Diagnostic Ultrasound in the Assessment of the Adnexal Mass
Authors
INTRODUCTION
The limitations of pelvic examination in accurately identifying adnexal masses, even with the luxury of general anesthesia, have been studied by Padilla and colleagues.1 In contrast, diagnostic ultrasound is highly sensitive for identifying adnexal masses. However, it is at best only 50% sensitive for Stage I epithelial ovarian cancer, and is further limited by poor specificity in accurately differentiating benign from malignant pathology.2 Ultrasound screening for ovarian cancer in asymptomatic women can result in large numbers of false-positive results and operations for benign pathology, ranging from 3 to 50 surgical operations for every case of ovarian cancer detected.3
It is estimated that 1.6% of women will develop an ovarian malignancy during their lifetime.4 Another 10% will have a benign tumor of the ovary requiring surgery. The percentage of women requiring surgery for adnexal pathology during their lifetime is even higher if endometriotic cysts, symptomatic hemorrhagic and follicular cysts, and residuals of pelvic inflammatory diseases are included. Although exact numbers are difficult to calculate, many women with benign physiologic cysts, hemorrhagic cysts, residuals of pelvic inflammatory disease, hydrosalpinges, and pedunculated leiomyomas undergo surgery when expectant management could have been used.
Physicians wishing to learn about ultrasound of the adnexal mass are faced with hundreds of recent articles but little interpretive consensus. The role of color and power Doppler velocimetry is hotly debated. This chapter reviews a diagnostic paradigm that can be learned readily and is simple to apply. Certain goals can be set for diagnostic ultrasound of the adnexa:
- Identify hemorrhagic corpus luteum, functional cysts, hydrosalpinges, and peritoneal adhesive disease and avoid operative interventions for them if possible.
- Differentiate benign from malignant disease of the ovary and Fallopian tube.
- Discriminate gastrointestinal and uterine patho-logy from ovarian or Fallopian tube processes.
The patient's age, history, and pelvic examination are important in the diagnostic paradigm. The obstetrician-gynecologist who is properly trained in diagnostic ultrasound (including transvaginal studies) therefore has a distinct advantage because of his or her clinical knowledge of female reproductive tract pathophysiology. As in all areas of medicine, practitioners are strongly advised not to overstep their diagnostic acumen and to liberally seek the advice of experts.
GRAY-SCALE ULTRASOUND
Many different scoring systems exist for discriminating benign from malignant adnexal masses. These scoring systems evaluate masses for solid elements, cyst wall thickness, number, thickness, and irregularity of septations, and the presence of ascitic fluid. Numerical scores are applied and masses that score higher than a certain cutoff are considered potentially malignant.5, 6, 7 As recently discussed by Jermy and colleagues, the application of these numerical systems is complex.8
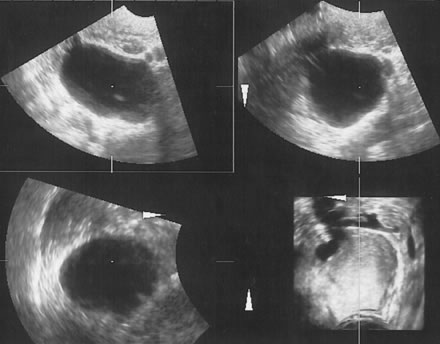
It is easier to assign the adnexal mass to one of to the five categories described by Osmers and coworkers9:
- Cystic
- Biloculated
- Multiloculated
- Complex
- Solid.
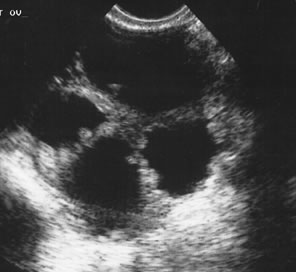
Examples of these categories are shown in Figures 1, 2, 3, 4, and 5. A mass is considered complex if it contains solid elements or thick or irregular septations, or if ascites is noted. Recent logistic regressions by Tailor and coworkers and Schelling and associates have confirmed that the presence of solid elements within a mass is the most predictive in identifying malignancy.10, 11 As shown in Table 1, most malignancies in Osmers's series occurred in the complex group. Osmers's system has a degree of subjectivity; a 5-cm thin-walled cystic mass with one or two thin septations could be categorized as either cystic or complex. This distinction ultimately depends on the informed judgment of the sonologist.
The management of adnexal masses has been reviewed in a ACOG practice bulletin from 2007.12
|
|
Table 1. Tumor Staging by Sonomorphologic Characteristics
Simple | |||||
Staging | Cystic | Bilocular | Multilocular | Complex | Solid |
Benign | 636 | 84 | 114 | 175 | 17 |
Borderline | 3 | - | 1 | 5 | - |
Malignant | 2 | - | 2 | 31 | 2 |
Osmers R, Osmers M, von Maydell B et al: Preoperative evaluation of ovarian tumors in the premenopause by transvaginosono-graphy. Am J Obstet Gynecol 175:428, 1996.
EXPECTANT MANAGEMENT OF PREMENOPAUSAL CYSTIC MASSES
Careful review of Osmers's series9 is informative. The study comprised 1072 premenopausal women referred with adnexal masses greater than 3 cm. The protocol included re-scanning all masses after 6 weeks to check for resolution of functional cysts. In the study, 53% of the masses proved to be functional or hemorrhagic cysts, 25% endometriotic cysts, 18% benign neoplasms, and 4% malignant neoplasms. Operations were avoided in 90% of the women with functional or hemorrhagic cysts. Of the remaining 10%, half required surgery for peritoneal irritation, and in the other half the referring physicians operated without waiting the 6 weeks even though their patients were asymptomatic.
Expectant management of physiologic functional cysts and hemorrhagic cysts is critical in avoiding unnecessary surgery (Fig. 6). As shown by Schulman and colleagues, 15% of asymptomatic premenopausal women and 5% of menopausal women will have cystic masses greater than 2.5 cm.13
THE ROLE OF EXPECTANT MANAGEMENT OF MENOPAUSAL CYSTS
The role of expectant management of simple menopausal cysts less than 5 cm has been studied by Bailey and colleagues.14 These cystic masses are estimated to occur in 3% to 5% of menopausal women. The American College of Obstetricians and Gynecologists (ACOG) has issued guidelines for the expectant management of these masses (Table 2).15 The malignant potential is believed to be less than 1%.
Table 2. Indications for surgery of Asymtomatic Menopausal Adnexal Mass
Solid mass
Complex mass
Increasing size of mass
Presence of ascites
Fixed mass or cul-de-sac nodularity
Elevated CA-125 level
American College of Obstetricians and Gynecologists, Criteria Set No. 15. April 1996.
ECHO-PATTERN RECOGNITION
Once the mass has been characterized into one of Osmers's five categories, a histologic differential should be provided if appropriate. Papers by Milad and Cohen16 and Jermy and colleagues8 have noted that many persistent ovarian cysts found in premenopausal women can be accurately identified by their characteristic echo-patterns as endometriomas or cystic teratomas using transvaginal ultrasound. In nonpregnant women from age 20 to 40, functional cysts, hemorrhagic cysts, endometriomas, and cystic teratomas will account for close to 90% of the ovarian masses seen in the average clinician's office practice.
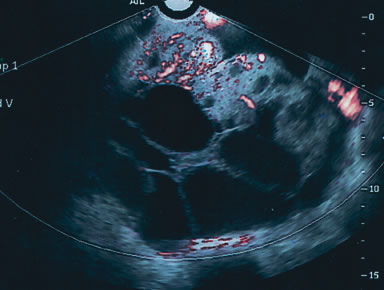
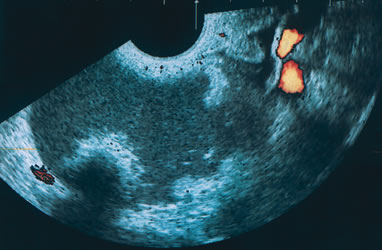
Endometriomas are typically cystic or multiloculated (Fig. 7). They can be filled with varying degrees of internal low echogenicity. Pascual and coworkers recently reviewed this.17 Cystic teratomas are the most common benign tumor of the ovary in women between ages 20 and 40, although they can be found in any age group. The tumors usually contain varying degrees of cystic and highly dense echogenic components. The dense component displays acoustic shadowing secondary to the presence of hair, sebaceous material, teeth, and the like. The transvaginal ultrasound features of these tumors have been described by Cohen and associates (Fig. 8).18
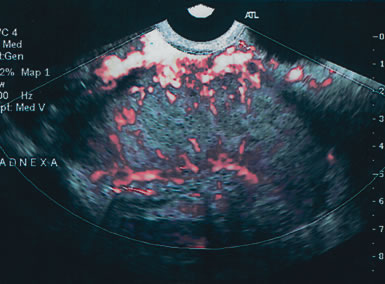
Serous cystadenomas are the most common benign tumor after age 40. They can present as simple cystic locules and may appear as highly loculated complex masses (see Fig. 3). As wall thickness and degree of complexity increase and excrescences are noted, borderline carcinomas of the ovary must be considered (Fig. 9). There can be considerable echo-pattern overlap between benign adenofibromas of the ovary and borderline tumors of the ovary, as discussed in the Doppler section of this chapter. Echo-pattern recognition also allows for the prediction of paramesonephric cysts, pedunculated leiomyomas, hydrosalpinges, and adhesions and residuals of pelvic inflammatory disease (Fig. 10).19
|
|
Unfortunately, there remains some diagnostic overlap between malignant and benign pathology. Papillary adenocarcinomas and malignant germ cell tumors have been misdiagnosed as cystic teratomas. Granulosa cell tumors can be confused with endometriomas. Benign adenofibromas are frequently confused with malignant papillary adenocarcinomas or borderline tumors. The addition of color or power Doppler as a secondary test can be helpful in differentiating these entities, and is reviewed in the following section.
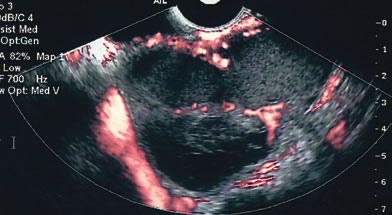
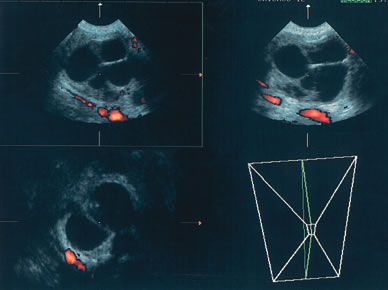
COLOR AND POWER DOPPLER
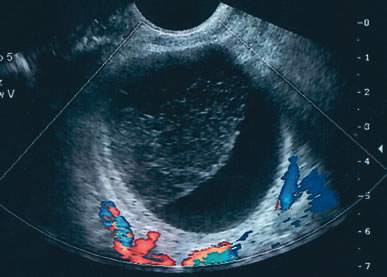
The published literature is replete with contrasting views of the efficacy of color and power Doppler in assessing the malignant nature of an adnexal mass. It is beyond the scope of this chapter to completely review this subject. Tailor and coworkers have clearly shown that 100% of malignant tumors and 80% of benign tumors display vascular flow.20 In that study, neither resistive indices (RI), pulsatility indices, or temporal average mean velocity measurements were specific enough to differentiate well between benign and malignant adnexal masses. The initial finding reported by Kurjak and colleagues21 that a resistance index less than 0.4 was highly sensitive and specific in predicting ovarian malignancy has not been confirmed. Using regression analysis, both Tailor and coworkers and Schelling and associates have found that the most important variable in assessing the risk for malignancy is the presence or absence of solid elements within tumors with vascular flow.10, 11 Using power Doppler as a secondary test, Guerriero and associates found that the specificity for correctly identifying benign disease was improved from 80% to 90%.22 This system merely scores the solid elements of a tumor for the presence or absence of central vascularity. This was also confirmed in the previously mentioned series of Schelling and colleagues11 and a paper by Cohen and associates using 3-dimensional power Doppler.23 Guerriero in a 2007 publication found that in masses with central vasculariazation that the addition of 3-D quantification did not furtherimprove diagnostic accuracy.24 Sladkevicius in 2007 explored the use of a 3-D morphologic assessment of the vascular vessel tree in ovarian masses. It could be used to distinguish benign from malignant masses but did not add to much to the impression from gray-scale imaging.25
OVARIAN CANCER SCREENING
There is no evidence to date that ultrasound can be used effectively to screen for Stage I ovarian cancer in high risk women. 26There is data from a Japanese study by Sato, among 183,034 women screened with transvaginal ultrasound, that found that 77% of ovarian cancers were detected at FIGO Stage I. Approximately fifteen surgeries for benign pathology were performed for evey case of ovarian cancer detected.27 The National Institutes of Health Consensus Conference currently recommends routine annual pelvic examination rather than ultrasound to evaluate the ovaries. This is based on the low annual prevalence of ovarian cancer (1/2500 for women age 40 and above) and the suboptimal accuracy of ultrasound.28 The series by von Nagell and coworkers followed 14,469 women with annual ultrasounds; 25 ovarian cancers occurred during the 12-year study, for which 21 patients were scanned within protocol.29 Sensitivity was 81% for all stages of ovarian cancer. The sensitivity for Stage I disease was 52%. However, this dropped to 31% if palpably enlarged borderline tumors and granulosa-cell tumors were excluded.2 Approximately 10 operations were performed for every ovarian cancer detected. In a follow-up paper publised by van Nagell in 2007, the number of false positive had decreased markedly. By following simple ovarian cysts less than 5 cm., they increased there positive predictive value for operating on women with malignancy from 8.8% to 27.1%. 30
The outcomes of two major screening trials in postmenopausal patients, one American and one British should be available in the next 5 years.31, 32
CONCLUSION
Even with the system described above, errors will occur in the prediction of benign and malignant processes. The assessment of an adnexal mass depends on physical examination, the patient's history and age, and the findings on ultrasound examination. The three-part assessment consists of a morphologic description, an echo-pattern assessment of possible histology, and power or color Doppler as a secondary test. It is anticipated that the addition of new serum tumor markers will enhance the clinician's ability to discriminate between benign and malignant disease.
REFERENCES
Padilla LA, Radosevich DM, Milad MP: Accuracy of the pelvic examination in detecting adnexal masses. Obstet Gynecol 96: 593, 2000 |
|
Fishman DA, Cohen LS: Editorial: Is transvaginal ultrasound effective for screening asymptomatic women for the detection of early-stage epithelial ovarian carcinoma: Gynecol Oncol 77:347, 2000 |
|
Bell R, Petticrew M, Sheldon T: The performance of screening tests for ovarian cancer: Results of a systematic review. Br J Obstet Gynaecol 105: 1136, 1998 |
|
Greenlee RT, Murray T, Bolden S et al: Cancer Statistics, 2000. CA Cancer J Clin 50: 7, 2000 |
|
Sassone AM, Timor-Tritsch IE, Artner A et al: Transvaginal sonographic characterization of ovarian disease: Evaluation of a new scoring system to predict malignancy. Obstet Gynecol 78: 70, 1991 |
|
Lerner JP, Timor-Tritsch IE, Federman A et al: Transvaginal ultrasonographic characterization of ovarian masses with an improved, weighted score. Am J Obstet Gynecol 170: 81, 1994 |
|
Ferrazzi E, Zanetta G, Dordoni D et al: Transvaginal ultrasonographic characterization of ovarian masses: A comparison of five scoring systems in a multicenter trial. Ultrasound Obstet Gynecol 10: 192, 1997 |
|
Jermy K, Luise C, Bourne T: The characterization of common ovarian cysts in premenopausal women. Ultrasound Obstet Gynecol 17: 140, 2001 |
|
Osmers R, Osmers M, von Maydell B et al: Preoperative evaluation of ovarian tumors in the premenopause by transvaginosonography. Am J Obstet Gynecol 175: 428, 1996 |
|
Tailor A, Jurkovic D, Bourne T: Sonographic prediction of malignancy in adnexal masses using multivariate logistic regression analysis. Ultrasound Obstet Gynecol 10: 41, 1997 |
|
Schelling M, Braun M, Kuhn W et al: Combined transvaginal B-mode and color Doppler sonography for differential diagnosis of ovarian tumors: Results of a multivariate logistic regression analysis. Gynecol Oncol 77: 78, 2000 |
|
ACOG Practice Bulletin #83. Management of adnexal masses. Obstetrics and Gynecology 2007: 83:201-214. |
|
Schulman H, Conway C, Zalud I et al: Prevalence in a volunteer population of pelvic cancer detected with transvaginal ultrasound and color flow Doppler. Ultrasound Obstet Gynecol 4: 414, 1994 |
|
Bailey C, Ueland F, Land G et al: The malignant potential of small cystic ovarian tumors in women over 50 years of age. Gynecol Oncol 69: 3, 1998 |
|
American College of Obstetricians and Gynecologists, Criteria Set No. 15. April 1996 |
|
Milad M, Cohen L: Preoperative ultrasound assessment of adnexal masses in premenopausal women. Int J Gynaecol Obstet 66: 137, 1999 |
|
Pascual MA, Tressera F, Lopez-Marin L et al: Role of color Doppler ultrasonography in the diagnosis of endometriotic cyst. J Ultrasound Med 19: 695, 2000 |
|
Cohen L, Sabbagha R: Echo-patterns of benign cystic teratomas by transvaginal ultrasound. Ultrasound Obstet Gynecol 3: 120, 1993 |
|
Molander P, Sjoberg J, Paavonen J et al: Transvaginal power Doppler findings in laparoscopically proven acute pelvic inflammatory disease. Ultrasound Obstet Gynecol 17: 233, 2001 |
|
Tailor A, Juirkovic D, Bourne T et al: Comparison of transvaginal color Doppler imaging and color Doppler energy for assessment of intraovarian blood flow. Obstet Gynecol 91: 561, 1998 |
|
Kurjak A, Zalud I, Alfirevic Z: Evaluation of adnexal masses with transvaginal color ultrasound. J Ultrasound Med 10: 295, 1991 |
|
Guerriero S, Ajossa S, Risalvato A et al: Diagnosis of adnexal malignancies by using color Doppler energy imaging as a secondary test in persistent masses. Ultrasound Obstet Gynecol 11: 277, 1998 |
|
Cohen L, Escobar P, Scharm C et al: Three-dimensional power Doppler ultrasound improves the diagnostic accuracy for ovarian cancer prediction. Gynecol Oncol 82: 40, 2001 |
|
Guerriero S, Ajossa S, Piras S et al. Three-dimensional quantification of tumor vascularity as a tertiary test after B-Mode and power Doppler evaluation for detection of ovarian cancer. J Ultrasound Med 2007;26:1271-1278. |
|
Sladkevicius P, Jokubiene, and Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnosis of malignancy in ovarian masses. Ultrasound Obstet Gynecol 2007;30:874-882. |
|
Fishman D, Cohen L, Blank S et al. The role of ultrasound in the detection of early stage ovarian cancer. AJOG 2005:192:1214-22 |
|
Sato S, Yokoyama Y, Sakamoto T, et al. Usefullness of mass screening for ovarian carcinoma using transvaginal ultrasound. Cancer 2000; 89:582-588. |
|
NIH Consensus Development Panel on Ovarian Cancer: Ovarian cancer: Screening, treatment and follow-up. JAMA 273:491, 1995 |
|
Van Nagell Jr JR, DePriest PD, Reedy MB et al The efficacy of transvaginal screening in asymptomatic women at risk for ovarian cancer. Gynecol Oncol 77:350, 2000 |
|
Van Nagell JR, DePreist PD, and Ueland FR. Ovarian cancer screening with annual transvaginal sonography. Cancer 2007;109:1887-1896 |
|
Buys SS, Patridge E, Greene MH. Ovarian cancer screening in the PLCO cancer screening trial. AJOG 2005:1630-1639. |
|
Jacobs IJ, Skates SS, MacDonald N, et al. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet 1999;353:1207-1210. |