Dilatation and Curettage
Authors
INTRODUCTION
Dilatation and curettage (D&C) is a technique whose indications have changed greatly in the past 20 years with the advent of newer techniques for accomplishing both the diagnostic and therapeutic aims of the procedure. Not all physicians have access to or training in some of these newer techniques; therefore, the standard D&C remains an important part of our specialty.
TECHNIQUE
The D&C is a surgical procedure during which the physician explores a cavity beyond his or her view. It is therefore an art dependent on the surgeon's sense of palpation and knowledge of probable findings. Variations in the shape, size, and consistency of the uterus can be determined by history and physical examination, and there are hazards of misdiagnosis of these factors. Before beginning a D&C, these variations should be ascertained and anticipated. The technique must be adjusted to new variations as they are perceived by the surgeon, sensitive to the messages transmitted through the instruments to his or her hands. Unless one is continually thinking of possible variations and complications, it is easy to perforate the uterus.
Endocrine and Anatomic Variations
EXTERNAL OS.
The external os is the only visible structure in the procedure. It may be patulous or stenosed, depending on the patient's reproductive and surgical history (e.g., prior cervical conization). These conditions are easily seen and dealt with and are independent of the condition of the internal os or uterus.
CERVICAL CANAL.
The cervical canal is a fairly voluminous hollow structure lined with secretory glands folded in many rugae. The canal usually offers no resistance to instrumentation until after menopause, when it may become more atrophic and rigid.
INTERNAL OS.
The internal os is an active “sphincter” of the lower uterine cavity. It is relaxed in the presence of estrogen (the first 2 weeks of the cycle, or in unopposed prolonged estrogen stimulation) and is constricted under progesterone influence (in the secondary phase or in early pregnancy). In postmenopausal women, the internal os may be very tightly closed and may resist probing and dilation to the point of possible laceration or perforation. Patients with a history of diethylstilbestrol exposure in utero may have a stricture at this point. Difficulty encountered in the office may require a hospital D&C under general anesthesia. In contrast, the internal os is open and soft in pregnancy-associated problems such as incomplete abortion or bleeding in the postpartum period.
MYOMETRIUM.
The uterine musculature responds to hormones in a manner opposite to the internal os: contracted and firm under estrogen influence, relaxed and easily perforated under progesterone influence. It is also thinner and more fibrous after menopause.
UTERUS.
The uterus is most commonly found in the anteverted, partially anteflexed position. Traction on the cervix will correct the anteversion and partially correct the anteflexion as well. In about one third of normal women, the uterus is retroverted. In a much smaller number, it is extremely retroflexed. These conditions can also be corrected with traction. It has been estimated in hysteroscopy that about 15% of patients have extreme anteflexion or retroflexion, making visualization of the entire uterus difficult. During a D&C, this exaggerated flexion can cause perforation.
In certain disease states, such as fibroids or endometrial carcinoma, the uterus may be distorted and difficult to outline by palpation with the sound or curet. As a result, significant pathology can be missed by blind curettage alone. Uterine anomalies such as unicornuate uterus or septate uterus may yield confusing findings during the initial palpation; these anomalies must be kept in mind as explanations for these findings. Undiagnosed, such anomalies may prevent a thorough exploration of the endometrial cavity.
Risks of the Technique
Because of the variations in anatomy and endocrinology, there are a number of known risks that the physician should keep in mind while doing the procedure. These are arranged in order of frequency.
- Laceration of the cervix. As a result of resistance to dilation at the internal os, the tenaculum holding the cervix may tear through and cause bleeding. This can be minimized by using less force over a longer time during the dilation effort.
- Tears of the internal os. It is quite easy to tear this structure rather than dilate it. Occasionally such tears result in severe hemorrhage from damaged uterine vascular branches or misdiagnosis of the tear as a submucous fibroid.1
- Fundal perforation. Under the influence of progesterone, the myometrium may be surprisingly soft and easily perforated, such as in the management of an incomplete first-trimester abortion.
- Perforation due to flexion. If anteflexion or retroflexion of the uterus is not appreciated and corrected by traction, dilators or curets may go through the anterior or posterior wall beyond the internal os and reach the peritoneal cavity. Intrauterine devices were found lying in the anterior or posterior wall in as many as 2% of women in some early series, illustrating how easily such perforations can occur. These transmigrations of intrauterine devices happened without excessive pain or bleeding at the time of insertion and were otherwise asymptomatic.
- False passage at the internal os. If the internal os is tight or atrophic, a false passage leading from the cervical canal just before the internal os into the peritoneal cavity can occur. This would lead to an absence of curettings at best and possible damage to adnexal vessels, bladder, or bowel at worst.
Techniques to Minimize Risks
The physician should evaluate these variations and keep the risks in mind. A D&C should then be performed in a systematic manner, following the “ritual” outlined in 1958.2
- Catheterization. The patient should be catheterized before bimanual pelvic examination. A preoperative enema or laxative to clear the pelvis of feces is also of significant benefit. Before an office procedure, the patient should void spontaneously just before getting on the examining table, to eliminate the need for catheterization.
- Bimanual pelvic examination. The pelvis is examined bimanually to evaluate the size, shape, mobility, and position of the uterus and to detect the presence of extrauterine masses. A rectovaginal examination, especially in retroversion, is very useful at this point.
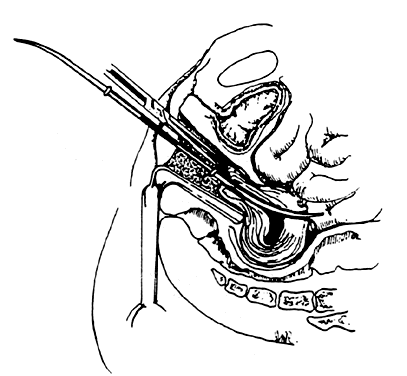
- Grasping the cervix. To visualize the cervix, the blade of a weighted speculum (Auvard) is inserted into the vagina to retract the posterior wall and assist in the exposure of the cervix. The single-toothed uterine vulsellum, the less traumatic Littlewood forceps, or the double-toothed Jacobs tenaculum can be used (Fig. 1). I prefer to put one tenaculum deep into the cervical canal, ensuring a large grasp of the anterior cervix. Other techniques include one tenaculum at the 3 o'clock position and one at the 9 o'clock position, or one tenaculum sideways over the external os to avoid the canal. A retroverted uterus may be corrected more completely by placing the tenaculum on the posterior cervix. With a shallow, superficial grasp, there is a greater risk of tearing during any resistance to dilation at the internal os. The middle finger of the hand holding the tenaculum should be placed between it and the patient's pubis to absorb the resistance to dilation and avoid trauma to the delicate clitoris and urethra.
- Uterine sound. The uterine sound is held like a pen, not a skewer. The sound should be used as a probe to gently and delicately determine the location and direction of the internal os and then the location and direction of the uterine cavity. It is helpful for the operator's hand to be on the patient's buttocks to steady the arm and increase the tactile sensitivity of the fingers holding the sound. Traction on the cervix by the tenaculum will help correct uterine flexion. The curve of the malleable sound should be adjusted and rotated to determine the exact direction and location of the internal os and uterine cavity. This finding will determine the direction for inserting the dilators and curets.
The sound should be passed until it meets the resistance of the fundus of the uterus, such that further pressure on the sound will cause the tenaculum holding the cervix to move. At this point, the depth of penetration by the sound should be marked with an index finger and the size of the uterus measured. The adult multiparous normal uterine cavity can be 7 to 9 cm long, and the hypoplastic or postmenopausal cavity can be as small as 5 cm or less.
I cannot stress enough that to avoid perforation, gentle technique is required in performing this procedure on the thin or atrophic uterus. Sounding should be avoided in the postpartum or pregnant uterus. Difficulty in passing the standard 3-mm-diameter sound may require using the smallest-diameter dilators in the internal os until the sound can again be used.
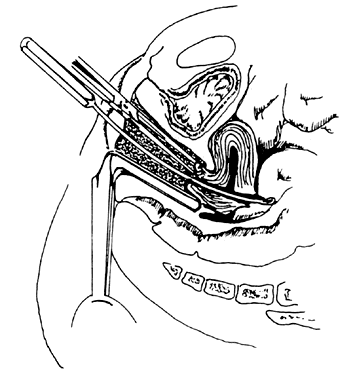
Once the sound is in the uterine cavity, it should be gently moved from side to side to determine the size, symmetry, and presence of distorting lesions of the endometrial cavity. Variations such as uterine septa, leiomyomata, synechiae, and the bicornuate uterus should be kept in mind so they can be diagnosed at this time. - Endocervical curettage. In postmenopausal bleeding or suspected malignancy, the endocervical canal should be curetted before cervical dilation. A sharp, narrow-tipped curet of the Kevorkian-Young or Duncan type is preferred (Fig. 2). The specimen should be collected on a small piece of Teflon-coated gauze that has been inserted onto the weighted speculum blade and tucked under the external os. All endocervical tissue can thus be obtained and separately labeled.
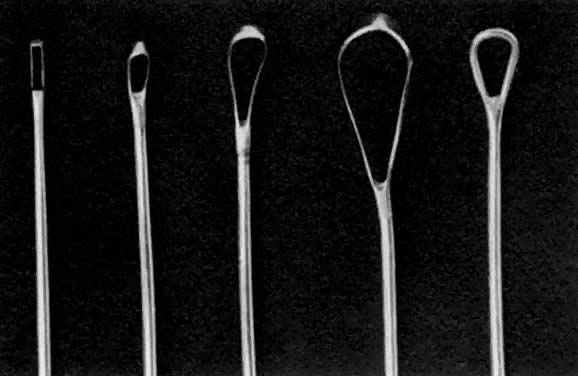
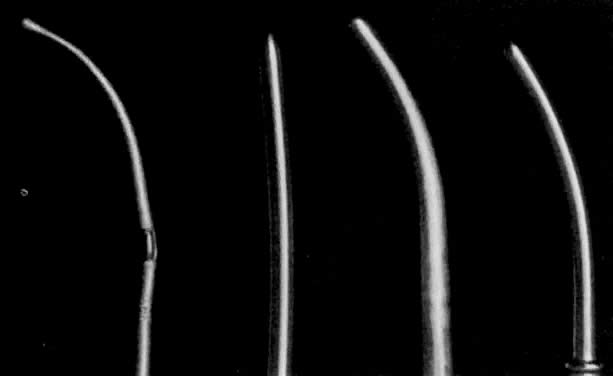
- Dilation of the internal os. Hagar dilators are round-tipped and short and are useful in the small or atrophic uterus. Pratt dilators have a long, tapered tip that makes dilation easier and less traumatic but may perforate the smaller uterus. Hanks dilators have a stop at 8 cm, the depth of most adult uteri, to minimize the risk of perforation (Fig. 3). For ordinary diagnostic curettage, dilation of 8 to 10 cm is all that is necessary. Dilators are measured in millimeters of diameter (Hagar) or “French” circumference in millimeters (Pratt). To convert French markings to diameter, divide the French value by pi, or roughly by three. Thus, a 31 French Pratt will dilate the cervix to about 10 mm.Dilation of the internal os should proceed slowly, allowing the fibers of the internal os to stretch over the dilator for several seconds. A rapid series of insertions may lead to a tear of these fibers. The dilators should again be held like a pen, with the operator's fourth and fifth fingers on the patient's buttocks. These fingers will act as a stabilizer to maximize proprioceptor sensitivity and act as a stop to minimize the risk of forceful perforation of the fundus when resistance is suddenly overcome. If a particular dilator has met with much resistance, it is advisable to leave it in the internal os for several seconds to allow the fibers to expand around it.
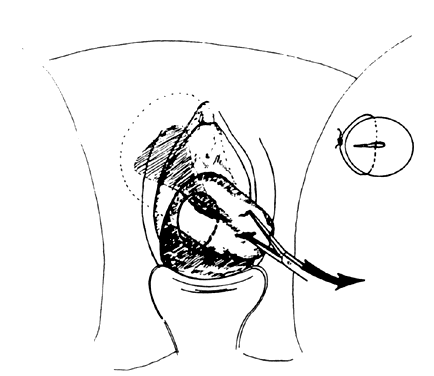
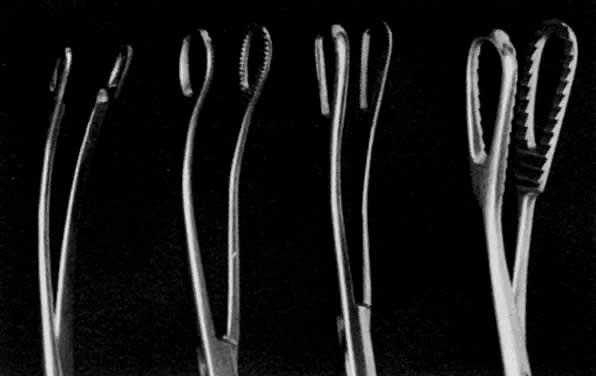
- Exploration with forceps. Especially for menometrorrhagia, the uterus should be explored at this point with a small Randall kidney forceps or a small Ring forceps (Fig. 4) to look for polyps, fragments of placenta, or submucous fibroids that may be better felt by two-point palpation. These large instruments also further define the cavity and thus minimize the risk of perforation by the smaller curet. The forceps should be widely opened inside the cavity, closed, twisted 180°, and then withdrawn to permit avulsion and removal of endometrial polyps or placental fragments.
- Uterine curettage. The standard Sims uterine curet comes in many sizes, but the small or medium is adequate for most D&Cs (see Fig. 2). The Heaney curet has a serrated cup, which permits ridges of decidua basalis to regenerate after vigorous curettage. The curet should have a slight curve modeled after the final curve of the malleable sound. The curet should be held by the entire hand for a firm grip during the “backstroke” coming out of the uterine cavity, but should be inserted gently to allow palpation of the fundus of the uterus. It should always be inserted in the direction of the flexion of the uterus. A fresh 4″
![]() 4″ gauze should be in between the vaginal retractor and the external cervical canal to receive the endometrial curettings. A systematic curettage should consist of directing the tip of the curet to one of the uterine horns and pulling with a continuous stroke from this area out of the uterine cavity to obtain a strip of endometrium to be placed on the gauze. The curet is reinserted, the contralateral uterine horn palpated, and another continuous stroke made down to the outside. This should be repeated with insertion in the anterior flexed position, rotating the curet inside the uterus to address the posterior wall of the fundus, with two or three more strips attempted from the posterior wall. After four or six strips of endometrium are attempted, the cavity should be curetted in its entirety, with frequent withdrawals of the curet to bring the specimen into the waiting sponge. To ensure thorough curettage, the curet is passed from one cornu to the other over the fundus of the uterus. A small, sharp curet might be useful to reach the cornual regions. Only after the tissue is evacuated should the curet be used as a scrub brush to denude the endometrium down to the decidual layer and possibly to detect irregularities suggestive of submucous fibroids hiding underneath the endometrium. The end point of this scrubbing should be the detection of a scratching sensation or sound (the “uterine cry”), which represents a sharp curet running over myometrium. Too vigorous a pursuit of this end point may lead to formation of synechiae (Asherman's syndrome) (see below).
4″ gauze should be in between the vaginal retractor and the external cervical canal to receive the endometrial curettings. A systematic curettage should consist of directing the tip of the curet to one of the uterine horns and pulling with a continuous stroke from this area out of the uterine cavity to obtain a strip of endometrium to be placed on the gauze. The curet is reinserted, the contralateral uterine horn palpated, and another continuous stroke made down to the outside. This should be repeated with insertion in the anterior flexed position, rotating the curet inside the uterus to address the posterior wall of the fundus, with two or three more strips attempted from the posterior wall. After four or six strips of endometrium are attempted, the cavity should be curetted in its entirety, with frequent withdrawals of the curet to bring the specimen into the waiting sponge. To ensure thorough curettage, the curet is passed from one cornu to the other over the fundus of the uterus. A small, sharp curet might be useful to reach the cornual regions. Only after the tissue is evacuated should the curet be used as a scrub brush to denude the endometrium down to the decidual layer and possibly to detect irregularities suggestive of submucous fibroids hiding underneath the endometrium. The end point of this scrubbing should be the detection of a scratching sensation or sound (the “uterine cry”), which represents a sharp curet running over myometrium. Too vigorous a pursuit of this end point may lead to formation of synechiae (Asherman's syndrome) (see below). - Repeat forceps exploration. After the curettage is complete, the Randall kidney clamp may reveal an avulsed polyp or placental fragment that had escaped the efforts to be withdrawn by a curet.
- Repeat uterine sound. As a final step, the uterus should be gently resounded to ensure that perforation has not occurred.
PREOPERATIVE CARE
Because most D&Cs are performed under local anesthesia, preoperative medication should consist of an antiprostaglandin (ibuprofen), 400 to 600 mg taken 1 or 2 hours before the procedure, and possibly an analgesic (oxycodon with aspirin [Percodan]) given 1 hour before.3 The patient should otherwise have taken no food or fluids orally for 4 hours before the procedure. A laxative the evening before or an enema the morning of the procedure may help evacuate the bowel contents, and the patient should spontaneously void to empty the bladder immediately before the procedure. With the conscious patient, ordinary stirrups will suffice for holding the lithotomy position. Under general anesthesia, straps to hold the legs in a lithotomy position are commonly used. Obstetric-type leg holders, providing support under the calves behind the knees, are more comfortable and secure for patients under local or general anesthesia.
No shaving of the perineum is necessary. The vagina should be disinfected with a standard povidone preparation, keeping in mind that the bacteria that are normal flora in the endocervical canal will not be affected by this disinfection and will provide bacteria to be brought into the sterile uterine cavity during the D&C. The uterus handles these bacteria of the patient's own flora remarkably well, and the uterine cavity is usually sterile again within 72 hours.
ANESTHESIA
Some D&Cs are performed in the hospital because of difficulty experienced during an attempt at biopsy in the office. For these patients, general anesthesia is necessary. However, most D&Cs are performed in the office or in the hospital under some local anesthesia. Immediate preoperative medication (in addition to ibuprofen to minimize postoperative pain) should include a short-acting analgesic such as fentanyl or midazolam intravenously, the dosage depending on the patient's level of anxiety. To prevent a vagal reflex, atropine or an antihistamine such as promethazine (Phenergan) is recommended.3 For a paracervical block, I prefer injecting a total of 20 mL of 1% lidocaine subvaginally, 5 mL each in the four quadrants of the cervicovaginal folds (“squaring the circle”), and waiting at least 2 minutes before proceeding with tenaculum manipulation or sounding. Short-acting intravenous analgesics have a risk of transient respiratory depression. However, if a vagal reflex occurs during dilation or curettage, the combination of respiratory depression, bradycardia, and hypotension may lead to unconsciousness and cardiac arrest. Prompt intravenous administration of atropine and cardiopulmonary resuscitation will make this emergency brief, with a complete recovery. This is best monitored and prevented in the office setting by the surgeon or assistant simply maintaining a conversation with the patient to ensure consciousness and adequate cerebral perfusion.
POSTOPERATIVE CARE
The patient may be uncomfortable with cramps from the cervical and uterine irritation, which may lead to a delayed vagal reflex of hypotension and bradycardia. Atropine available in the postoperative area will help manage persistent vagal reactions.
Bleeding from cervical tenaculum punctures is common and usually subsides in 5 minutes with observation alone or gentle pressure with sponge forceps. Chemical cautery (e.g., silver nitrate [AgNO3]) may also be useful. Puncture bleeding rarely requires suturing. If a cervical laceration continues to bleed, suturing may be required. The patient can expect to have some bleeding for 3 or 4 days until the endometrial cavity is relined with endometrium. In an outpatient setting, it is advisable to have the patient leave with another responsible adult, because the effects of medication or vagal reflex make driving hazardous.
MANAGEMENT OF COMPLICATIONS
Listed in order of frequency, three immediate complications can occur.
Laceration of the Cervix by the Tenaculum
Cervical laceration occurs when the resistance of cervical dilation exceeds the strength of the external os where the tenaculum is placed. Because this happens before the curettage is completed, the immediate management should be placing another tenaculum or perhaps two over the tear and finishing the procedure. An ovum or sponge forceps may be required to control bleeding at this point. If the cervix is still bleeding at the end of the procedure, a suture may be required for hemostasis. Arterial pumping may require a suture before completing the D&C. If the tear results in distortion and avulsion of the cervical tissue, a suture may be necessary to reapproximate normal anatomy. If, however, there is no bleeding and the torn cervix is reasonably well approximated, no suture is required. These tears realign themselves; the damage is well below the internal os and will cause no functional problem, and it will usually heal with a barely detectable scar.
Uterine Perforation
In an analysis of 7,000 D&Cs, a 1% occurrence of recognized perforation was noted.2 In this report, seven hysterectomies were performed, although “in none did the findings in the peritoneal cavity warrant hysterectomy…. It is believed that these patients would have done just as well had they been treated conservatively.” Of the laparotomies performed, only three were deemed necessary to manage bowel damage, for an incidence of 0.04%.
Recent experience with diagnostic laparoscopy has taught us that uterine sounds are found penetrating normal uteri with surprising frequency. The incidence of unrecognized perforation with a sound during D&C is probably in the 3% to 4% range. As observed at laparoscopy, these perforations are benign, seldom bleed for more than 5 minutes, if at all, and are more a cause of embarrassment to the physician than risk to the patient. If the perforation is known to have occurred with a blunt instrument such as a sound or dilator without a possible curet or forceps exploration of the peritoneum, observation of vital and peritoneal signs for 4 hours is all that is necessary. A laparoscopy may be necessary, however, to complete the curettage with visual monitoring (Fig. 5). Ultrasound may also provide a noninvasive method for visual monitoring.
If the suspicion is strong that a curet has entered the abdominal cavity, or if the curet or forceps has brought back adult fat, mesenteric damage must be ruled out and a laparoscopy should be performed (Fig. 6). A two-puncture technique, using a grasping forceps to mobilize the bowel, maximizes exploration. Steep Trendelenburg will display most of the pelvic viscera. Mesenteric damage will manifest as a disruption of the smooth peritoneum of a mesenteric surface, with possible bleeding. If some fat is devitalized or a small area of ecchymosis is visible but there is no active bleeding, the patient can be observed overnight with antibiotics, because of the vaginal flora that have been introduced. If there is persistent or active bleeding, an attempt can be made to control this with bipolar coagulation by laparoscopy, taking great care to avoid the bowel itself. Most gynecologists would be more comfortable doing a laparotomy to control bleeding at this point and would use that opportunity to “run the bowel” to look for bowel damage. Bowel itself is tough and slippery and is rarely injured enough during these perforations to warrant repair and anastomosis. However, some adhesions may hold the bowel fixed for perforation and prevent laparoscopic visualization of the damage. Exploration with laparotomy in the presence of such adhesions is indicated.
Tears of the Internal Os
The cervix may be torn rather than dilated. These tears generally go laterally, most often in the 9 o'clock direction (to the patient's right). Although the entire thickness of the lateral wall may be torn, these injuries usually are unrecognized, are uneventful, and heal without functional defect such as incompetent os.1 Rarely, they can injure a cervical artery or vein and cause vigorous bleeding. If this occurs, the curettage itself should be completed rapidly. Then, drawing the cervix further down into the vagina, a 0 chromic or Dexon suture is placed through the vagina high up and deep into the lateral cervix from about the 11 o'clock to the 7 o'clock position. It is important that the suture arc include the cervical stroma and not paracervical tissues, to avoid ureteral injury. Tying should compress the bleeding point (Fig. 7).
Late Complication: Asherman's Syndrome
Delayed postpartum bleeding is best managed by a D&C to remove retained secundines. The myometrium is soft, and the scratching sensation of myometrium against the curet (to ensure completion) may not occur. As a result, the physician may curet too vigorously and destroy the decidua basalis of the endometrium. Healing will result in synechiae between the walls of the uterus that present as amenorrhea or secondary infertility. Diagnosis is made by hysterosalpingography, and therapy is hysteroscopic lysis. Although pregnancy rates after therapy are good, the thin endometrium increases the risk of placenta accreta. Myometrial thinning to the point of uterine sacculation during pregnancy has also been reported.4 This suggests myometrial destruction beyond the decidua at the time of curettage.
To minimize these risks, vigorous scrubbing of the postpartum myometrium should be avoided by using a dull, large “banjo” curet with gentleness to free the uterine wall of retained products of conception while avoiding decidual and myometrial destruction.
INDICATIONS FOR D&C
The diagnostic indications are as follows:
Abnormal bleeding
Intermenstrual bleeding
Postmenopausal bleeding (rule out endometrial carcinoma)
Menometrorrhagia
Abnormal cytology (endocervical curettage, cone biopsy for cervical carcinoma)
Ruling out disease of the endometrium (endometritis, malignancy) at time of hysterectomy
Ruling out pregnancy at time of laparoscopic sterilization
Dysmenorrhea
Oligomenorrhea and amenorrhea
Infertility
The therapeutic indications are as follows:
Menometrorrhagia
Dysmenorrhea
Suspected intrauterine pathology (polyps, incomplete abortion, molar pregnancy)
Postpartum bleeding and retained secundines
Hematometra or hematocolpos
Retrieval of “lost” intrauterine device
Insertion of radioactive carriers for management of uterine or cervical malignancy
This list includes all possible indications for a D&C. However, with today's technologies, many alternatives have evolved. In conditions in which intrauterine pathology is almost certain (e.g., postpartum bleeding or suspected incomplete abortion), D&C is the method of choice to stop the bleeding rapidly and to obtain a diagnosis. The roles of aspiration biopsy, hysteroscopy, abdominal and vaginal ultrasonography, and saline infusion sonohysterography will be discussed in the context of the indications for D&C.
Menometrorrhagia: Medical Management
The D&C has long been held to be therapeutic for menometrorrhagia, because about half the patients improve afterwards. However, this was probably because half the cases were due to an endocrine abnormality that would probably have run its course by the time of the D&C. Medical management is currently used initially to manage menometrorrhagia in women under age 35 or 40, with hormonal cycling for several cycles, rather than D&C.5
The therapeutic value of a D&C as an initial management for menometrorrhagia has been studied.6 Over half the patients continue to lose blood in the same quantity and pattern after this procedure as before, even though subjective complaints may be fewer. These women usually have anatomic causes (polyps, submucous fibroids) for their abnormal bleeding. Current management may include an office screening procedure to rule out intrauterine pathology that would not respond to hormonal management. The roles of office hysteroscopy, vaginal sonography, and saline infusion hysterography to screen all patients are currently being evaluated.
In women who continue to bleed after a course of medical management, hysteroscopy has emerged as the gold standard for the diagnosis of intrauterine pathology against which other procedures, including D&C, have been measured (see Polyps or Submucous Fibroids below).
Ruling Out Endometrial Carcinoma
In several large studies,7,8,9,10,11,12,13,14,15,16 the sensitivity of the D&C to rule out endometrial carcinoma was compared with the recently developed office aspiration biopsy or brush techniques, which require little dilation and anesthesia and can be performed during an office visit. The specimen obtained was adequate for histologic diagnosis in 77% to 94% of D&Cs and in 85% to 99% of aspiration techniques.16 These techniques appear to be more accurate than curettage in detecting carcinoma of the endometrium. Most of these series do not report detection of endometrial carcinoma under the age of 40 or 45. Such evidence suggests that especially in younger women, a simple office aspiration to rule out carcinoma may be preferable to the D&C in terms of accuracy as well as cost.16,17 Candidates for hospital D&C include women who do not tolerate office aspiration, whose cervix is stenosed, who have complicating medical problems, or whose prior aspirations were technically inadequate, with no material obtained.
The development of high-resolution vaginal ultrasonography has led to the evaluation of this less invasive and less painful tool as a screening method to rule out endometrial carcinoma. Initial studies18,19 suggested that an endometrial strip 4 mm wide or less (a “pencil line”) could rule out endometrial stimulation with the risk of carcinoma; measurements over 5 mm suggested hormonal stimulation and possible cancer. Goldstein and colleagues18 found such a pencil line in 11 of 28 (39%) women with postmenopausal bleeding, and Varner and associates19 in 60 of 80 (75%) women, of whom 65 were asymptomatic. Varner observed that “a sonographically measured endometrial thickness of 4 mm or less exhibited a 96.7% sensitivity, 100% specificity, and 100% positive predictive value for…low estrogen stimulation.” All carcinomas were associated with a substantially thicker endometrium.
Thus, screening to rule out endometrial carcinoma can rely on vaginal ultrasonographic findings. A postmenopausal endometrium thickness of 3 to 4 mm or less rules out carcinoma as consistently as endometrial aspiration, and could eliminate the need for the more painful endometrial aspiration procedure or D&C in almost half the symptomatic patients and the great majority of asymptomatic patients. These findings and recommendations were further substantiated recently by Van Den Bosch20 and Dijkhuizen21 and their colleagues.
Managing Postmenopausal Bleeding
Patients with postmenopausal bleeding may be managed in the office by cervical and endocervical cytology (and possibly colposcopy) to rule out carcinoma of the cervix, followed by endocervical curettage and endometrial aspiration. A formal D&C can be reserved for technical difficulties in obtaining a sample or patient discomfort justifying general anesthesia. Vaginal ultrasonography can rule out endometrial carcinoma but may be less accurate than hysteroscopy to rule out other causes of such bleeding such as focal hyperplasia or submucous fibroids or polyps. Office hysteroscopy may be feasible and accurate (in detecting an early focal lesion) if the patient is already on estrogen replacement and the internal os is still dilated.
Ruling Out Carcinoma Before Elective Hysterectomy
In an analysis of pathology specimens when a “ritual” curettage was performed immediately before hysterectomy to rule out carcinoma, less than half of the uterine cavity had been curetted in 60% of the specimens.22 This has led to the practice of replacing the D&C at surgery with screening (before admission) by aspiration or cytology brush to rule out malignant changes before an otherwise routine hysterectomy.
Ruling Out Pregnancy Before Elective Sterilization
A routine D&C just before laparoscopic sterilization to detect and abort a possible luteal-phase pregnancy has been performed in some institutions. However, a review of the results of this approach23 revealed that such a D&C diminishes, but does not eliminate, the possibility of a pregnancy continuing after the procedure. The recently developed rapid and sensitive enzyme-linked immunosorbent assay pregnancy tests (e.g., ICON) detect such possible luteal-phase pregnancies and are replacing the routine D&C for this purpose.
Ruling Out Polyps or Submucous Fibroids
In women whose metrorrhagia does not respond to D&C or hormonal therapy, intrauterine anatomic factors, such as polyps, fibroids, or retained products of conception, are likely. In several independent studies, the efficacy of hysteroscopy was recently compared with D&C (Table 1).24,25,26,27,28,29 Polyps and fibroids were often missed at D&C but found at hysteroscopy, resulting in an overall sensitivity (ability to detect disease) of 65% to 78% for D&C and 93% to 98% for hysteroscopy. Submucous fibroids detected at D&C are known to “disappear” at hysteroscopy,28 a false-positive risk for D&C rare with hysteroscopy. The false-negative rate (missing pathology) is 10% to 34% with D&C, compared to 1% to 9% with hysteroscopy. Most of the pathology missed by hysteroscopy alone is histologic (endometritis, hyperplasia). Focal lesions such as early endometrial carcinoma are detected more accurately with hysteroscopic localization than with blind curettage.25
TABLE 1. Comparison of Sensitivity of False-Negative Rates for D&C and Hysteroscopy- Directed Biopsies (Four Independent Series)
|
|
| Sensitivity | False-negatives | ||
Author | No. of Cases | Indicators | D&C | Hysteroscopy | D&C | Hysteroscopy |
Mohr | 60 | Postmenopausal bleeding | 65% | 96% | 33% | 3% |
Gimpelson | 51 | Menometrorrhagia | 68% | 93% | 34% | 9% |
Wamsteker | 196 | Menometrorrhagia | 78% | 96% | 10% | 2% |
Loffer | 151 | Menometrorrhagia | 68% | 98% | 13% | 1% |
Saline Infusion Sonohysterography
Most recently, saline solutions have been instilled into the uterine cavity by means of a small intrauterine catheter during vaginal ultrasonography. Fluid enhances the outlines of soft-tissue sonodensities; the amniotic fluid in intrauterine pregnancies is a familiar example. Saline infusion with a catheter adds somewhat to the invasive and uncomfortable nature of sonohysterography, but adds significantly to the enhancement of the sonographic image and therefore the sensitivity and specificity in diagnosing polyps and fibroids. Currently called “saline infusion sonohysterography,”30 it was first described in 1986 by Randolph and colleagues,31 who correctly diagnosed 53 of 54 intrauterine abnormalities (missing a noncommunicating uterine horn) with abdominal sonography. An early comparative study of abdominal sonography and hysteroscopy using this method suggested diagnostic accuracy comparable to that of hysteroscopy.32 Since then, several series have studied the accuracy of vaginal sonohysterography verified at surgery33,34 or by hysteroscopy.35 In a series of 113 patients, Widrich and associates35 found that the sensitivity and specificity of “saline infusion sonography” was the same as that of hysteroscopy. A good current review of the technique has recently appeared.30
With increasing interest in diagnostic procedures that can be performed in the office rather than hospital operating rooms, significant new technologies for the diagnosis and management of intrauterine disease have become competitive with the traditional D&C. Ultrasonography is minimally invasive and not painful, and many gynecologists have this tool already in their office. It requires considerable manual and visual skill, however, especially if saline infusion is added. Office hysteroscopy remains a gold standard for the diagnosis of intrauterine abnormalities but may never become a standard of care for gynecologists due to the technical demands to minimize the discomfort of the procedure. These procedures are not taught in every residency training program, and the reliable aspiration biopsy and D&C, with tissue diagnosis, will remain an acceptable standard of care until the new superior technologies are more widely accepted.
ALTERNATIVE METHODS OF CERVICAL DILATION
Laminaria
Laminaria tents derived from seaweed were first described in 1863. This technique has had very limited use in gynecology, usually reserved for induced abortions. Recently, however, synthetic polymers have been developed to overcome the potential hazard of spores present in the compressed seaweed that survive disinfection. Lamicel is a polyvinyl alcohol polymer sponge impregnated with 450 mg magnesium sulfate. It is compressed to a diameter of 3 or 5 mm, and when inserted into the cervical canal swells to a maximum diameter of 12 to 20 mm by absorbing water from the cervical tissue.
Dilapan dilators were made of a hydrogel similar to the material used in soft contact lenses. They resulted in a more consistent degree of dilation and were superior to the laminaria tents36,37 and Lamicel.38 However, the manufacturer has been unable to obtain FDA approval for continuing sale, and the device is no longer available in the United States. A recent comparison of laminaria tents and two available synthetic osmotic devices demonstrated the superiority of the thick laminaria tents for dilatation of the pregnant cervix.39 These devices are useful in managing cervical stenosis before D&C: the device is inserted the afternoon before surgery, allowing gradual dilatation overnight.
Other Mechanical Dilators
Various mechanical devices have been proposed as an alternative to the sets of dilators with gradually increasing diameters. However, compared with sets of dilators, these instruments are complex, more difficult to clean, traumatic, and marginally efficient.
Prostaglandins
Prostaglandins have been widely studied for their ability to induce uterine contraction for abortion. They also have a direct relaxation effect on the sheep cervix, and possibly in humans. Studies in humans have demonstrated cervical dilation in pregnancy up to 40 weeks with vaginal suppositories, but simultaneous induction of uterine contractions may account for a good deal of the cervical dilation observed in these pregnant patients.40,41 Prostaglandin E2, which is present in semen, has been demonstrated to induce relaxation of the nonpregnant human cervix without contractions.42 However, chemical dilation of the nonpregnant cervix with prostaglandins has not become clinically available, possibly because of the side effects of these compounds.
Misoprostol is a synthetic prostaglandin E1 analog developed and sold (as Cytotec) to prevent gastric ulcers in patients taking ulcer-inducing medications. It is remarkably well tolerated, without the side effects or contraindications of the other prostaglandins. It also appears to be effective when used vaginally in inducing uterine contractions in the early pregnant uterus43 as well as ripening the cervix and inducing labor at term.44,45,46,47,48,49 There have been no reports of efficacy of cervical dilatation in the nonpregnant cervix, however.
Vasopressin
A drug increasingly used in the United States by gynecologists to decrease operative blood loss has been found to soften the nonpregnant cervix significantly and consistently during dilatation. Using minute amounts (20 cc of normal saline containing 1 unit vasopressin) injected as a paracervical injection at 4 o'clock and 8 o'clock beneath the vaginal mucosa (similar to a paracervical block), a randomized double-blind study in 52 patients undergoing hysteroscopy demonstrated clear and consistent ease of cervical dilatation to 11 mm when vasopressin had been used.50 I have consistently used 4 units in 20 cc of 1% lidocaine for paracervical blocks for local anesthesia to minimize blood loss in certain procedures with no harmful side effects.
Estrogen
The clinician can sometimes take advantage of the cyclic, natural dilation of the cervix resulting from unopposed estrogen present during the first 2 weeks of the menstrual cycle. Procedures to rule out intrauterine abnormalities can be scheduled for the second week after a menstrual period for maximum unopposed estrogen effect and for the most comfortable natural relaxation of the cervix. In clinical situations in which the endocrine condition of the uterus is not in question and the cycle is uncertain, 5 days of pretreatment with relatively high doses of oral estrogens may similarly dilate the cervix and allow an office procedure to proceed more comfortably.
ALTERNATIVES TO CURETTAGE
It is not within the scope of this chapter to discuss the technique of vacuum aspiration, which is dealt with elsewhere in these volumes. Nevertheless, early studies of the techniques with the nonpregnant uterus in 196851 revealed that with negative pressures of 500 to 600 mm Hg, a 5-mm suction cannula would yield better histologic appearance than curettage, with a consistent cleavage plane leaving the decidua basalis of the endometrium intact. The author concluded that vacuum aspiration was a suitable substitute for conventional sharp curettage, with somewhat less dilation being required to obtain an ample histologic sample. More recent studies with smaller vacuum aspiration catheters have led to the increased use of office aspiration methods as an alternative to D&C.
CONCLUSION
Recent developments in endocrinology, aspiration techniques, ultrasonography, and hysteroscopy have provided alternatives to the traditional D&C in the United States. However, many physicians are not trained or equipped to offer these approaches, and many patients are not suitable for these approaches. For these, the D&C will continue to serve as the standard diagnostic or therapeutic method for bleeding problems. Because gynecologists perform fewer classical D&Cs today, more attention must be paid to the details of technique during each D&C to ensure that complications from this useful tool remain infrequent.
REFERENCES
Hulka JF, Higgins G: Tears of the internal cervical os during dilation for routine curettage. Am J Obstet Gynecol 82: 913, 1961 |
|
Word B, Gravlee LC, Wideman GL: The fallacy of simple uterine curettage. Obstet Gynecol 12: 642, 1958 |
|
Penfield HJ: Gynecologic Surgery Under Local Anesthesia. Baltimore, Urban & Schwarzenberg, 1986 |
|
Friedman A, DeFazio J, DeCherney A: Severe obstetric complications after aggressive treatment of Asherman syndrome. Obstet Gynecol 67: 864, 1986 |
|
Speroff L, Glass RH, Kase NG: Clinical Gynecologic Endocrinology and Infertility. Baltimore, Williams & Wilkins, 1973 |
|
Haynes PJ, Hodgson H, Anderson AB et al: Measurement of menstrual blood loss in patients complaining of menorrhagia. Br J Obstet Gynaecol 84: 763, 1977 |
|
MacKenzie IZ, Bibby JG: Critical assessment of dilation and curettage in 1029 women. Lancet 2: 566, 1978 |
|
Teare AJ, Rippey JJ: Dilatation and curettage. S Afr Med J 55: 535, 1979 |
|
Kovacs GT, Bishop GJ: Diagnostic vacuum curettage of the uterus. Med J Aust 2: 321, 1980 |
|
Kerr-Wilson RH, Chaudhury N, Harris VG et al: Outpatient curettage: A comparison of Vabra and Gravlee methods. Scott Med J 26: 49, 1981 |
|
Wren BG: A comparison of the Vabra aspirator and the Mimark endometrial sampler in the screening of postmenopausal women. NZ J Obstet Gynaecol 22: 43, 1982 |
|
Sonnedecker EWW, Simon GB, Sevitz H et al: Diagnostic accuracy of the Accurette endometrial sampler. S Afr Med J 61: 109, 1982 |
|
Kriseman MM: Description of a new disposable uterine sampler (the Accurette) for endometrial cytology and histology. S Afr Med J 61: 107, 1982 |
|
Holst J, Koskela O, Von Schoultz B: Endometrial findings following curettage in 2018 women according to age and indications. Ann Chir Gynaecol 72: 274, 1983 |
|
Polson DW, Morse A, Beard RW: An alternative to the diagnostic dilation and curettage-endometrial cytology. Br Med J 288: 981, 1984 |
|
Grimes DA: Diagnostic dilation and curettage: A reappraisal. Am J Obstet Gynecol 142: 1, 1982 |
|
Smith JJ, Schulman H: Current dilatation and curettage practice: A need for revision. Obstet Gynecol 63: 516, 1985 |
|
Goldstein SR, Nachtigall M, Nachtigall L: Endometrial assessment by vaginal ultrasonography before endometrial sampling in patients with postmenopausal bleeding. Am J Obstet Gynecol 163: 119, 1990 |
|
Varner RE, Sparks JM, Cameron CD et al: Transvaginal sonography of the endometrium in postmenopausal women. Obstet Gynecol 78: 195, 1991 |
|
Van den Bosch I, Vandendael A, Van Schoubroeck D et al: Combining vaginal ultrasonography and office endometrial sampling in the diagnosis of endometrial disease in postmenopausal women. Obstet Gynecol 85: 349, 1995 |
|
Dijkhuizen FPHLJ, Brölmann HAM, Potters AE et al: The accuracy of transvaginal ultrasonography in the diagnosis of endometrial abnormalities. Obstet Gynecol 87:345, 1996 |
|
Stock RJ, Kanbour F, Kanbour A: Prehysterectomy curettage. Obstet Gynecol 45: 537, 1957 |
|
Grubb GS, Peterson HB: Luteal phase pregnancy and tubal sterilization. Obstet Gynecol 66: 784, 1985 |
|
Mohr JW: Hysteroscopy as a diagnostic tool in postmenopausal bleeding. In Phillips JM (ed): Endoscopy in Gynecology, p 347. Downey, CA, American Association of Gynecologic Laparoscopists, 1977 |
|
Valle RF: Hysteroscopic evaluation of patients with abnormal uterine bleeding. Surg Gynecol Obstet 153: 521, 1981 |
|
Wamsteker K: Hysteroscopy in the management of abnormal uterine bleeding in 199 patients. In Siegler AM, Lindemann HJ (eds): Hysteroscopy: Principles and Practice. Philadelphia, JB Lippincott, 1984 |
|
Gimpelson RJ: Panoramic hysteroscopy with directed biopsies vs. dilatation and curettage for accurate diagnosis. J Reprod Med 29: 575, 1984 |
|
Goldrath MH, Sherman AI: Office hysteroscopy and suction curettage: Can we eliminate the hospital diagnostic dilatation and curettage? Am J Obstet Gynecol 152: 220, 1985 |
|
Loffer FD: Hysteroscopy with selective endometrial sampling compared to dilation and curettage in abnormal uterine bleeding: The value of negative hysteroscopic view. Obstet Gynecol 73: 16, 1989 |
|
Goldstein SR: Saline infusion sonohysterography. Clin Obstet Gynecol 39: 248, 1996 |
|
Randolph JR Jr, Ying YK, Maier DB et al: Comparison of real-time ultrasonography, hysterosalpingography, and laparoscopy/hysteroscopy in the evaluation of uterine abnormalities and tubal patency. Fertil Steril 46: 828, 1986 |
|
Bonilla-Musones F, Simón C, Serra V et al: An assessment of hysterosalpingosonography (HSSG) as a diagnostic tool for uterine cavity defects and tubal patency. J Clin Ultrasound 20: 175, 1992 |
|
Parsons AK, Lense JJ: Sonohysterography for endometrial abnormalities: Preliminary results. J Clin Ultrasound 21: 87, 1993 |
|
Goldstein SR: Use of ultrasonohysterography for triage of perimenopausal patients with unexplained uterine bleeding. Am J Obstet Gynecol 170: 565, 1994 |
|
Widrich T, Bradley LD, Mitchinson AR, Collins RL: Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol 174: 1327, 1996 |
|
Grimes DA, Ray IG, Middleton CJ: Lamicel versus laminaria for cervical dilation before early second-trimester abortion: A randomized clinical trial. Obstet Gynecol 69: 887, 1987 |
|
Darney PD, Dorward K: Cervical dilation before firsttrimester elective abortion: A controlled comparison of Metemprost, laminaria, and Hypam. Obstet Gynecol 70: 397, 1987 |
|
Wells E, Hulka JF: Cervical dilation: A comparison of Lamicel and Dilapan. Am J Obstet Gynecol 161: 1124, 1989 |
|
Kline SB, Meng H, Munsick RA: Cervical dilation from laminaria tents and synthetic osmotic dilators used for 6 hours before abortion. Obstet Gynecol 86: 931, 1995 |
|
Hulka JF, Chepko M: Vaginal prostaglandin E, analogue (ONO-802) to soften the cervix in first-trimester abortion. Obstet Gynecol 69: 57, 1987 |
|
Dingfelder JR, Brenner WE, Hendricks CH et al: Reduction of cervical resistance by prostaglandin suppositories prior to dilatation for induced abortion. Am J Obstet Gynecol 122: 25, 1975 |
|
Coutinho EM, Darze E: Spontaneous contractility and the response of the human uterine cervix to prostaglandins F2α and E2 during the menstrual cycle. Am J Obstet Gynecol 126: 224, 1976 |
|
Creinin MD, Darney PH: Methotrexate and misoprostol for early abortion. Contraception 48: 339, 1993 |
|
Sanchez-Ramos L, Kaunitz AM, Del Valle GO et al: Labor induction with the prostaglandin E1 methyl analogue misoprostol versus exytocin: A randomized trial. Obstet Gynecol 81: 332, 1993 |
|
Fletcher HM, Mitchell S, Simenon D et al: Intravaginal misoprostol as a cervical ripening agent. Br J Obstet Gynaecol 100: 641, 1993 |
|
Fletcher HE, Mitchell S, Simenon D et al: Intravaginal misoprostol versus dinoprostone as cervical ripening and labor-inducing agents. Obstet Gynecol 83: 244, 1994 |
|
Wing DA, Jones MM, Rahall A et al: A comparison of misoprostol and prostaglandin E2 gel for preinduction cervical ripening and labor induction. Am J Obstet Gynecol 172: 1804, 1995 |
|
Varaklis K, Gumina R, Stubblefield PG: Randomized controlled trial of vaginal misoprostol and intracervical prostaglandin E2 gel for induction of labor at term. Obstet Gynecol 86: 541, 1995 |
|
Sanchez-Ramos L, Chen AH, Kaunitz AM et al: Labor induction with intravaginal misoprostol in term premature rupture of membranes: A randomized study. Obstet Gynecol 89: 909, 1997 |
|
Phillips DR, Nathanson HG, Milim SJ, Haselkorn JS: The effect of dilute vasopressin solution on the force needed for cervical dilation: A randomized controlled trial. Obstet Gynecol 89: 507, 1997 |
|
Marik JJ, Tatarun IV: Dilation, fractional curettage, and nonpregnant vacuum aspiration. In Symonds EM, Zuspan FP (eds): Clinical and Diagnostic Procedures in Obstetrics and Gynecology. New York, Marcel Dekker, 1984 |



 4″ gauze should be in between the vaginal retractor and the external cervical canal to receive the endometrial curettings. A systematic curettage should consist of directing the tip of the curet to one of the uterine horns and pulling with a continuous stroke from this area out of the uterine cavity to obtain a strip of endometrium to be placed on the gauze. The curet is reinserted, the contralateral uterine horn palpated, and another continuous stroke made down to the outside. This should be repeated with insertion in the anterior flexed position, rotating the curet inside the uterus to address the posterior wall of the fundus, with two or three more strips attempted from the posterior wall. After four or six strips of endometrium are attempted, the cavity should be curetted in its entirety, with frequent withdrawals of the curet to bring the specimen into the waiting sponge. To ensure thorough curettage, the curet is passed from one cornu to the other over the fundus of the uterus. A small, sharp curet might be useful to reach the cornual regions. Only after the tissue is evacuated should the curet be used as a scrub brush to denude the endometrium down to the decidual layer and possibly to detect irregularities suggestive of submucous fibroids hiding underneath the endometrium. The end point of this scrubbing should be the detection of a scratching sensation or sound (the “uterine cry”), which represents a sharp curet running over myometrium. Too vigorous a pursuit of this end point may lead to formation of synechiae (Asherman's syndrome) (see below).
4″ gauze should be in between the vaginal retractor and the external cervical canal to receive the endometrial curettings. A systematic curettage should consist of directing the tip of the curet to one of the uterine horns and pulling with a continuous stroke from this area out of the uterine cavity to obtain a strip of endometrium to be placed on the gauze. The curet is reinserted, the contralateral uterine horn palpated, and another continuous stroke made down to the outside. This should be repeated with insertion in the anterior flexed position, rotating the curet inside the uterus to address the posterior wall of the fundus, with two or three more strips attempted from the posterior wall. After four or six strips of endometrium are attempted, the cavity should be curetted in its entirety, with frequent withdrawals of the curet to bring the specimen into the waiting sponge. To ensure thorough curettage, the curet is passed from one cornu to the other over the fundus of the uterus. A small, sharp curet might be useful to reach the cornual regions. Only after the tissue is evacuated should the curet be used as a scrub brush to denude the endometrium down to the decidual layer and possibly to detect irregularities suggestive of submucous fibroids hiding underneath the endometrium. The end point of this scrubbing should be the detection of a scratching sensation or sound (the “uterine cry”), which represents a sharp curet running over myometrium. Too vigorous a pursuit of this end point may lead to formation of synechiae (Asherman's syndrome) (see below).