION
For optimum ultrasonographic visualization, certain mechanical, physical, and ultrasonographic principles must be understood. The quality of an image ultimately depends on the degree of resolution. In general, the closer the transducer tip is to the imaging target, the greater the resolution, and, therefore, the clearer the image. Every effort must be made to avoid interference with transmission of ultrasonographic energy.11 A thorough understanding of the longitudinal (sagittal), cross-sectional (axial), and coronal anatomy of the pelvis is necessary for optimal image interpretation. Whereas scanning planes for transabdominal scanning are classically described as sagittal, axial, and oblique, the transvaginal approach offers the additional coronal scanning plane.12,13 Accurate orientation is a necessity for the appropriate interpretation of pelvic imaging. Because much of the imaging is performed with the use of “organ-specific” planes, the practitioner must be aware of the scanning orientation to achieve an accurate interpretation of the findings noted on the image.13
In addition to understanding the need for close approximation of the imaging target by the transducer, realize that the quality of the image is influenced by the frequency of the transducer, pulse repetition frequency, and image processing. For a more in-depth analysis of these imaging principles, the reader is referred to standard texts on ultrasonography.
DOPPLER ULTRASONOGRAPHIC SCANNING
The direction, velocity, and variability of blood flow can be evaluated ultrasonographically by using the principle of the Doppler effect. By displaying the Doppler frequency shifts that result from calculating the difference between the emitted frequency and the frequency of echoes returning from a moving target, a flow velocity waveform is created. This can be described in terms of velocity (in centimeters per second) and by using descriptive ratios depicting the difference in the systolic velocity and diastolic velocity.
The S/D ratio (also referred to in some literature as the A/B ratio) is calculated by dividing the systolic maximum velocity by the diastolic minimum velocity. As the diastolic velocity approaches zero, however, this relationship becomes less accurate. To avoid this problem, other ratios also are used. The pulsatility index is calculated by dividing the difference between the systolic and diastolic velocities by the mean velocity ([S - D]/mean velocity). The resistance index divides the same numerator by the systolic velocity.
The velocity of blood flow is influenced by myocardial contractile force, vessel diameter, and downstream resistance. The interpretation of flow velocity waveforms requires a realization that blood flow velocity and volume of flow are not synonymous because of the effect of vasoconstriction.
As is addressed later, the ability to visualize small arteries with color Doppler interrogation offers the potential for further evaluation of the physiologic and pathophysiologic conditions that may occur in the female genital tract. When ordering and interpreting Doppler studies of the ovary and uterus, the practitioner should be cognizant of the effect of estrogen and progesterone on the blood flow velocity waveform. In general, during the periovulatory and luteal phases, there is an increasing diastolic velocity (reduced S/D ratio, pulsatility index, or resistance index). This realization is necessary for the correct interpretation of blood flow velocity waveforms in the premenopausal patient. Considering these principles, the potential improvement in diagnostic accuracy in the assessment of functional, as well as pathologic, conditions in the gynecologic patient can be seen.14,15,16
NORMAL PELVIC ANATOMY
Uterus
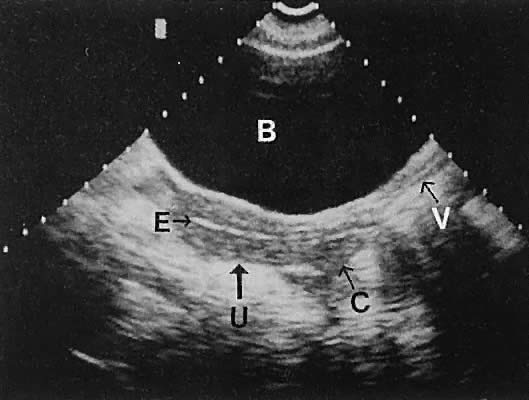
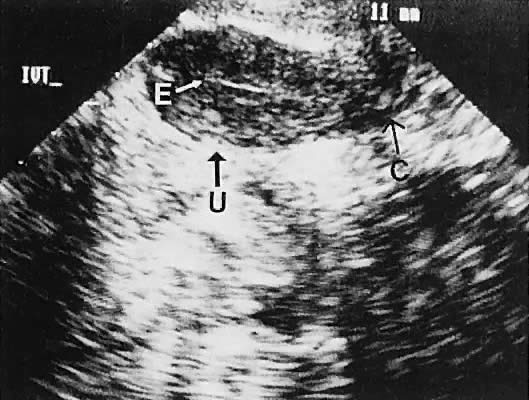
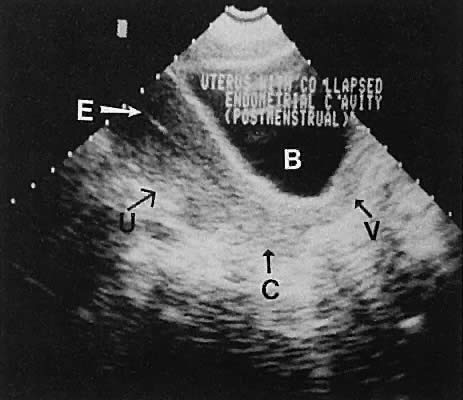
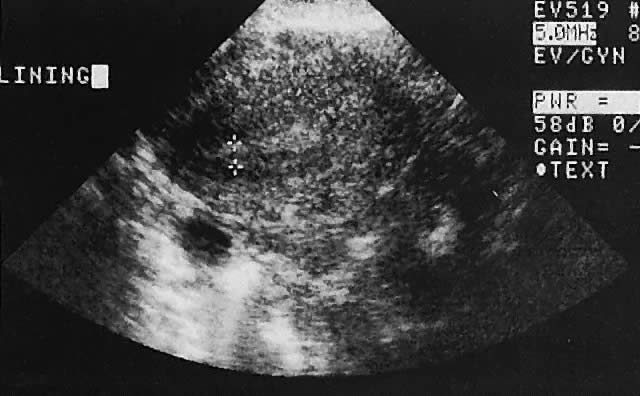
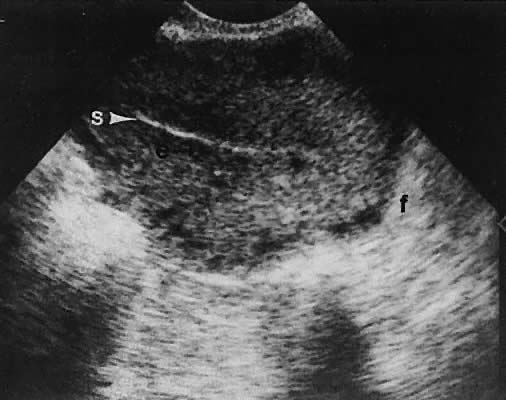
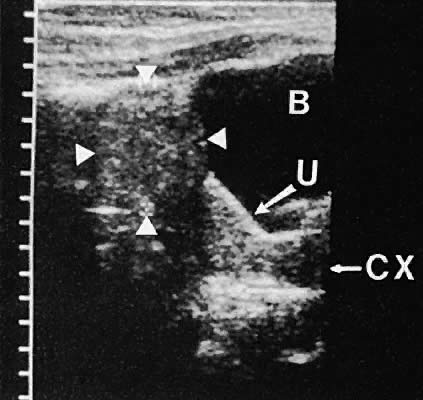
Regardless of the scanning approach used, a reliable landmark for orientation is the uterus (Fig. 1). Therefore, it is more difficult to scan posthysterectomy patients than those with a uterus in situ. The uterus should be readily seen in the midplane of the pelvis and normally exhibits an echo density that is clearly distinguishable from surrounding pelvic viscera (Fig. 2). The endometrial echo has a variable density, depending on water content and cellular density, that fluctuates with the hormonal status of the patient (Fig. 3). The changes noted in endometrial ultrasonographic appearance have been characterized. The endometrium has a trilaminar preovulatory appearance, then thickness becomes more homogeneous after ovulation. Progressive echogenicity of the functional zone (compactum and spongiosum) occurs with completion of the preovulatory phase and during the secretory phase.17 The thickness of the endometrium correlates with the histologic response to estrogenic stimulation.18 The relative position of the uterus to the cervix and to the axis of the vagina should be noted. Retrodisplacement of the uterus usually produces a less clearly defined image on transabdominal scanners but does not interfere with uterine delineation significantly using the transvaginal approach.19 The shape or symmetry of the uterus also should be assessed during the scanning session.
|
|
CERVIX.
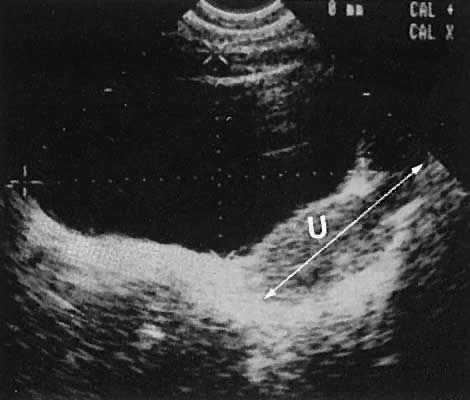
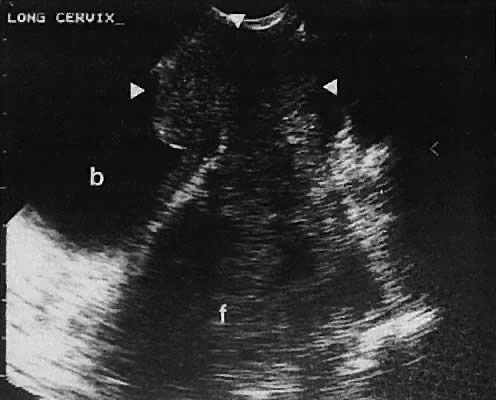
The uterine cervix is visible and may be measured with a great degree of accuracy, especially with the transvaginal technique. Remember that with the transvaginal approach, the cervix may not be seen if the scanning tip is placed in either the anterior or posterior fornix. Therefore, careful scanning during insertion and removal of the scanning transducer is advisable.
Urinary Bladder
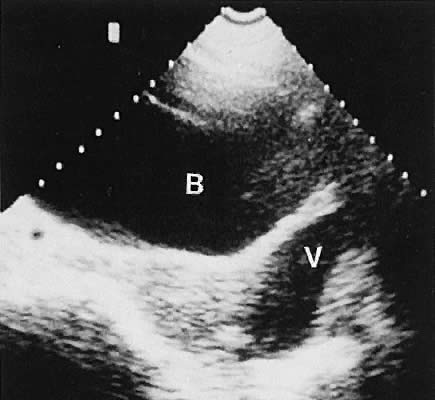
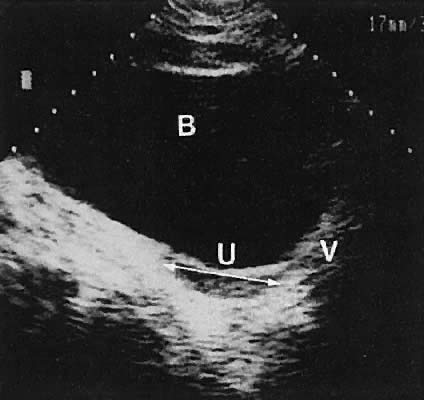
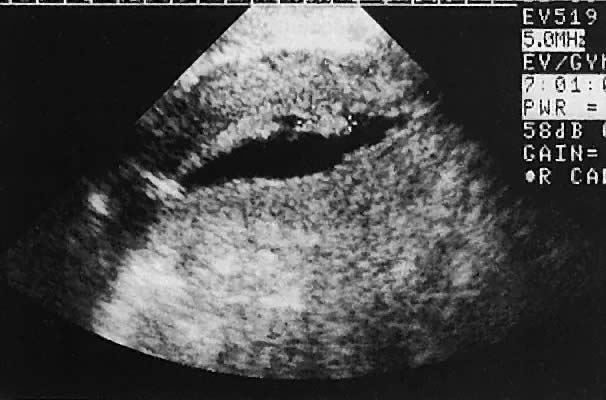
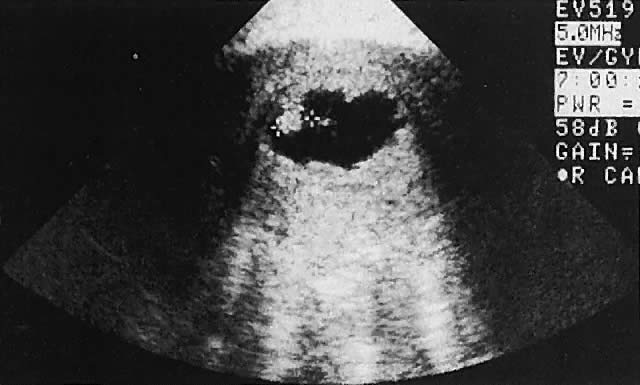
The urinary bladder usually is clearly seen and represents another landmark for anatomic orientation in transvaginal and transabdominal scanning. The bladder should be partially distended before attempting transabdominal scanning. Caution must be used to differentiate a full urinary bladder from a unilocular, anechoic-type ovarian cyst that may lie anterior to the uterus. If any question regarding this possibility exists, a postvoid scan is advisable for definitive evaluation.
Excessive filling of the urinary bladder displaces the uterus so posteriorly that not only does the patient experience undue discomfort, but adequate imaging is difficult. Conversely, in the interpretation of transabdominal images with inadequate bladder filling, significant posterior uterine wall or fundal disease may be missed. The appropriate amount of urine in the bladder for optimal visualization varies from patient to patient.
During insonation of unilocular cystic structures, a proximal artifact may occur as a result of near-field sensitivity or of the “gain setting,” producing near-field reverberation artifact. To the uninitiated, this echo may appear to represent intracystic echo-dense areas. Variation of the sensitivity (gain setting) of the equipment allows these areas to be differentiated from more significant findings.
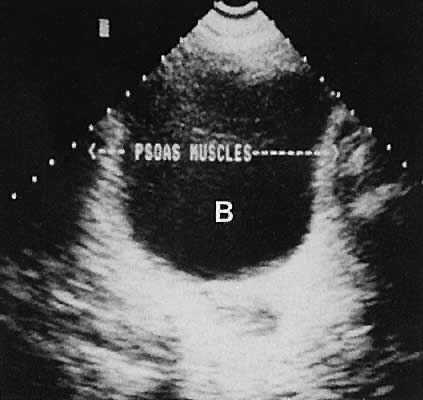
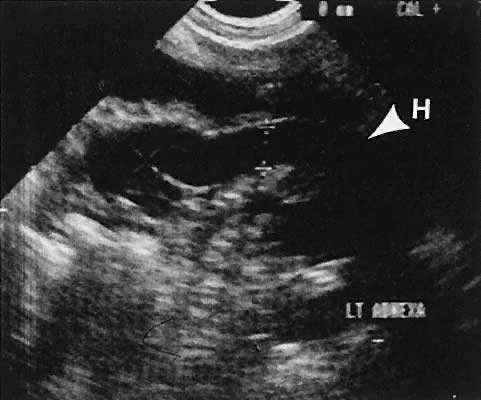
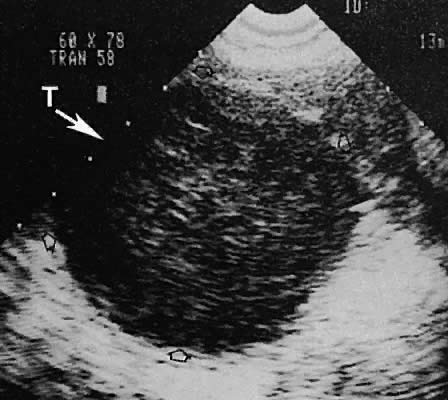
On either side of the urinary bladder in the anterolateral pelvic area are the iliopsoas muscles. These areas should not be confused with pathologic pelvic masses (Fig. 4).
Frequently, the urethra and the urethrovesical junction can be visualized. Transvaginal or perineal (introital) scanning enhances the imaging of these structures.
Vagina
The vagina appears as a collapsed tubular structure lying inferior to the urinary bladder and distal to the uterine cervix by transabdominal scanning. Transvaginal ultrasonography does not delineate the vagina as well as the transabdominal or perineal (introital) approach. Anomalies of vaginal development are discussed later.
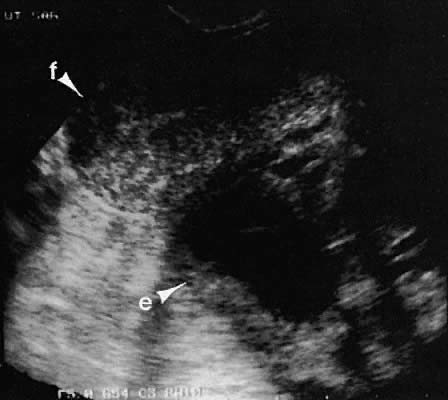
Occasionally, with overdistention of the urinary bladder, urine may accumulate in the vagina (Fig. 5). Likewise, the presence of tampons or menstrual blood may be discerned.
|
Adnexa
The adnexa include the ovaries, fallopian tubes, blood vessels, supporting ligaments, and peritoneal folds of the lateral pelvis. The main structures that are recognizable with ultrasonography include the ovary, fallopian tube, and vascular anatomy.
OVARY.
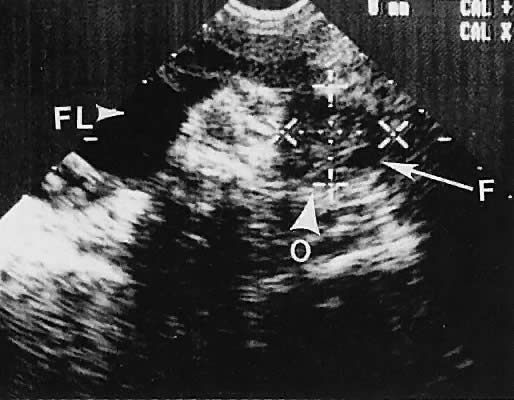
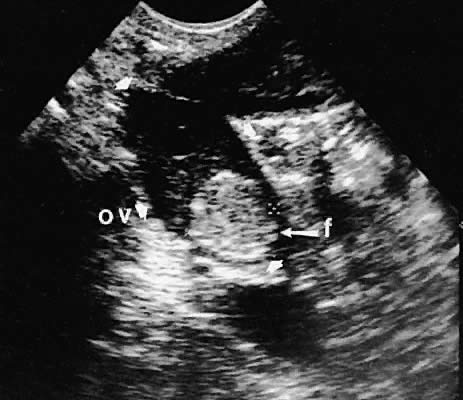
The position of the ovary is variable, depending on the length of the infundibulopelvic ligament, the presence or absence of adhesions, and other anatomic abnormalities that may displace the ovary. Usually, the ovaries lie in a lateral position to the uterus and are identifiable by scanning in transverse or longitudinal planes lateral to the uterine corpus. Identification of the internal iliac vessels with transvaginal ultrasonography is helpful in identifying the appropriate location of the ovary, but manipulation of the scanning transducer to bring out the full extent of the ovarian echo frequently is necessary. During transvaginal scanning, the manipulation should be performed slowly, and patient cooperation is helpful. In the absence of pelvic adhesive disease, the ovary moves in response to transducer manipulation.
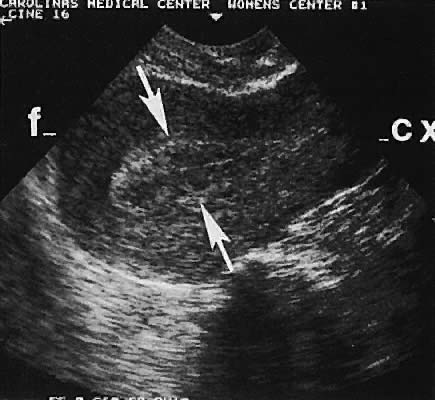
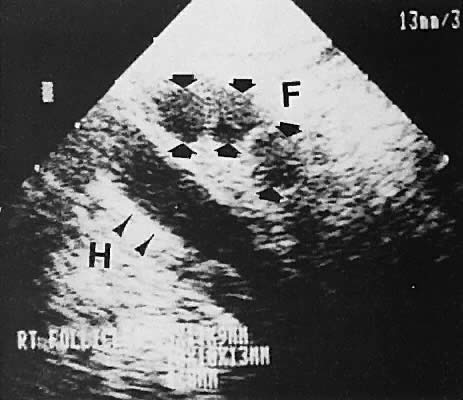
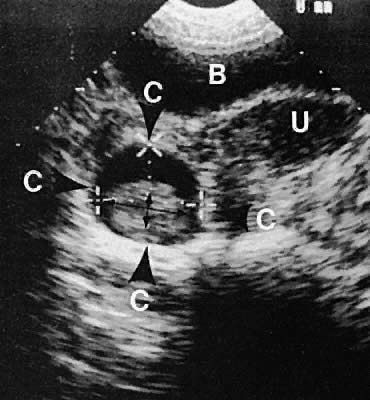
With high-resolution ultrasonography, the ability to monitor follicular development exists. Follicles are clearly visible in most ovaries in women of reproductive age and appear as echo-sparse, well-circumscribed areas within the ovarian stroma, varying between 5 and 20 mm in diameter (Fig. 6). Ultrasonographic follicular monitoring has become an integral aspect of ovulation induction protocols by allowing correlation of serum estradiol levels with follicular diameter during gonadotropin stimulation. A follicular diameter of 18 to 22 mm is characteristic of a periovulatory follicle.20,21
|
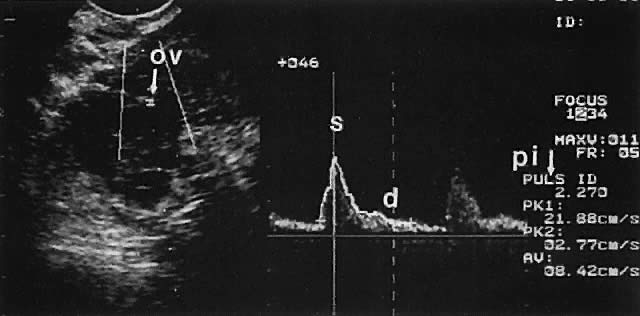
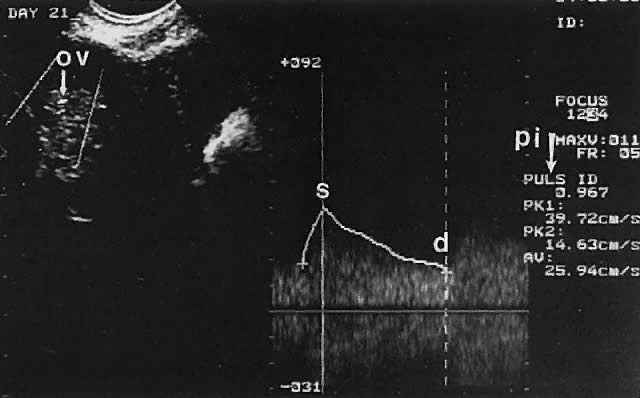
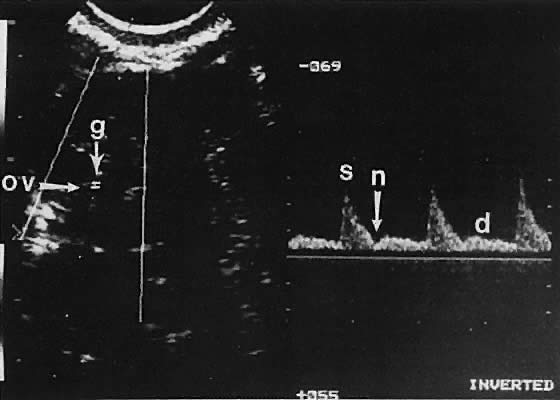
Ovarian blood is supplied mostly by the ovarian artery coursing through the infundibulopelvic ligament. Velocity waveform analysis reveals increasing diastolic velocity in the periovulatory and luteal phase (Figs. 7 and 8).
FALLOPIAN TUBE.
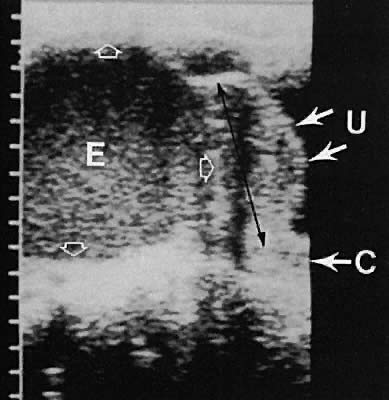
The fallopian tube is difficult to visualize in the normal state. Frequently, in cases of abnormal tubal morphologic conditions such as after the development of a hydrosalpinx or neoplasm, the tube may be more clearly defined. Transvaginal ultrasonography results in a higher frequency of tubal visualization. A hydrosalpinx typically is a convoluted, anechoic tubular structure (Fig. 9). Frequently, the tube and ovary form a complex, echo-dense adnexal mass in cases of adhesive inflammatory disease of the pelvis or a neoplastic process.
Other Pelvic Findings
TRUE AND FALSE LIGAMENTS OF THE UTERUS.
The supporting uterine ligaments rarely are clearly visualized with ultrasonography. The folds of peritoneum covering the vascular and lymphatic supply to the ovary and uterus (infundibulopelvic and broad ligaments) are not true ligaments and are not seen; the uterosacral ligaments also are not usually seen. The round ligament, which is a tubular structure composed of smooth muscle, may be seen. Variable echo densities and reflected echoes may result from inflamed peritoneal surfaces involved in adhesive pelvic disease. In most instances, however, the echo pattern is such that although a more echo-dense peritoneal area may be visualized, a definitive diagnosis usually is not possible.
BOWEL CHARACTERISTICS.
The presence of gas and feces in the bowel produces a variably dense echo return. Peristalsis is seen easily. Frequently, gas-filled bowel has proximal echoes with poor distal echoes from gas attenuation of the ultrasound energy. Occasionally, a distended loop of bowel may be confused with a complex cystic or solid adnexal mass. The possibility of a primary bowel process must be considered in the diagnosis of adnexal processes.
URETERS.
The ureters rarely are visualized with ultrasonography unless they are specifically searched for and dilated. In transverse section, the ureter may be seen juxtaposed near the lateral border of the uterine cervix. Most ureteral imaging using either the transabdominal or transvaginal route is done when there is a concern regarding a potential ureteral dilation, as in patients with parametrial extension of cervical carcinoma.
PELVIC VASCULAR ANATOMY.
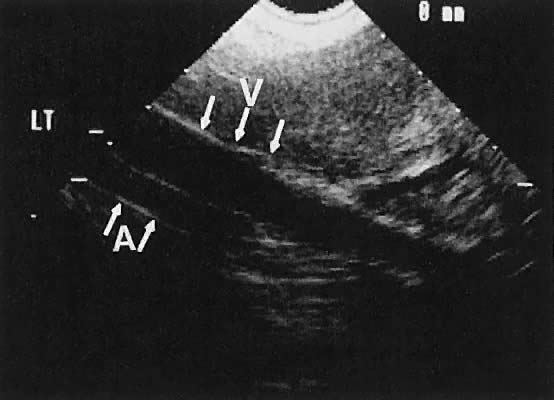
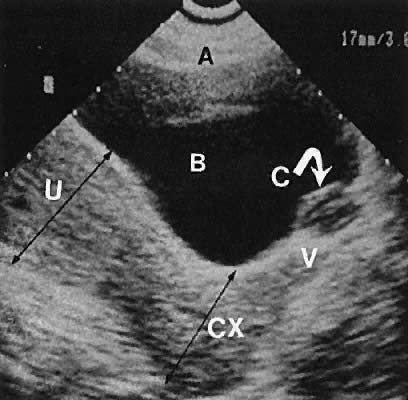
The internal iliac vessels, as previously noted, are landmarks for ovarian location (Fig. 10). The uterine arteries are visualized occasionally and frequently exhibit prominent pulsations in early pregnancy. Pelvic vessels that are amenable to insonation for Doppler study include the ovarian and uterine arteries, as well as vascular structures within the stroma of pelvic masses.
CUL-DE-SAC FLUID ACCUMULATION.
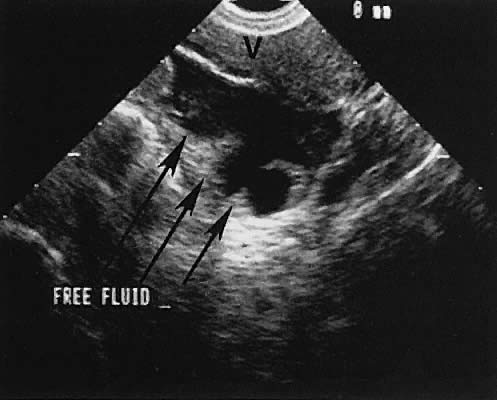
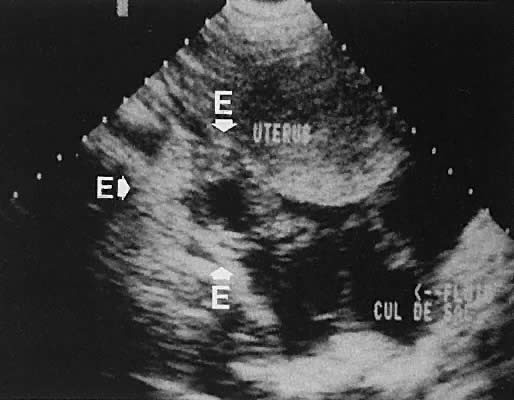
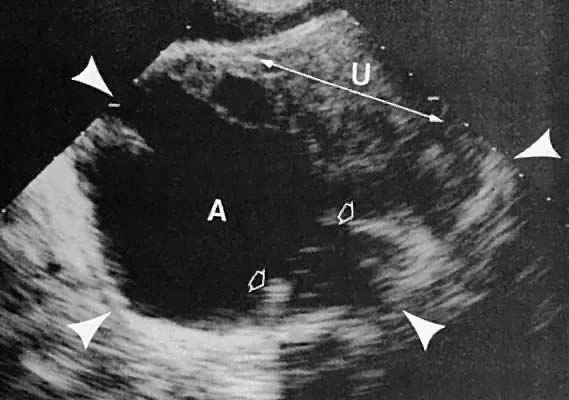
The presence of fluid in the cul-de-sac is a frequent finding. Small amounts of peritoneal fluid accumulate in the inferior-most portion of the cul-de-sac as a result of the menstrual cycle. Massive accumulations of fluid may exist in cases of ovarian carcinoma (Fig. 11). The hemoperitoneum of ruptured tubal pregnancy is apparent during transabdominal or transvaginal scanning (Fig. 12).
SCANNING TECHNIQUES
Two basic scanning approaches are applicable to gynecologic ultrasound imaging: transabdominal and transvaginal. The transabdominal technique refers to insonation of the pelvis through a partially distended urinary bladder to minimize the acoustic impedance of interposed bowel gas. This scanning technique may be performed with static scanning or real-time equipment offering sagittal, axial, and oblique planes of pelvic anatomy for evaluation.
Static scanners produce repetitive still images of pelvic anatomy. Echoes that return from multiple acoustic interfaces are detected by a manually maneuvered transducer and are electronically combined to produce the final display. Real-time scanners differ from static scanners in that the image approximates actual motion. The image is created by displaying multiple returning images at a rapid frame rate to reflect the activity.22
The evaluation of blood flow depends on the imaging of a flow velocity waveform depicting an upstroke (systolic) velocity generated by myocardial systole and interval velocity, which is a function of downstream resistance. The vessels to be insonated are readily delineated by color Doppler and studied by specific vessel insonation to establish the Doppler waveform mentioned earlier (Fig. 13).
The transvaginal technique, which uses a transducer inserted into the vagina, has emerged as a separate scanning technique applicable to gynecologic ultrasonography.23 Sagittal, coronal, and oblique images of the pelvic viscera may be obtained, as demonstrated in Figures 14 and 15. Transvaginal ultrasonography offers several advantages over transabdominal ultrasonography. First, the scanning tip may be placed closer to the scanning target, thus enhancing resolution. Second, a full urinary bladder is not needed, resulting in a procedure that is more acceptable to patients. Third, transvaginal ultrasonography interfaces nicely with the pelvic examination, which can be performed at the same sitting. Fourth, the image quality of transvaginal ultrasonography has been found superior to transabdominal ultrasonography in most instances, although the depth of penetration is comparatively limited. Finally, the transvaginal approach offers superior tissue characterization of the uterus and ovaries.24
|
|
A complete ultrasonographic evaluation of the pelvis, however, should include transabdominal ultrasonography if any question exists regarding the possibility of pedunculated masses, ovarian cysts that may be on long pedicles, or other masses that may not be readily visible by the vaginal route. Similarly, a postvoid transabdominal scan may allow visualization of previously unapparent masses.25
With either technique, the ultrasonographer must use a deliberate scanning technique that allows interpretation of echoes in an organized and thorough manner, paying constant attention to orientation. The entire pelvis should be explored with documentation of the appearance of the adnexa, uterus, cervix, and any other significant pelvic structures. Although Doppler insonation is feasible with either technique, most favor the transvaginal Doppler approach because of its ability to approximate the pelvic vasculature.
MINIMIZING ARTIFACTS
Artifactitious echoes may produce confusing images. Procedures that minimize artifacts include avoiding interspersed air between the scanning tip and the imaging target, using the appropriate sensitivity setting of the scanning instrument, using variable bladder distention, and carefully integrating ultrasonographic images with knowledge of the expected pelvic anatomy. Nonetheless, the ultrasonographer should realize the potential for a certain echo to be artifactitious. The realization of this possibility minimizes the likelihood that an artifact will be interpreted as a significant pathologic process. A repeat study after bowel evacuation may be helpful.
CHARACTERIZATION OF PELVIC MASSES
Ultrasonographically detected masses should be classified as predominantly cystic or solid. Cystic masses produce anechoic or hypoechoic images with excellent through-transmission of sound, resulting in a bright, distal surface (acoustic enhancement). Solid masses attenuate the sound energy and result in poor penetration. Masses containing gas also demonstrate poor sound transmission with clear proximal borders and indistinct distal boundaries.26 Cystic masses containing blood, tissue fragments, or other material are described as complex because they produce a cystic appearance as judged by sonic transmission, but the echo density of the intracystic material is greater than that of fluid alone.27 This type of echo pattern frequently is referred to as low-level echo density.
In addition to ultrasound characteristics, masses should be categorized by the suspected site of origin or location (e.g., uterine, ovarian, adnexal, cul-de-sac). If the site of origin is unclear, then a statement delineating separate, noninvolved organs frequently is helpful (e.g., an adnexal mass that does not appear to arise from either ovary).
The size of pelvic masses usually is measured along specified scanning planes to allow volume assessment if desired. In addition, the character of a cyst wall (smooth versus irregular) and intracystic anatomic appearance (e.g., septated, papillary) also assists in establishing the likelihood of a neoplastic or reactive (inflammatory or endometriotic) process, as opposed to a functional process.
GYNECOLOGIC ABNORMALITIES
Congenital Abnormalities
MÜLLERIAN (PARAMESONEPHRIC).
Müllerian anomalies of the reproductive tract are divided into two broad categories:
- Patients with a normal 46,XX karyotype who exhibit abnormalities of the reproductive tract secondary to partial or complete failure of müllerian development or fusion
- Patients with abnormal karyotypes, including lack of an X chromosome (45,X), presence of a Y chromosome or portion of a Y, or a mosaic combination of these karyotypes
Patients With Normal Chromosomes.
Anomalous development in patients with a normal 46,XX karyotype usually involves failure of canalization of the developing müllerian tubercle as it meets the invaginating urogenital sinus or incomplete fusion of the paired müllerian ducts. Anomalies resulting from abnormal müllerian tubercle fusion with the urogenital sinus and canalization include imperforate hymen and transverse vaginal septum. During attempted transvaginal scanning, a hematocolpos may be noted as cystic dilation of the superior vagina with cephalad displacement of the uterus and other pelvic viscera28,29,30,31,32,33,34 (Figs. 16 and 17). Ultrasonographic guidance may offer intraoperative assistance in correcting these anomalies.35
|
Patients with failure of müllerian differentiation to the degree where there is no discernible uterus or cervix and only small fibromuscular condensations of tissue near the gonad may represent the Mayer-Rokitansky-Küster-Hauser syndrome ( müllerian agenesis).36 Ultrasound evaluation of these patients reveals no apparent uterus, but gonadal development is noted in the adnexal areas.33
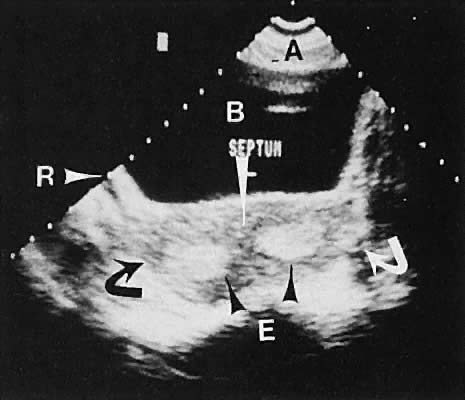
Abnormalities of müllerian fusion in chromosomally normal 46,XX patients may result in a variety of uterine abnormalities, ranging from a true bicornuate uterus to uterine septation.37,38,39,40 The presence of two distinct endometrial cavities is discernible by ultrasonography; however, some difficulty may be encountered in establishing whether a septate or bicornuate uterus exists (Fig. 18). The association of müllerian anomalies with renal anomalies must be kept in mind.
Patients With Abnormal Chromosomes.
Patients with androgen insensitivity syndrome, who manifest a 46,XY karyotype, exhibit no uterine development and have intra-abdominal testes. The shallowness of the vagina in these patients, along with sparse pubic hair, chromosomal constitution, and serum androgen concentration, is helpful in the differential diagnosis of this syndrome and müllerian agenesis. Gonads containing a Y chromosomal component or a portion of a Y chromosome should be removed because of the risk of gonadoblastoma development in the gonad.41
Patients with 45,X gonadal dysgenesis have a sexually infantile body habitus and prepubertal uterine morphologic characteristics42 (Fig. 19). Monitoring of the change in uterine morphologic features secondary to estrogen replacement therapy reveals a transformation of the uterus into a more adult-type appearance with fundal dominance (Fig. 20).
In summary, transabdominal and transvaginal ultrasonography are excellent additions to the pelvic examination in patients believed to have anomalous reproductive tract development. The transperineal technique may be beneficial in the peripubertal patient who is difficult to examine and who has signs and symptoms of possible cryptomenorrhea.37
WOLFFIAN (MESONEPHRIC).
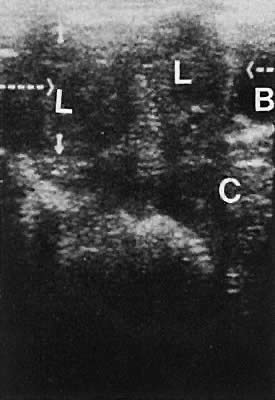
Nonanomalous embryologic remnants of the wolffian ducts are seen occasionally. Epoophoron cysts or hydatids of Morgagni produce small anechoic areas adjacent to the ovary. Gartner duct cysts are located in the anterolateral areas of the vagina and also are anechoic and unilocular (Fig. 21). These structures are of minimal clinical significance and rarely warrant significant management.
OTHER ABNORMALITIES OF DEVELOPMENT.
Uterine and cervical abnormalities from in utero exposure to diethylstilbestrol have been documented. The depiction of this abnormality by ultrasonography has not been as clear as with hysterosalpingography.43
Gynecologic Conditions of the Uterus
MYOMETRIUM.
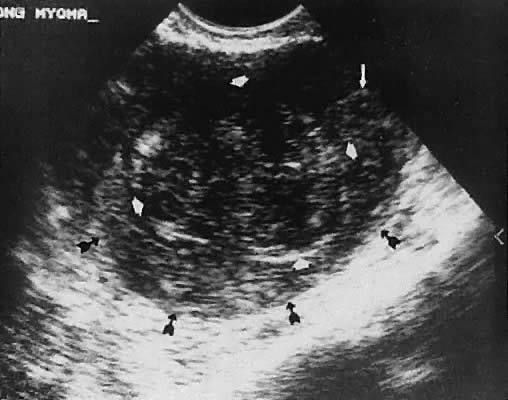
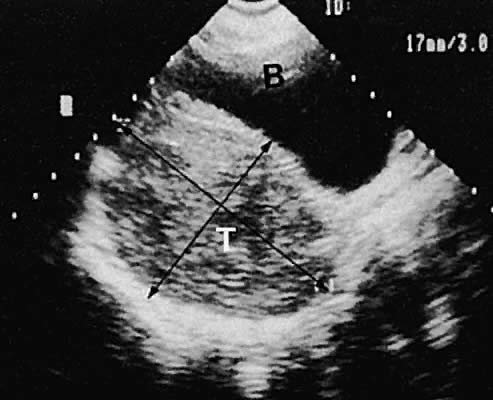
Leiomyoma. Uterine enlargement that is not caused by pregnancy most often is a result of uterine leiomyomata. Uterine leiomyomata represent proliferations of smooth muscle and are benign neoplasms. Leiomyomata are classified by their location as subserous, intramural, or submucous. Ultrasonographically, leiomyomata exhibit poor sound transmission because much of the sonic energy is attenuated by the solid consistency of the mass. Uterine contour irregularity is one of the most consistent findings44; however, a variety of findings may occur.45 Ultrasonography offers the potential of measuring uterine leiomyomata in patients in whom a conservative or nonsurgical management plan is initiated46 (Figs. 22 and 23). In addition, ultrasonography may detect early signs of degeneration or calcification of uterine leiomyomata. The effect of gonadotropin-releasing hormone suppression of uterine leiomyomata is easily monitored by ultrasonography. Occasionally, leiomyomata may undergo degeneration and mimic other cystic pelvic masses.47,48
|
Leiomyosarcoma.
Rarely, a malignant leiomyosarcoma may be found. Leiomyosarcomas present a complex echo pattern analogous to uterine leiomyomata with degeneration. Differential diagnosis of these clinical phenomena requires more extensive analysis. Rarely, 2D imaging is diagnostic of this process, but evidence of increased diastolic velocity with Doppler ultrasound may assist in the diagnosis.49
ENDOMETRIUM.
The endometrium is clearly imaged with pelvic ultrasonography, especially the transvaginal technique. Endometrial thickness correlates well with the endometrial response to estrogen stimulation. Morcos and associates50 noted that an endometrial echo greater than 1.5 cm had a sensitivity of 94% and specificity of 93% in predicting a withdrawal bleeding response to progesterone in amenorrheic reproductive-age women.
The instillation of a small quantity of sterile saline into the endometrial cavity (saline infusion sonography) creates an ultrasonographic interface between solid tissue and liquid. This results in improved visualization of the endometrium in general and the endometrial surface in particular. This procedure also has been termed hysterosonography, sonohysterography, or hydrosonography.
A small polyethylene catheter or pediatric Foley catheter is inserted through the cervix into the endometrial cavity after appropriate cleansing with an antiseptic solution. Concurrent real-time imaging using the transvaginal or transabdominal technique allows visualization of the separation of the endometrial surfaces as the fluid is instilled (Figs. 24 to 26).
|
|
|
Using this technique, the ultrasonographer can achieve better delineation of endometrial polyps, submucous leiomyomata, and the configuration of the endometrial cavity. There also is a potential for this technique to enhance the endometrial assessment of patients on tamoxifen therapy.
Using this technique to evaluate small endoluminal masses within the endometrium, Dubinsky and colleagues51 detected 19 endoluminal masses in 48 patients with an endometrial thickness of 5 to 10 mm. These masses were diagnosed clearly as either endometrial polyps or submucous leiomyomata.
Concurrent transabdominal scanning and transcervical instillation of saline is another technique used to visualize the endometrial anatomy. Cicinelli and coworkers52 report a sensitivity of 100% in the diagnosis of submucous leiomyomata with this technique. An excellent description of a variety of hydrosonograpic findings was reported by Goldstein.53
Transvaginal ultrasonography offers the opportunity to visualize the endometrium (Figs. 27 and 28). To select patients at an increased risk of endometrial disease, several authors have evaluated the reliability of inferring endometrial histologic changes from measuring the width of the endometrial echo (Fig. 29).
Granberg and associates54 evaluated 205 women with postmenopausal bleeding and noted benign endometrial histologic change with an endometrial width (thickness) of less than 9 mm. The mean endometrial width in patients with endometrial cancer was 18.2 ± 6.2 mm compared with 3.4 ± 1.2 mm in those with an atrophic histologic type. Malpani and coworkers55 reviewed endometrial thickness measurements in patients with endometrial hyperplasia compared with those with normal histopathologic features. The mean width of the endometrial echo in patients with hyperplasia was 18.8 mm compared with 5.4 mm in the benign group. Varner and colleagues56 also noted benign findings in patients with an endometrial thickness of less than 5 mm.
To compare the diagnostic accuracy of transvaginal ultrasonography, endometrial cytologic study, and histologic assessment of specimens obtained using dilation and curettage, Karlsson and associates57 performed all three of these techniques on 105 patients with postmenopausal bleeding. The sensitivity and specificity of these three modalities essentially were the same (sensitivity 81%, specificity 58%), leading these authors to conclude that the method of transvaginal scanning and endometrial thickness measurement was a “valuable adjunct” in managing these patients.
Realizing that false-positive and false-negative findings exist for most testing schemes, notice that the patient's response to therapy and persistence of concerning symptoms must be addressed with more in-depth study. To emphasize this point, a study from Norway by Dorum and coworkers58 noted that 3 of 54 patients with endometrial carcinoma had an endometrial thickness of less than 5 mm. Two patients had stage I adenocarcinoma of the endometrium; the third had endometrial involvement by a malignant lymphoma.
The likelihood of a malignancy in a patient with a thin endometrium, normal endometrial biopsy specimen, or normal specimen obtained through dilation and curettage is low. Persistent bleeding, failure of expected response to therapy, development of new symptoms, or persistence of concerning symptoms require further evaluation. Therefore, a postmenopausal patient with a thin (less than 5 mm) endometrial width may be managed without endometrial sampling or curettage for abnormal bleeding, assuming an appropriate response to therapy follows.59
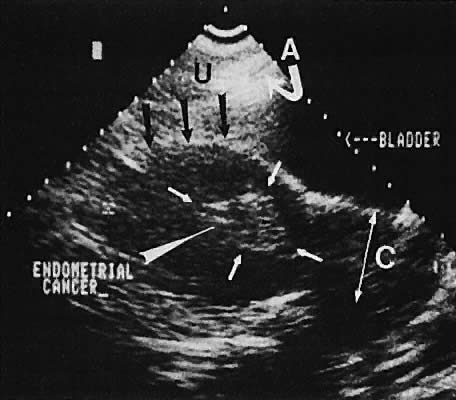
The value of ultrasound assessment of endometrial carcinoma, particularly the depth of invasion of the myometrium, is under investigation. Endometrial carcinoma produces an irregularity of the endometrial cavity in advanced cases60,61,62,63 (Fig. 30). Of 25 cases of endometrial carcinoma, the correct depth of invasion was predicted in 21.64 In a separate study, Conte and associates65 confirmed these data, predicting the correct amount of myometrial invasion in 18 of 20 patients.
The finding of endometrial fluid should heighten the ultrasonographer's suspicion of unapparent abnormalities of the genital tract. Of 17 postmenopausal patients with endometrial fluid collection, 5 had malignancies: 2 had ovarian cancer, 1 had tubal carcinoma, 1 had endometrial carcinoma, and 1 had cervical carcinoma.66
CERVIX.
In addition to visualization of the volume of cervical cancer,67 the evaluation of parametrial involvement also has been performed (Fig. 31). Using a transrectal probe, Yamamoto and Kitao68 evaluated the accuracy of ultrasonographic prediction of parametrial involvement compared with computed tomography and Federation of International Gynecology and Obstetrics staging and noted a sensitivity of 83% and specificity of 97%. The potential also exists to assess Doppler flow characteristics of the uterine arteries in cervical malignancy.
GYNECOLOGIC CONDITIONS OF THE ADNEXA
Functional Ovarian Processes
FOLLICULAR MATURATION.
As noted earlier, in addition to the delineation of specific ovarian masses, follicular maturation may be monitored by serial transabdominal or transvaginal ultrasonography.69 Preovulatory follicular development is seen clearly with progression of follicle diameter to the 18 to 22 mm range at midcycle. Follicular dominance and progression are monitored easily by real-time ultrasonography in correlation with serum estradiol concentration to induce ovulatory ovarian function70,71,72,73,74,75 (Figs. 32 and 33). Transvaginal-directed oocyte retrieval for assisted reproductive techniques is the preferred method of acquiring oocytes.
|
POLYCYSTIC OVARY SYNDROME.
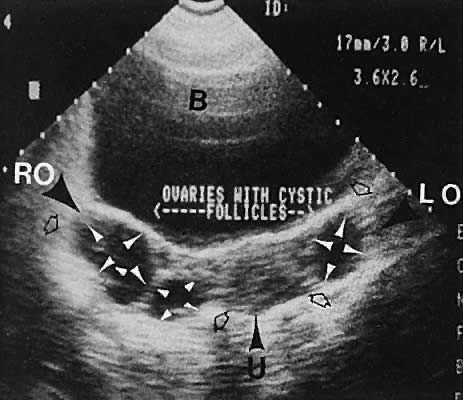
Polycystic ovary syndrome produces a wide spectrum of menstrual disturbances and manifestations of androgen excess. The ovary also may exhibit a variety of appearances, ranging from multiple cystic follicles (up to 2.5 cm) of varying sizes to only a few follicle cysts (larger than 2.5 cm)76,77,78,79,80,81,82 (Fig. 34). In cases of hyperthecosis, in which fewer follicles are developed, the ovary would be expected to exhibit a more solid echo pattern. Monitoring of the changes in ovarian volume with suppressive treatment with gonadotropin-releasing hormone analogs has been described.83
OVARIAN MASSES.
The ovary has the capacity to develop many different cystic and solid masses. Transvaginal and transabdominal ultrasonography can categorize adnexal masses into various echo densities and morphologic types, thereby narrowing the differential diagnosis. The reliability of ovarian size assessment has been documented to be high, with a correlation coefficient of 0.960 between observers documented by Higgins and coworkers.84
Ovarian Cysts.
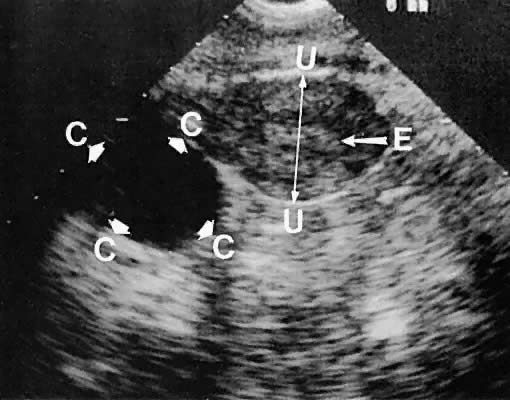
Unilocular cystic masses with few intracystic echoes are most likely serous-type cysts of the ovary (cystic follicles or follicular cysts) or serous cystadenomas (Fig. 35). Cystic masses with complex echogenic intracystic echoes are more commonly hemorrhagic in origin (hemorrhagic corpus luteum cysts or endometriomata).85
A more conservative management approach is feasible in patients with reassuring intracystic morphology (e.g., anechoic, smooth wall, no papillary excrescences). The decreased need for surgery in children was demonstrated retrospectively in 1989 by Thind and colleagues,86 who reviewed the cases of 64 children with ovarian cysts. In addition, the potential also exists for therapeutic cyst aspiration using ultrasound guidance of selected cysts in young women.87,88 In patients for whom expectant management is elected, comparative studies after subsequent cycles or hormonal therapy is feasible, especially with the transvaginal technique.
The differentiation between endometriomas and nonendometriotic ovarian cysts was investigated by Guerriero and associates,89 who evaluated 251 premenopausal, nonpregnant patients using transvaginal ultrasound. Of the 31 diagnosed as ovarian endometriomas, 24 were confirmed as endometriomas by histologic assessment. The sensitivity of transvaginal ultrasound in differentiating endometriotic cysts from cysts of other etiologies was 83%, and the specificity, 89%. The authors observe that this was compatible with the diagnostic accuracy of magnetic resonance imaging.
Controversy exists regarding the significance of cystic masses in the postmenopausal age group. Fleischer and associates90 published an excellent correlation of ultrasonographic findings with subsequent histopathologic features of 67 ovaries in 37 postmenopausal patients. A positive predictive value of 92% and a negative predictive value of 92% were noted in this highly selected population. Luxman and colleagues91 noted that 2 of 29 ovarian cancers were not detected by transabdominal ultrasonography. Goldstein and coworkers92 and Andolf and Jorgensen93 noted no malignancies in hypoechoic cysts smaller than 5 cm in more than 190 patients.
Therefore, small, nonsuspicious cystic areas in postmenopausal patients may be followed conservatively with surgical evaluation for persistent cysts or those that progressively increase in size. The absolute maximum cystic diameter that may be followed safely is debatable but appears to be in the 3.5- to 5-cm range. If a noninterventional posture is selected, the ultrasonographer must realize that a small likelihood of an unapparent malignancy exists and that close follow-up is mandatory.
Ovarian Neoplasms.
Germ Cell Tumors. The most common neoplasms of reproductive-age women are germ cell tumors. Cystic teratomas are wellcircumscribed, complex, cystic masses that usually are unilateral and exhibit a variably complex, intracystic echo pattern94,95,96 (Figs. 36 and 37). This type of tissue characterization is important because these complex cystic masses require surgical evaluation for definitive therapy more often than the smaller, unilocular, serous-type cysts, which may be followed expectantly. Rupture of cystic teratomas during pregnancy is a major complication that should be avoided.97 Although the specific pathologic diagnosis of an ovarian mass cannot be made ultrasonographically, ultrasound can be used to categorize and describe these masses to establish a more precise differential diagnosis.98
|
Stromal Neoplasms.
Solid ovarian neoplasms include those arising from ovarian stromal cells (fibroma and thecoma)99 (Fig. 38) and the Brenner cell tumor.100
|
Ovarian Carcinoma (Epithelial Ovarian Tumors).
Ovarian malignant neoplasms produce a variety of ultrasonographic patterns that typically produce an image of mixed solid and cystic components, irregular septations, and coexistent ascites, and they are frequently bilateral101,102 (Figs. 39 and 40).
|
The role of ultrasonography in screening for ovarian cancer is controversial. An ever-increasing body of literature continues to question the cost-effectiveness of ultrasound as a screening modality. A thorough review of this controversy is beyond the scope of this chapter. Ultrasound screening for ovarian cancer is not widely accepted. The combined use of tumor-associated antigens (e.g., CA-125) with ovarian imaging by 2D technique and Doppler may offer an enhanced potential for diagnosis of ovarian malignancies in high-risk groups.14,103,104
The salient question that must be answered before recommending universal ovarian cancer screening with this modality encompasses the reliability and cost-effectiveness of the screening protocol to detect early (stage I or II) disease. The realization that CA-125 levels are not elevated in many cases of early stage disease raises a serious question regarding the efficacy of this serum test as a screening component. The question of routine ovarian cancer screening remains unanswered. The role of ultrasonography in staging and follow-up of ovarian cancer continues to expand. Conte and coworkers105 describe the potential value of ultrasound study in the preoperative staging of ovarian carcinoma. Although ultrasonography can detect macroscopic residual disease with acceptable accuracy, its limitation in detecting small residual disease in lieu of second-look laparotomy has been demonstrated by Murolo and associates.106
Breast cancer and gastrointestinal tract cancer may metastasize to the ovary. Other ovarian neoplasms have been described ultrasonographically, including Brenner and Krukenberg tumors.107,108 The Krukenberg tumor has been characterized ultrasonographically as a complex solid mass and a cystic mass but is unlike the ultrasound picture of primary ovarian carcinoma.109 Ovarian neoplasms may be imaged more precisely by the vaginal approach. Figure 41 shows the image quality of an ovarian thecoma by transvaginal ultrasonography.
Many articles have been published further describing the ultrasonographic 2D image characteristics of ovarian cancer. In addition, Doppler interrogation of small vessels detected by color Doppler imaging reveals increased diastolic flow velocities and evidence of neovascularization secondary to tumor angiogenesis. The resultant calculations of waveform flow velocity ratios are therefore decreased.
Multiple scoring systems for 2D findings and 2D and Doppler findings also have been created. The details of these systems are beyond the scope of this chapter. The various scoring systems offer the potential of assigning numeric values to the well-known findings of ovarian malignancy, such as thickened cyst walls, septations, papillary excrescences, complex intratumoral echoes, and disorganized echo texture. The addition of Doppler waveform analysis frequently confirms the concern of nonreassuring 2D findings but rarely increases concern in patients with benign-appearing cysts on 2D findings.
Brown and coworkers110 evaluated 44 masses with 2D and color Doppler imaging. They noted significant overlap in the resistance index and pulsatility index between benign and malignant masses. This finding contrasts with that of other investigators.111
FALLOPIAN TUBE CARCINOMA.
Although less common than ovarian carcinoma, cancer of the fallopian tube exhibits a similar clinical course. The complex echo pattern of tubal carcinoma diagnosed by transvaginal ultrasonography has been described.112,113,114
Pelvic Inflammatory Disease and Endometriosis
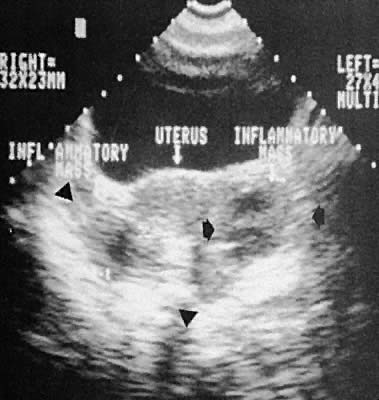
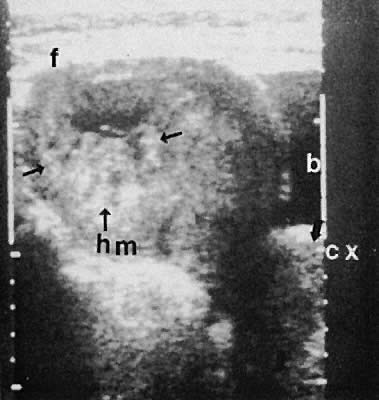
Other adnexal processes exclusive of neoplastic changes include pelvic inflammatory disease and endometriosis. Pelvic inflammatory disease most often results from a primary salpingo-oophoritis that has progressed to some degree of hydrosalpinx formation with adhesive pelvic disease. A variety of ultrasonographic findings with differing types of complex adnexal masses result with frequent visualization of tubal dilation and intrafallopian tube fluid (hydrosalpinx formation).115 The ability to diagnose and guide aspiration of tubo-ovarian abscesses with transvaginal ultrasonography has been documented.116,117,118,119,120
Frequently, it is difficult to establish the precise etiology of complex adnexal masses on an endometriotic or inflammatory basis.121,122,123 Endometriosis also produces complex adnexal masses with distortion of the pelvic anatomy typical of adhesive disease (Figs. 42 to 45). In some instances, endometriotic involvement of a nongynecologic structure, such as the urinary bladder or liver, has been noted.124,125,126
|
Intrauterine Devices
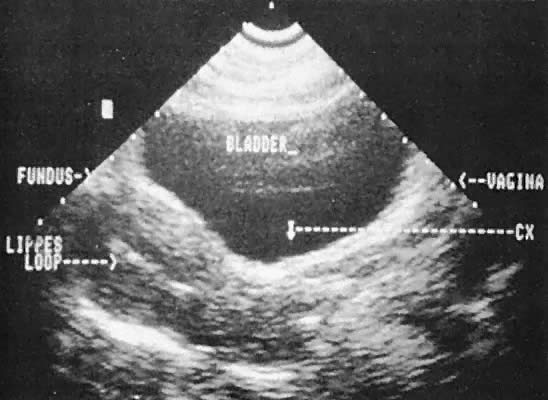
Ultrasonographic localization of intrauterine devices has been possible for years (Fig. 46). The advent of transvaginal scanning increases the accuracy of intrauterine device localization.127 Removing intrauterine devices under transabdominal ultrasound guidance offers a more precise and potentially less traumatic removal in difficult clinical settings.128,129 The ability to extract an intrauterine device that is juxtaposed, but inferior to, an early intrauterine pregnancy also is occasionally enhanced by transabdominal ultrasound guidance.
Early Pregnancy
INTRAUTERINE PREGNANCY.
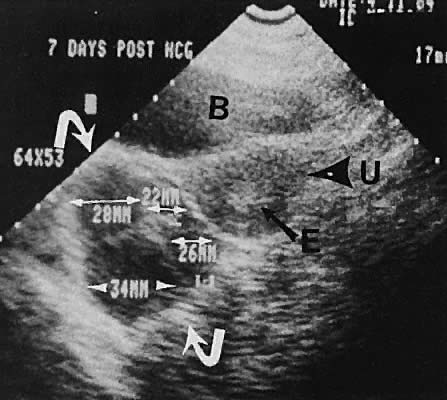
The most common cause of uterine enlargement is early pregnancy. The advent of transvaginal ultrasonography offers the capability of extremely early diagnosis of pregnancy. The prominent echogenicity of decidualized endometrium is seen clearly on transabdominal or transvaginal scanning before the visualization of a gestational sac.130,131 With currently available transvaginal imaging techniques, a gestational sac that is located within the uterine cavity and is developing normally should be clearly visible, concomitant with a serum human chorionic gonadotropin (hCG) concentration of 1000 mIU/mL (Second International Standard [2nd IS]).132 Using the transabdominal technique, the same authors report the discriminatory zone to be 1800 mIU/mL (2nd IS).133 Notice that the First International Preparation (1st IRP) standard results in hCG values that are approximately twice the 2nd IS values. Other publishedanalyses of transvaginal ultrasound show lower discriminatory zones of 300 mIU/mL (2nd IS),134 450 to 750 mIU/mL (presumed 2nd IS),135 and 1100 mIU/mL (1st IRP).136 From a clinical standpoint, if a quantitative hCG exceeds 1000 mIU/mL and an intrauterine gestational sac cannot be seen with transvaginal scanning, the suspicion of an ectopic pregnancy must increase, and serial follow-up or further evaluation is indicated. Studies of serial scanning of patients conceiving during in vitro fertilization cycles reveal the presence of a gestational sac at even lower hCG concentrations.137
The correlation of gestational sac visualization and serum hCG concentration offers the practicing gynecologist a powerful tool for early diagnosis of extrauterine pregnancy. The principles of management, evaluation, and diagnosis presuppose knowledge of the rate of increase of hCG concentration in normal pregnancy and correlation with ultrasonographic findings. As a general rule, hCG should double every 2 to 3 days during the early first trimester.138,139,140,141 Many ectopic pregnancies exhibit a subnormal rise in hCG.
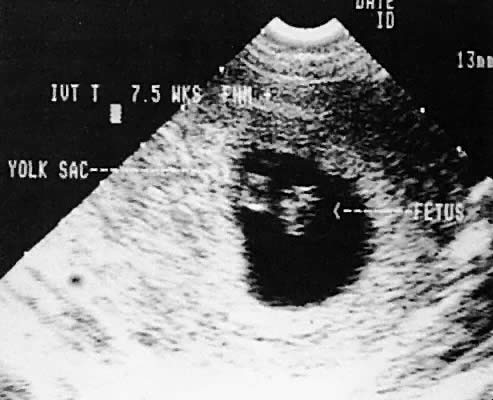
Early intrauterine pregnancy scanning displays the developing amniotic membrane, chorion, and yolk sac.130,142 Before visualization of the fetal pole, the assessment of pregnancy status depends on the rate of growth of the sac and correlation with hCG levels. Early in gestation, the mean sac growth is 0.9 to 1.13 mm/day.136 A yolk sac should be seen when the mean sac diameter exceeds 2 cm, and a fetal pole should be visible after the sac exceeds 2.5 cm143 (Fig. 47) using the transabdominal approach. Transvaginal scanning allows visualization of the embryo with a mean sac diameter of 1.2 cm.144 As the first trimester progresses, the fetus can be outlined easily, and the cephalic pole, caudal pole, limb buds, and umbilical cord can be delineated.135 The presence of fetal cardiac activity in early pregnancy scanning indicates an excellent prognosis for the pregnancy. Fetal cardiac flicker can be seen in embryos of at least 5 mm with transvaginal scanning and in embryos of at least 9 mm with the transabdominal approach.144 In published series, the likelihood of spontaneous abortion of ultrasonographically normal pregnancies in the 8- to 12-week range approximates 2% to 4%.145 Before cardiac flicker becomes apparent, correlation of the gestational sac size with quantitative hCG assessment provides important predictive information. If the hCG concentration is clearly abnormal for the stated gestational sac size, the likelihood of pregnancy progression is unlikely. The presence of a normal hCG concentration for a specified gestational sac measurement does not necessarily predict a successful outcome.146
|
The accuracy of diagnosing pregnancy failure has been enhanced by ultrasonography. Incomplete spontaneous abortion is characterized by disorganized intrauterine echoes, irregularity of the gestational sac, frequent eccentric sac location, and the evidence of intradecidual hemorrhage.147 The anembryonic pregnancy (blighted ovum) is characterized by a visible gestational sac (chorion) but no apparent fetal pole. Frequently, the sac is smaller than expected.143,148,149 When the mean sac diameter minus the crown-rump length is less than 5 mm, 94% of pregnancies were lost.150 Retention of a discernible embryo that has undergone an early death has been termed a missed abortion.
First-trimester hydatidiform mole may produce bizarre echoes in early pregnancy or may appear initially as an unremarkable gestational sac.149. Robinson HP: The diagnosis of early pregnancy failure by sonar. Br J Obstet Gynaecol 82:849, 1975
149 The ultrasound characterization of hydatidiform mole has been well described. The early diagnosis of molar pregnancy enhances appropriate management and hopefully minimizes the likelihood of malignant sequelae. Although variable, the echo pattern suggestive of a molar pregnancy consists of irregular solid and cystic interfaces within the endometrial cavity in a patient with signs and symptoms of early pregnancy (Fig. 48).
EXTRAUTERINE PREGNANCY.
Pregnancies located outside of the intrauterine cavity are referred to as extrauterine pregnancies. Although these pregnancies include ovarian pregnancies, abdominal pregnancies, and cervical pregnancies, the most common location is in the fallopian tube. This section addresses the preclinical diagnosis of unruptured tubal pregnancies using a combined approach of ultrasonographic findings correlated with serum hCG concentration.
As noted earlier, a discriminatory zone of approximately 1000 mIU/mL is being used through transvaginal ultrasonography as the level at which a normal gestational sac should be seen.132 The lack of visualization of the gestational sac with hCG concentrations greater than 1000 mIU/mL or an inadequate rate of increase of hCG is suggestive of the diagnosis of extrauterine pregnancy and warrants further evaluation.132,151
Using transvaginal ultrasound and an hCG discriminatory zone of 1500 mIU/mL (1st IRP), Barnhart and colleagues152 report that all viable intrauterine pregnancies that were visualized by ultrasound demonstrated hCG values in excess of 1500 mIU/mL. In a study of 1253 patients, there were 205 ectopic pregnancies diagnosed by the combined use of serial hCG measurements and transvaginal ultrasound. Fifty-nine percent of ectopic pregnancies never produced hCG concentrations greater than 1500 mIU/mL. In their experience, the sensitivity of this combined protocol was 100%, and the specificity, 99%.
A word of caution: multifetal pregnancies typically demonstrate a higher discriminatory zone of hCG. Kadar and coworkers153 report that a discriminatory zone of 3000 mIU/mL is required to avoid misdiagnosis of early, potentially viable multifetal pregnancy.
Because the hCG doubling time is relatively short (2 to 3 days), this difference in discriminatory hCG levels does not translate into a long time, but as noted in the study mentioned earlier,152 most ectopic pregnancies do not achieve an hCG level of 1500 mIU/mL, much less 3000 mIU/mL. To minimize the risk of intervening in a potentially normal intrauterine pregnancy, a discriminatory zone of 3000 mIU/mL and expected doubling time of 72 hours is preferable.
If this protocol is adopted, the physician must be assured of the patient's reliability and potential for follow-up, having the ability to intervene rapidly if concerning symptoms develop. This diagnostic protocol should be applied to patients who are asymptomatic or minimally symptomatic. Patients with an acute abdomen, vasomotor instability, or evidence of hemoperitoneum on transvaginal scanning should be treated surgically.
Using the transvaginal approach, an extrauterine conceptus frequently is delineated with ultrasonography, at which time definitive surgical therapy may be instituted.154,155 As noted earlier, this technique allows assessment of the cul-de-sac for fluid accumulation. If evidence of a possible hemoperitoneum is seen, transvaginally directed culdocentesis can be readily accomplished.
Rarely, a combined intrauterine and extrauterine pregnancy (heterotopic pregnancy) is seen.156,157,158,159 The incidence of heterotopic pregnancy has been noted to be more frequent in patients undergoing in vitro fertilization and warrants heightened awareness.160
The role of Doppler assessment to increase diagnostic accuracy is being evaluated. A sensitivity of 73% for early evaluation was noted in a study of 96 ectopic pregnancies by Taylor and coworkers161 using Doppler technology. Early diagnosis results in less morbidity, potential tubal salvage, and the possibility of medical therapy.162
The assessment of eccentric intrauterine implantations is more difficult. The diagnosis of a cornual or isthmic implantation site frequently is possible, and in these cases, the early diagnosis alleviates the potential for catastrophic uterine rupture.163 Chronic and interstitial ectopic pregnancies producing complex adnexal echogenic masses have been described.164,165,166,167
THREE-DIMENSIONAL ULTRASOUND
The use of three-dimensional (3D) ultrasonography in obstetrics and gynecology was first described by Kuo and colleagues in 1992.168 It has since been applied in many areas of obstetrics and gynecology; however, its use still is being investigated.
Pretorius and Nelson169 found that evaluation of the fetal face was improved with the use of 3D ultrasonography. Liang and coworkers,170 with the use of upper arm volume measurement, were able to predict fetal weight more accurately with 3D than with traditional 2D ultrasonography. Lee and colleagues171 also describe accurate fetal birth weight predictions at term; however, they recognize the question of practical clinical application.
Harika and associates172 used 3D imaging for early ectopic pregnancy in 12 asymptomatic patients before 6 weeks of amenorrhea who had no evidence of ectopic on traditional 2D ultrasound. The 3D ultrasonography showed a small ectopic gestational sac in four of nine laparoscopy-proven ectopic pregnancies.
In patients with postmenopausal bleeding, Gruboeck and coworkers173 showed that endometrial volume was superior to that of endometrial thickness as a diagnostic test for the detection of endometrial cancer in women not on hormone replacement. With a cutoff level of 13 mL for endometrial volume, sensitivity was 100% and the positive predictive value 92%. In addition to endometrial disease, volume estimation of cervical carcinoma has been studied using 3D ultrasonography.174
In differentiating benign from malignant adnexal masses, Chan and associates175 found in eight women, 3D ultrasonography confirmed the preoperative diagnoses. Wu and colleagues176 studied women with polycystic ovarian disease and compared them with routine controls. They found that stroma and volume determinations could be obtained more accurately by 3D images than by traditional ultrasonography. Not surprisingly, they found that women with polycystic ovarian disease have ovaries that are larger in size, area, and volume. Dolz and coworkers177 had similar findings.
Kupesic and Kurjak178 describe the use of different ultrasonographic imaging modalities in the evaluation of septate uterus and rate of obstetrical complications.
As 3D ultrasonography becomes more available, its application and use in clinical obstetrics and gynecology will continue to be studied.
USE OF REAL-TIME ULTRASONOGRAPHY BY GYNECOLOGISTS
Real-time ultrasonography, and more specifically, intravaginal or transvaginal real-time ultrasonography, interfaces well with the pelvic examination and offers the potential for enhanced diagnostic accuracy of gynecologic conditions. As noted earlier, the knowledge of sagittal, coronal, and axial anatomy; attention to maximizing image detail; and correlation with the patient's history and physical examination offer a comprehensive evaluation of the gynecologic patient. The role of gynecologic scanning will continue to expand. The correlation of palpable pelvic findings with visual images of tissue texture should enhance the diagnostic acumen of the clinical gynecologist. However, clinicians should continue to delineate the appropriate use of this modality, limiting it to clinical situations when the cost-benefit ratio clearly warrants its use.
In closing, a word should be mentioned regarding training and experience of the gynecologic ultrasonographer. No specific guidelines exist regarding the educational experience necessary for assurance of competence in gynecologic imaging. Prerequisites to the use of this technique are a thorough knowledge of gynecologic physiologic and pathologic features; the ability to access or obtain a thorough gynecologic history and physical examination; and experience in acquisition, display, and documentation of ultrasonographic images. Attention to continuing education through reading periodicals and taking postgraduate courses is necessary for the physician to stay abreast of this rapidly expanding field.
REFERENCES
O'Brien WF, Buck DR, Nash JD: Evaluation of sonography in the initial assessment of the gynecologic patient. Am J Obstet Gynecol 149: 598, 1984 |
|
Granberg S, Wikland M: A comparison between ultrasound and gynecologic examination for detection of enlarged ovaries in a group of women at-risk for ovarian carcinoma. J Ultrasound Med 7: 59, 1988 |
|
Wade RV, Smythe AR, Watt GW et al: Reliability of gynecologic sonographic diagnosis, 1978-1984. Am J Obstet Gynecol 153: 186, 1985 |
|
Thompson HE, Holmes JH, Gottesfeld KR et al: Ultrasound as a diagnostic aid in diseases of the pelvis. Am J Obstet Gynecol 98: 472, 1967 |
|
Queenan JT, Kubarych SF, Douglas DL: Evaluation of diagnostic ultrasound in gynecology. Am J Obstet Gynecol 123: 453, 1975 |
|
Sack RA, Maharry JM: Misdiagnoses in obstetric and gynecologic ultrasound examinations: Causes and possible solutions. Am J Obstet Gynecol 158: 1260, 1988 |
|
Willson JR: Ultrasonography in the diagnosis of gynecologic disorders. Am J Obstet Gynecol 164: 1064, 1991 |
|
Council on Scientific Affairs, American Medical Association: Gynecologic sonography: Report of the Ultrasonography Task Force. JAMA 265:2851, 1991 |
|
Timor-Tritsch IE: Is office use of vaginal ultrasonography feasible? Am J Obstet Gynecol 162: 983, 1990 |
|
Goldstein SR: Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol 162: 625, 1990 |
|
Ziskin MC: Basic physics of ultrasound. In Sanders RD, James AE (eds): The Principles and Practice of Ultrasonography in Obstetrics and Gynecology, pp 6–7. 2nd ed. New York, Appleton-Century-Crofts, 1980 |
|
Dodson MG, Deter RL: Definition of anatomical planes for use in transvaginal sonography. J Clin Ultrasound 18: 239, 1990 |
|
Rottem S, Thaler I, Goldstein SR et al: Transvaginal sonographic technique: Targeted organ scanning without resorting to “planes.” J Clin Ultrasound 18: 243, 1990 |
|
Bourne T, Campbell S, Steer C et al: Transvaginal colour flow imaging: A possible new screening technique for ovarian cancer. Br Med J 299: 1367, 1989 |
|
Kurjak A, Zalud I, Alfirevic A et al: The assessment of abnormal pelvic blood flow by transvaginal color and pulsed Doppler. Ultrasound Med Biol 16: 437, 1990 |
|
Hata T, Hata K, Senoh D et al: Doppler ultrasound assessment of tumor vascularity in gynecologic disorders. J Ultrasound Med 8: 309, 1989 |
|
Grunfeld L, Walker B, Bergh PA et al: High-resolution endovaginal ultrasonography of the endometrium: A noninvasive test for endometrial adequacy. Obstet Gynecol 78: 200, 1991 |
|
Goldstein SR, Nachtigall M, Snyder JR et al: Endometrial assessment by vaginal sonography before endometrial sampling. Am J Obstet Gynecol 163: 119, 1990 |
|
Lamki N, Athey P, Dunn G et al: Transvaginal sonographic evaluation of the retrodisplaced uterus. Can Assoc Radiol J 41: 291, 1990 |
|
Schwimer SR, Lebovic J: Transvaginal pelvic ultrasonography: Accuracy in follicle and cyst size determination. J Ultrasound Med 4: 61, 1985 |
|
Defacio J, Speroff L: Ultrasonography's role in infertility. Contemp Obstet Gynecol 25: 194, 1985 |
|
Kremkau FW: Dynamic imaging instruments. In Kremkau FW (ed): Diagnostic Ultrasound: Principles, Instrumentation, and Exercises, pp 112–113. 2nd ed. New York, Grune & Stratton, 1984 |
|
Schwimer SR, Lebovic J: Transvaginal pelvic ultrasonography. J Ultrasound Med 3: 381, 1984 |
|
Mendelson EB, Bohm-Velez M, Joseph N et al: Gynecologic imaging: Comparison of transabdominal and transvaginal sonography. Radiology 166: 321, 1988 |
|
Hill LM, Breckle R: Value of a postvoid scan during adnexal sonography. Am J Obstet Gynecol 152: 23, 1985 |
|
Bushong SC: The physics and biology of diagnostic ultrasound. In Athey PA, Hadlock FD (eds): Ultrasound in Obstetrics and Gynecology, pp 315–316. 2nd ed. St Louis, CV Mosby, 1985 |
|
Athey PA: Adnexa: Nonneoplastic cysts. In Athey AP, Hadlock FD (eds): Ultrasound in Obstetrics and Gynecology, pp 206–207. 2nd ed. St Louis, CV Mosby, 1985 |
|
Malini S, Valdes C, Malinak LR: Sonographic diagnosis and classification of anomalies of the female genital tract. J Ultrasound Med 3: 397, 1984 |
|
Janus C, Godine L: Newborn with hydrometrocolpos and ambiguous genitalia: Clinical significance. J Clin Ultrasound 14: 739, 1986 |
|
Davis GH, Wapner RJ, Kurtz AB et al: Antenatal diagnosis of hydrometrocolpos by ultrasound examination. J Ultrasound Med 3: 371, 1984 |
|
Ngo C, Verma RC, Wong L et al: Simulation of a hydronephrotic pelvic kidney by an unusual pelvic mass. J Clin Ultrasound 15: 126, 1987 |
|
Siegberg R, Tenhunen A, Ylostalo P: Diagnosis of mucocolpos and hematocolpos by ultrasound: Two case reports. J Clin Ultrasound 13: 421, 1985 |
|
Swayne LC, Rubenstein JB, Mitchell B: The MayerRokitansky-Kuster-Hauser syndrome: Sonographic aid to diagnosis. J Ultrasound Med 5: 287, 1986 |
|
Graham D, Nelson MW: Combined perineal-abdominal sonography in the evaluation of vaginal atresia. J Clin Ultrasound 14: 735, 1986 |
|
Sherer DM, Beyth Y: Ultrasonographic diagnosis and assisted surgical management of hematotrachelos and hematometra due to uterine cervical atresia with associated vaginal agenesis. J Ultrasound Med 8: 321, 1989 |
|
Blask AR, Sanders RC, Rock JA: Obstructed uterovaginal anomalies: Demonstration with sonography. Part II: Teenagers. Radiology 179: 84, 1991 |
|
Stangl W, Frank RC, Frank W et al: Sonographic findings in a case of uterine and vaginal duplication (didelphys) with unilateral hematocolpometrasalpinx. J Clin Ultrasound 11: 40, 1983 |
|
Worthen NJ, Gonzalez F: Septate uterus: Sonographic diagnosis and obstetric complications. Obstet Gynecol 64 (Suppl): 34S, 1984 |
|
McArdle CR, Berezin AF: Ultrasound demonstration of uterus subseptus. J Clin Ultrasound 8: 139, 1980 |
|
Russ PD, Zavitz WR, Pretoruis DH et al: Hydrometrocolpos, uterus didelphys and septate vagina: An antenatal sonographic diagnosis. J Ultrasound Med 5: 211, 1986 |
|
Schwimer SR, Rubinstein L, Lebovic J: Sonographic evaluation of the testicular feminization syndrome. J Ultrasound Med 4: 503, 1985 |
|
Shawker TH, Garra BS, Loriaux DL et al: Ultrasonography of Turner's syndrome. J Ultrasound Med 5: 125, 1986 |
|
Kaufman RH, Binder GL, Gray PM et al: Upper genital tract changes associated with exposure in utero to diethylstilbestrol. Am J Obstet Gynecol 128: 51, 1977 |
|
Gross BH, Silver TM, Jaffe MH: Sonographic features of uterine leiomyomas: Analysis of 41 proven cases. J Ultrasound Med 2: 401, 1983 |
|
Borgstein RL, Shaw JJ, Pearson RH: Uterine leiomyomata: Sonographic mimicry. Br J Radiol 62: 1019, 1989 |
|
Aharoni A, Reiter A, Golan D et al: Patterns of growth of uterine leiomyomas during pregnancy: A prospective longitudinal study. Br J Obstet Gynaecol 95: 510, 1988 |
|
Nocera RM, Fagan CJ, Hernandez JC: Cystic parametrial fibroids mimicking ovarian cystadenoma. J Ultrasound Med 3: 183, 1984 |
|
Friedman AJ: Treatment of leiomyomata uteri with short-term leuprolide followed by leuprolide plus estrogen-progestin hormone replacement therapy for 2 years: A pilot study. Fertil Steril 51: 526, 1989 |
|
Hata K, Hata T, Makihara K et al: Sonographic findings of uterine leiomyosarcoma. Gynecol Obstet Invest 30: 242, 1990 |
|
Morcos RN, Leonard MD, Smith M et al: Vaginosonographic measurement of endometrial thickness in the evaluation of amenorrhea. Fertil Steril 55: 543, 1991 |
|
Dubinsky TJ, Parvey HR, Gormaz G et al: Transvaginal hysterosonography in the evaluation of small endoluminal masses. J Ultrasound Med 14: 1, 1995 |
|
Cicinelli E, Romano F, Anastasio PS et al: Transabdominal sonohysterography, transvaginal sonography, and hysteroscopy in the evaluation of submucous myomas. Obstet Gynecol 85: 42, 1995 |
|
Goldstein SR: Sonohysterography as an office procedure. Contemp Obstet Gynecol 35: 9, 1995 |
|
Granberg S, Wikland M, Karlsson B et al: Endometrial thickness as measured by endovaginal ultrasonography for identifying endometrial abnormality. Am J Obstet Gynecol 164: 47, 1991 |
|
Malpani A, Singer J, Wolverson MK et al: Endometrial hyperplasia: Value of endometrial thickness in ultrasonographic diagnosis and clinical significance. J Clin Ultrasound 18: 173, 1990 |
|
Varner RE, Sparks JM, Cameron CD et al: Transvaginal sonography of the endometrium in postmenopausal women. Obstet Gynecol 78: 195, 1991 |
|
Karlsson B, Granberg S, Wikland M et al: Endovaginal scanning of the endometrium compared to cytology and histology in women with postmenopausal bleeding. Gynecol Oncol 50: 173, 1993 |
|
Dorum A, Kristensen GB, Langebrekke A et al: Evaluation of endometrial thickness measured by endovaginal ultrasound in women with postmenopausal bleeding. Acta Obstet Gynecol Scand 72: 116, 1993 |
|
Osmers R, Volksen M, Schauer A: Vaginosonography for early detection of endometrial carcinoma? Lancet 335: 1569, 1990 |
|
Fleischer AC, Kalemeris GC, Machin JE et al: Sonographic depiction of normal and abnormal endometrium with histopathologic correlation. J Ultrasound Med 5: 445, 1986 |
|
Obata A, Akamatsu N, Sekiba K: Ultrasound estimation of myometrial invasion of endometrial cancer by intrauterine radial scanning. J Clin Ultrasound 13: 397, 1985 |
|
Johnson MA, Graham MF, Cooperberg PL: Abnormal endometrial echoes: Sonographic spectrum of endometrial pathology. J Ultrasound Med 1: 161, 1982 |
|
McCarthy KA, Hall DA, Kopans DB: Postmenopausal endometrial fluid collections: Always an indicator of malignancy? J Ultrasound Med 5: 647, 1986 |
|
Gordon AN, Fleischer AC, Reed GW: Depth of myometrial invasion in endometrial cancer: Preoperative assessment by transvaginal ultrasonography. Gynecol Oncol 39: 321, 1990 |
|
Conte M, Guariglia L, Benedetti-Panici P et al: Transvaginal ultrasound evaluation of myometrial invasion in endometrial carcinoma. Gynecol Obstet Invest 29: 224, 1990 |
|
Carlson JA Jr, Arger P, Thompson S et al: Clinical and pathologic correlation of endometrial cavity fluid detected by ultrasound in the postmenopausal patient. Obstet Gynecol 77: 119, 1991 |
|
Osmers R, Bergholz M, Kuhn W: Vaginal sonographic visualisation of a cervical carcinoma. Int J Gynaecol Obstet 28: 283, 1989 |
|
Yamamoto K, Kitao M: The evaluation of transrectal radial ultrasonography on parametrial infiltration in untreated cervical carcinoma for more accurate staging. Nippon Sanka Jujinka Gakkai Zasshi 41: 487, 1989 |
|
Meldrum DR, Chetkowski RJ, Steingold KA et al: Transvaginal ultrasound scanning of ovarian follicles. Fertil Steril 42: 803, 1984 |
|
Smith DH, Picker RH, Sinosich M et al: Assessment of ovulation by ultrasound and estradiol levels during spontaneous and induced cycles. Fertil Steril 33: 387, 1980 |
|
Dornbluth NC, Potter JL, Shepard MK et al: Assessment of follicular development by ultrasound and total serum estrogen in human menopausal gonadotropin-stimulated cycles. J Ultrasound Med 2: 407, 1983 |
|
Adoni A, Milwidsky A, Palti Z: The role of ultrasound in ovulation induction by gonadotropins. Int J Fertil 31: 170, 1986 |
|
Cabau A, Bessis R: Monitoring of ovulation induction with human menopausal gonadotropin and human chorionic gonadotropin by ultrasound. Fertil Steril 36: 178, 1981 |
|
Ylostalo P, Ronnberg L, Jouppila P: Measurement of the ovarian follicle by ultrasound in ovulation induction. Fertil Steril 31: 651, 1979 |
|
Orsini LF, Rizzo N, Calderoni P et al: Ultrasound monitoring of ovarian follicular development: A comparison of real-time and static scanning techniques. J Clin Ultrasound 11: 207, 1983 |
|
El Tabbakh GH, Lotfy I, Azab I et al: Correlation of ultrasonic appearance of the ovaries in polycystic ovarian disease and clinical, hormonal and laparoscopic findings. Am J Obstet Gynecol 154: 892, 1986 |
|
Nicolini U, Ferrazze E, Bellotti M et al: The contribution of sonographic evaluation of ovarian size in patients with polycystic ovarian disease. J Ultrasound Med 4: 347, 1985 |
|
Parisi L, Tramonti M, Derchi LE et al: Polycystic ovarian disease: Ultrasonic evaluation and correlations with clinical and hormonal data. J Clin Ultrasound 12: 21, 1984 |
|
Yeh HC, Futterweit W, Thornton JC: Polycystic ovarian disease: US features in 104 patients. Radiology 163: 111, 1987 |
|
Swanson M, Sauerbrei EE, Cooperberg PL: Medical implications of ultrasonically detected polycystic ovaries. J Clin Ultrasound 9: 219, 1981 |
|
Ardaens Y, Robert Y, Lemaitre L et al: Polycystic ovarian disease: Contribution of vaginal endosonography and reassessment of ultrasonic diagnosis. Fertil Steril 55: 1062, 1991 |
|
Conway GS, Honour JW, Jacobs HS: Heterogeneity of the polycystic ovary syndrome: Clinical, endocrine and ultrasound features in 556 patients. Clin Endocrinol 30: 459, 1989 |
|
Jaffe R, Abramowicz J, Eckstein N et al: Sonographic monitoring of ovarian volume during LHRH analogue therapy in women with polycystic ovarian syndrome. J Ultrasound Med 7: 203, 1988 |
|
Higgins RV, van Nagell JR Jr, Woods CH et al: Interobserver variation in ovarian measurements using transvaginal sonography. Gynecol Oncol 39: 69, 1990 |
|
Reynolds R, Hill MC, Glassman LM: Sonography of hemorrhagic ovarian cysts. J Clin Ultrasound 14: 449, 1986 |
|
Thind CR, Carty HM, Pilling DW: The role of ultrasound in the management of ovarian masses in children. Clin Radiol 40: 180, 1989 |
|
Granberg S, Crona N, Enk L et al: Ultrasound-guided puncture of cystic tumors in the lower pelvis of young women. J Clin Ultrasound 17: 107, 1989 |
|
DeCrespigny LC, Robinson HP, Davoren RA et al: The “simple” ovarian cyst: Aspirate or operate? Br J Obstet Gynaecol 96: 1035, 1989 |
|
Guerriero S, Mais V, Ajossa S et al: The role of endovaginal ultra sound in differentiating endometriomas from other ovarian cysts. Clin Exp Obstet Gynecol 22: 20, 1995 |
|
Fleischer AC, McKee MS, Gordon AN et al: Transvaginal sonography of postmenopausal ovaries with pathologic correlation. J Ultrasound Med 9: 637, 1990 |
|
Luxman D, Bergman A, Sagi J et al: The postmenopausal adnexal mass: Correlation between ultrasonic and pathologic findings. Obstet Gynecol 77: 726, 1991 |
|
Goldstein SR, Subramanyam B, Snyder JR et al: The postmenopausal cystic adnexal mass: The potential role of ultrasound in conservative management. Obstet Gynecol 73: 8, 1989 |
|
Andolf E, Jorgensen C: Cystic lesions in elderly women, diagnosed by ultrasound. Br J Obstet Gynaecol 96: 1076, 1989 |
|
Bundy AL, Ritchie WGM, Fine C et al: Dermoid tumor and cystadenoma arising in the same ovary. J Clin Ultrasound 14: 727, 1986 |
|
Gargano G, De Leonardis A, Perrotti P et al: Ovarian bilateral cystic teratomas: Diagnosis and therapy in a young woman. Clin Exp Obstet Gynecol 17: 37, 1990 |
|
Sisler CL, Siegel MJ: Ovarian teratomas: A comparison of the sonographic appearance in prepubertal and postpubertal girls. Am J Roentgenol 154: 139, 1990 |
|
Schaffer RM, Cataldi GA, Shih YH: Sonographic demonstration of rupture of a cystic teratoma during pregnancy. J Ultrasound Med 3: 425, 1984 |
|
Rosenberg ER, Trought WS: The ultrasonographic evaluation of large cystic pelvic masses. Am J Obstet Gynecol 139: 579, 1981 |
|
Segal S, Feld R, Kurtz AB: Ultrasound case of the day: Ovarian fibroma. Radiographics 9: 959, 1989 |
|
Conforti S, Di Serio C, Donofrio V et al: Bilateral Brenner tumor. Eur J Gynaecol Oncol 10: 438, 1989 |
|
Wicks JD, Mettler FA, Hilgers RD et al: Correlation of ultrasound and pathologic findings in patients with epithelial carcinoma of the ovary. J Clin Ultrasound 12: 397, 1984 |
|
Paling MR, Shawker TH: Abdominal ultrasound in advanced ovarian carcinoma. J Clin Ultrasound 9: 435, 1981 |
|
Campbell S, Bhan V, Royston P et al: Transabdominal ultrasound screening for early ovarian cancer. Br Med J 299: 1363, 1989 |
|
van Nagell JR Jr, Higgins RV, Donaldson ES et al: Transvaginal sonography as a screening method for ovarian cancer: A report of the first 1000 cases screened. Cancer 65: 573, 1990 |
|
Conte M, Guariglia L, Benedetti-Panici PL et al: Ovarian carcinoma: An ultrasound study. Eur J Gynaecol Oncol 11: 33, 1990 |
|
Murolo C, Costantini S, Foglia G et al: Ultrasound examination in ovarian cancer patients: A comparison with second look laparotomy. J Ultrasound Med 8: 441, 1989 |
|
Athey PA, Siegel MF: Sonographic features of Brenner tumor of the ovary. J Ultrasound Med 6: 367, 1987 |
|
Athey PA, Butters HE: Sonographic and CT appearance of Krukenberg tumors. J Clin Ultrasound 12: 205, 1984 |
|
Shimizu H, Yamasaki M, Ohama K et al: Characteristic ultrasonographic appearance of the Krukenberg tumor. J Clin Ultrasound 18: 697, 1990 |
|
Brown DL, Frates MC, Laing FC et al: Ovarian masses: Can benign and malignant lesions be differentiated with color and pulsed Doppler US? Radiology 190: 333, 1994 |
|
Fleischer AC, Cullinan JA, Jones HW III et al:Serial assessment of adnexal masses with transvaginal color Doppler sonography. Ultrasound Med Biol 21: 435, 1995 |
|
Granberg S, Jansson I: Early detection of primary carcinoma of the fallopian tube by endovaginal ultrasound. Acta Obstet Gynecol Scand 69: 667, 1990 |
|
Ajjimakorn S, Bhamarapravati Y: Vaginal ultrasound and the diagnosis of fallopian tubal carcinoma. J Clin Ultrasound 19: 116, 1991 |
|
Kol S, Gal D, Friedman M et al: Preoperative diagnosis of fallopian tube carcinoma by transvaginal sonography and CA-125. Gynecol Oncol 37: 129, 1990 |
|
Hata K, Hata T, Aoki S et al: Ultrasonographic evaluation of pelvic inflammatory disease. Nippon Sanka Fujinka Gakkai Zasshi 41: 895, 1989 |
|
Teisala K, Heinonen PK, Punnonen R: Transvaginal ultrasound in the diagnosis and treatment of tubo-ovarian abscess. Br J Obstet Gynaecol 97: 178, 1990 |
|
Patten RM, Vincent LM, Wolner-Hanssen P et al: Pelvic inflammatory disease: Endovaginal sonography with laparoscopic correlation. J Ultrasound Med 9: 681, 1990 |
|
Gross BH, Chinn DH, Callen PW et al: Real-time vs static scanning in the diagnosis of abdominal and pelvic abscesses. J Ultrasound Med 2: 223, 1983 |
|
Amstey MS, Schaffer DL: Ultrasound in the identification of true pelvic abscesses. Infect Surg 2: 190, 1984 |
|
Mahony BS, Callen PW: Sonography in the detection of intraabdominal abscesses. Infect Surg 2: 691, 1984 |
|
Swayne LC, Love MB, Karasick SR: Pelvic inflammatory disease: Sonographic-pathologic correlation. Radiology 151: 751, 1984 |
|
Uhrich PC, Sanders RC: Ultrasonic characteristics of pelvic inflammatory masses. J Clin Ultrasound 4: 199, 1976 |
|
Spirtos NJ, Bernstine RL, Crawford WL et al: Sonography in acute pelvic inflammatory disease. J Reprod Med 27: 312, 1982 |
|
Vincent LM, Mittelstaedt CA: Sonographic demonstration of endometrioma arising in cesarean scar. J Ultrasound Med 4: 437, 1985 |
|
Kumar R, Haque AK, Cohen MS: Endometriosis of the urinary bladder: Demonstration by sonography. J Clin Ultrasound 12: 363, 1984 |
|
Grabb A, Carr L, Goodman JD et al: Hepatic endometrioma. J Clin Ultrasound 14: 478, 1986 |
|
Najarian KE, Kurtz AB: New observations in the sonographic evaluation of intrauterine contraceptive devices. J Ultrasound Med 5: 205, 1986 |
|
Reuter KL, Daly D: Ultrasonic monitoring under local anesthesia during removal of an embedded intrauterine contraceptive device. J Clin Ultrasound 14: 68, 1986 |
|
Ylostalo PR, Nilsson CG, Hieta-Heikurainen MH: Ultrasonically controlled removal of intrauterine contraceptive device. J Clin Ultrasound 12: 505, 1984 |
|
Cadkin AV, McAlpin J: The decidua-chorionic sac: A reliable sonographic indicator of intrauterine pregnancy prior to detection of a fetal pole. J Ultrasound Med 3: 539, 1984 |
|
Nelson P, Bowie JD, Rosenberg ER: Early intrauterine pregnancy or decidual cast: An anatomic-sonographic approach. J Ultrasound Med 2: 543, 1983 |
|
Nyberg DA, Mack LA, Laing FC et al: Early pregnancy complications: Endovaginal sonographic findings correlated with human chorionic gonadotropin levels. Radiology 167: 619, 1988 |
|
Nyberg DA, Filly RA, Mahony BS et al: Early gestation: Correlation of HCG levels and sonographic identification. Am J Roentgenol 144: 951, 1985 |
|
Bernaschek G, Rudelstorfer R, Csaicsich P: Vaginal sonography versus serum human chorionic gonadotropin in early detection of pregnancy. Am J Obstet Gynecol 158: 608, 1988 |
|
Timor-Tritsch IE, Farine D, Rosen MG: A close look at early embryonic development with the high-frequency transvaginal transducer. Am J Obstet Gynecol 159: 676, 1988 |
|
Daya S, Woods S, Ward S et al: Early pregnancy assessment with transvaginal ultrasound screening. Can Med Assoc J 144: 441, 1991 |
|
Timor-Tritsch IE, Rottem S, Thaler I: Review of transvaginal ultrasonography: Description with clinical application. Ultrasound Q 6: 1, 1988 |
|
Kadar N, Romero R: The timing of a repeat ultrasound examination in the evaluation for ectopic pregnancy. J Clin Ultrasound 10: 211, 1982 |
|
Fritz MA, Guo S: Doubling time of human chorionic gonadotropic (HCG) in early normal pregnancy: Relationship to HCG concentration and gestational age. Fertil Steril 47: 584, 1987 |
|
Mahony BS, Filly RA, Nyberg DA et al: Sonographic evaluation of ectopic pregnancy. J Ultrasound Med 4: 221, 1985 |
|
Adoni A, Milwidsky A, Hurwitz A et al: Declining á-HCG levels: An indicator for expectant approach in ectopic pregnancy. Int J Fertil 31: 40, 1986 |
|
Yeh HC, Goodman JD, Carr L et al: Intradecidual sign: A US criterion of early intrauterine pregnancy. Radiology 161: 463, 1986 |
|
Nyberg DA, Mack LA, Laing FC et al: Distinguishing normal from abnormal gestational sac growth in early pregnancy. J Ultrasound Med 6: 23, 1987 |
|
Pennell RG, Needleman L, Pajak T et al: Prospective comparison of vaginal and abdominal sonography in normal early pregnancy. J Ultrasound Med 10: 63, 1991 |
|
Wilson RD, Kendrick V, Wittmann BK et al: Spontaneous abortion and pregnancy outcome after normal first-trimester ultrasound examination. Obstet Gynecol 67: 352, 1986 |
|
Nyberg DA, Filly RA, Filho DLD et al: Abnormal pregnancy: Early diagnosis by US and serum chorionic gonadotropin levels. Radiology 158: 393, 1986 |
|
Jeong WG, Kim CH, Bernstine RL et al: Ultrasonic sonography in the management of incomplete abortion. J Reprod Med 26: 90, 1981 |
|
Donald I, Morley P, Barnett E: The diagnosis of blighted ovum by sonar. J Obstet Gynaecol Br Commonw 79: 304, 1972 |
|
Robinson HP: The diagnosis of early pregnancy failure by sonar. Br J Obstet Gynaecol 82: 849, 1975 |
|
Bromley B, Harlow BL, Laboda LA et al: Small sac size in the first trimester: A predictor of poor fetal outcome. Radiology 178: 375, 1991 |
|
Cacciatore B, Stenman UH, Ylostalo P: Diagnosis of ectopic pregnancy by vaginal ultrasonography in combination with a discriminatory serum hCG level of 1000 IU/l (IRP). Br J Obstet Gynaecol 97: 904, 1990 |
|
Barnhart K, Mennuti MT, Benjamin I et al: Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol 84: 1010, 1994 |
|
Kadar N, Bohrer M, Kemmann E et al: The discriminatory human chorionic gonadotropin zone for endovaginal sonography: A prospective, randomized study. Fertil Steril 61: 1016, 1994 |
|
Stiller RJ, Haynes-de-Regt R, Blair E: Transvaginal ultrasonography in patients at risk for ectopic pregnancy. Am J Obstet Gynecol 161: 930, 1989 |
|
Fleischer AC, Pennell RG, McKee MS et al: Ectopic pregnancy: Features at transvaginal sonography. Radiology 174: 375, 1990 |
|
Benacerraf BR, Rinehart JS, Schiff I: Sonographic diagnosis of simultaneous intrauterine and ectopic pregnancy. J Ultrasound Med 4: 321, 1985 |
|
van Dam PA, Vanderheyden JS, Uyttenbroeck F: Application of ultrasound in the diagnosis of heterotopic pregnancy: A review of the literature. J Clin Ultrasound 16: 159, 1988 |
|
Reuss ML: Delayed diagnosis of advanced combined pregnancy. J Clin Ultrasound 16: 187, 1988 |
|
Dunner PS, Glassman LM, Lipsit ER: Two unusual ectopic pregnancies. J Clin Ultrasound 12: 225, 1984 |
|
Rein MS, Di Salvo DN, Friedman AJ: Heterotopic pregnancy associated with in vitro fertilization and embryo transfer: A possible role for routine vaginal ultrasound. Fertil Steril 51: 1057, 1989 |
|
Taylor KJ, Ramos IM, Feyock AL et al: Ectopic pregnancy: Duplex Doppler evaluation. Radiology 173: 93, 1989 |
|
Stovall TG, Ling FW, Gray LA: Single-dose methotrexate for treatment of ectopic pregnancy. Obstet Gynecol 77: 754, 1991 |
|
Maher PJ, Grimwade JC: Cornual pregnancy-diagnosis before rupture: A report of two cases. Aust NZ J Obstet Gynaecol 22: 172, 1982 |
|
Jafri SZH, Loginsky SJ, Bouffard JA et al: Sonographic detection of interstitial pregnancy. J Clin Ultrasound 15: 253, 1987 |
|
Beckmann CRB, Sampson MB: Ultrasonographic diagnosis of interstitial ectopic pregnancy. J Clin Ultrasound 12: 304, 1984 |
|
Graham M, Cooperberg PL: Ultrasound diagnosis of interstitial pregnancy: Findings and pitfalls. J Clin Ultrasound 7: 433, 1979 |
|
Rivlin ME: Persistent ectopic pregnancy: Complication of conservative surgery. Int J Fertil 30: 10, 1985 |
|
Kuo HC, Chang FM, Wu CH et al: The primary application of three-dimensional ultrasonography. Am J Obstet Gynecol 166: 880, 1992 |
|
Pretorius DH, Nelson TR: Fetal face visualization using three-dimensional ultrasonography. J Ultrasound Med 14: 349, 1995 |
|
Liang RI, Chang FM, Yao BL et al: Predicting birth weight by fetal upper-arm volume with use of three-dimensional ultrasonography. Am J Obstet Gynecol 177: 632, 1997 |
|
Lee W, Comstock CH, Kirk JS et al: Birthweight prediction by three-dimensional ultrasonographic volumes of the fetal thigh and abdomen. J Ultrasound Med 16: 799, 1997 |
|
Harika G, Gabriel R, Carre-Pigeon F et al: Primary application of three-dimensional ultrasonography to early diagnosis of ectopic pregnancy. Eur J Obstet Gynaecol Reprod Biol 60: 117, 1995 |
|
Gruboeck K, Jurkovic D, Lawton F et al: The diagnostic value of endometrial thickness and volume measurements by three-dimensional ultrasound in patients with postmenopausal bleeding. Ultrasound Obstet Gynecol 8: 272, 1996 |
|
Chou CY, Hsu KF, Wang ST et al: Accuracy of three-dimensional ultrasonography in volume estimation of cervical carcinoma. Gynecol Oncol 66: 89– 93, 1997 |
|
Chan L, Lin WM, Uerpairojkit B et al: Evaluation of adnexal masses using three-dimensional ultrasonographic technology: Preliminary report. J Ultrasound Med 16: 349, 1997 |
|
Wu MH, Tang HH, Hsu CC et al: The role of three dimensional ultrasonographic images in ovarian measurement. Fertil Steril 69: 1152, 1998 |
|
Dolz M, Osborne NG, Blanes J et al: Polycystic ovarian syndrome: Assessment with color Doppler angiography and three-dimensional ultrasonography. J Ultrasound Med 18: 303, 1999 |
|
Kupesic S, Kurjak A: Septate uterus: Detection and prediction of obstetrical complications by different forms of ultrasonography. J Ultrasound Med 17: 631, 1998 |