Hormonal Therapy of Endometrial Carcinoma
Authors
INTRODUCTION
During the past 30 years there have been significant changes in the pathologic epidemiology and the clinical and pathologic classification of carcinoma of the endometrium. In 1975, 75% of tumors were confined to the uterus and were well-differentiated endometrioid endometrial carcinomas.1 Currently, many more tumors are less well differentiated. This is in part a result of an aging population, as well as to the more constrained use of estrogen for replacement therapy and the concomitant use of progestogens. There are therefore proportionately fewer patients with well-differentiated endometrioid endometrial carcinomas. Also, high-risk histologic entities such as papillary serous carcinoma (now called serous carcinoma) and clear cell carcinoma have been recognized to have a poor prognosis;2 these histologic entities may be disseminated at the time of diagnosis and have a high potential for recurrence even with surgical stage I disease. The staging system devised by the International Federation of Gynecologists and Obstetricians (FIGO) now requires surgical staging3 to ensure that a more precise histopathologic diagnosis is made and that subclinical metastases are more frequently recognized at the time of initial intervention. Most patients with grade 1 endometrioid endometrial carcinoma confined to the uterus are therefore likely to be cured by primary hysterectomy. Yet it is in such patients, if there are metastases at presentation or there is recurrence, that hormonal therapy is still most useful. The response rates of the various histologic grades of endometrial cancer have not changed. What has changed is that the tumors of higher histologic grade are more likely to be metastatic and to recur, and these are the variants that are less sensitive to progestational agents. Many investigators group all histologic types and grades together in assessing response;4 as a result, the commonly quoted response rate of 20–30% fails to identify the subgroup of grade 1 tumors that has a higher response rate.
This chapter discusses the hormonal treatment of endometrioid endometrial cancer. Nonhormonal chemotherapy is addressed elsewhere in this library. There are convincing data showing that uterine serous papillary carcinoma and clear cell carcinoma are hormone nonresponsive,5 and that the management of these entities deserves a discussion separate and distinct from that of uterine endometrioid tumors.
Since recurrence with grade 1 tumors is less common and since most advanced and recurrent disease is associated with higher tumor grade, the initial finding that about one third of tumors will respond to progestational agents may no longer be valid. Clinical experience and research have taught us which women are likely to have tumors that will respond, so we are in a position to define more precisely the indications for hormonal therapy. However, it is of concern that the use of progestational agents in grade 1 endometrial cancer is still widespread, hormonal therapy is used less frequently with grade 2 and particularly grade 3 tumors where there is still significant though occasional activity. This aspect of hormonal therapy deserves reevaluation.
HISTORICAL ASPECTS
In 1953, Wellenbach and Rakoff6 showed that endometrial hyperplasia, that had been induced in oophorectomized hamsters by the prolonged administration of estrogen, regressed when progesterone was given. If, however, the progesterone was given with the estrogen, the development of endometrial hyperplasia was prevented. It was also found that in patients with Stein-Leventhal syndrome (polycystic ovary syndrome), endometrial hyperplasia could be reversed following the return of ovulatory cycles after wedge resection of the ovaries.7 The use of progestational agents as a treatment for endometrial cancer was first suggested by Nathanson, but implementation of the idea was frustrated by the unavailability of a suitable medication.8 The action of progesterone was too brief, and the necessary daily injections were painful.
Potent, longer acting synthetic progestogens became available in 1959. Kistner9 was the first to report the use of progestogens in patients with cystic and adenomatous hyperplasia and carcinoma in situ of the endometrium. These patients were menopausal or postmenopausal, and glandular atrophy and stromal decidua were produced in each case. In 1960, Kelly and Baker10 reported on the use of 17α-hydroxyprogesterone caproate (Delalutin) in patients with metastatic carcinoma of the endometrium, and they subsequently described the results in 165 patients. They obtained a 34% response rate, especially among those with pulmonary and osseous metastases. In the tumors that responded, the disease was of long duration and slow growth, and the tumor was usually well differentiated. Kennedy11 and Varga and Henricksen12 soon added to these initial reports. Effective cancer therapy was being achieved without toxicity.
It is remarkable how these initial findings have been sustained in subsequent studies. The only impression that has not withstood the test of time is that lung and osseous metastases are more sensitive to therapy. Progestational steroid hormones were used clinically without much prior animal experimentation, perhaps because there is no good animal model of spontaneous endometrial carcinoma. Because of their low toxicity, progestational agents may, on occasion, be given too freely, and there is temptation to use the medication prophylactically. Instillation of progestational agents into the uterine cavity has been repeatedly described and shown to be locally effective, but local intrauterine therapy has not gained widespread use. Even now it deserves critical evaluation, perhaps as an alternative to intracavitary radium application in patients with well-differentiated tumors. Clearly, progestogens have not become the panacea that it was originally hoped they would be since they are not effective cancericidal agents for all patients with endometrial cancer. There may therefore be an indication for the use of conventional chemotherapy as initial treatment for advanced disease or recurrent cancer.
PROGESTATIONAL AGENTS
Progestational agents used in treatment of endometrial carcinoma are listed in Table 1 (Figs. 1–3).
Table 1. Progestational agents used in treatment of endometrial carcinoma
Nonproprietary name | Trade name | Loading dose | Maintenance dose |
Medroxyprogesterone | Depo-Provera | 400 mg three times per week for 12 weeks | 400 mg twice weekly |
| Provera | 200–300 mg daily for 12 weeks | 200 mg daily |
Megestrol acetate | Megace | 160 mg daily for 12 weeks | 80–160 mg daily |
17α-Hydroxyprogesterone caproate | Delalutin | 500 mg twice weekly | 500 mg twice weekly |
Medrogestone | Colprone | Not available | Not available |
Norethynodrel | Enovid |
|
|
Dimethisterone |
|
|
|
Ethynodiol diacetate |
|
|
|
The initial medication used was progesterone in oil. This was painful and messy to inject and never attained widespread use, although it certainly appears to be effective. The first potent oral synthetic progestogen was norethynodrel (Enovid), which in fact contains 1.5% ethinyl estradiol as an intended concomitant impurity. This was one of the drugs Kistner9 used in the patients with endometrial hyperplasia and carcinoma in situ. Wentz13 used oral dimethisterone (75 mg daily for 6 weeks) in patients with persistent adenomatous and atypical hyperplasia.
Neither norethynodrel nor any of the 19-nortestosterone progestational agents gained widespread use in the treatment of endometrial carcinoma. There is no reason to believe that they are less active than “true” progestational compounds, with a methyl group at position 19 and an acyl (CH3–CO) group at position 17β. In fact, ethynodiol diacetate and dimethisterone were effectively used for local instillation into the uterine cavity.
Kelly used 17α-hydroxyprogesterone caproate, which is injectable.10 Medroxyprogesterone acetate (Depo-Provera, Provera) which was initially used by Anderson14 as an oral preparation of 300 mg daily, was subsequently used mainly as an injectable depot preparation, and is being used as an oral preparation only outside the United States. The two injectable preparations were the most widely used progestogens in the treatment of endometrial carcinoma during the first decade of therapy. Medroxyprogesterone was given in clinical trials as an oral preparation,14, 15 but it has not been approved for use in endometrial cancer in the United States. Medrogestone (Colprone) and megestrol acetate (Megace) are the other two oral progestational agents that have been used in endometrial carcinoma and both are also “true” progestogens. Medrogestone was used only in clinical trials and has not become commercially available. Chlormadinone, another potent oral progestogen, was not approved for commercial use because it was shown to produce malignant nodules in the breast tissue of beagles. Its use in endometrial carcinoma has not been reported.
USE OF PROGESTATIONAL AGENTS IN ENDOMETRIAL CANCER
The progestogens most commonly used in endometrial carcinoma were medroxyprogesterone, 17α-hydroxyprogesterone caproate, and megestrol acetate. 17α-Hydroxyprogesterone (Delalutin) was used as a 250 mg/ml injection but has now been taken off the market and is no longer manufactured. Medroxyprogesterone (400 mg/ml) is still available as a depot injection. Oral medroxyprogesterone was used in clinical trials in the United States as a 50-mg tablet15 and was found to be effective in an oral dose of 200 mg daily. Although a 100-mg tablet is available for use in cancer in Europe and a 500-mg tablet is available in Australia, problems with the US Food and Drug Administration (FDA) precluded the use of these doses in cancer therapy in the United States. Megestrol acetate became available after only one clinical trial on 46 patients16 and is used as a 40-mg tablet, usually with a total dose of 160 mg/day, and sometimes 240 mg/day. The ready commercial availability of megestrol acetate, together with the certification problems with medroxyprogesterone, has allowed megestrol to capture the market as the only progestational cancer agent available in oral tablet form in the United States.
DOSAGE OF PROGESTOGENS
A survey of gynecologic oncologists in 1976 showed great variation in the use of agents available at that time.17 However, it appears that a minimal loading dose is necessary and that this is achieved with 3 g medroxyprogesterone given orally or parenterally during the first 3 weeks of therapy. With 17α-hydroxyprogesterone caproate, a similar dose is given parenterally. With megestrol, 160 mg should be given daily, at least for the first 3 weeks.
These practices are based on the fact that an adequate loading dose is necessary for response. Kistner18 found that all patients who had a response received at least 2 g medroxyprogesterone in the first 8 weeks of therapy. With megestrol, the response rate was dose dependent. Thus, there was one response (14.3%) out of seven patients given 40 mg megestrol,16 three responses (42.8%) out of seven patients given 80 mg, and 14 (48.3%) responses out of 29 patients given 160 mg. Similar data were reported by Gowan19 with ethynodiol diacetate: no responses were achieved with 10–40 mg daily for 4 weeks, whereas a dose of 100 mg daily given for 3 weeks induced histologic changes in four of five patients. It is important to note that these were mainly grade 1 tumors.
SERUM LEVELS OF PROGESTOGENS
Data on serum levels of gestagen related to administered dose show that there is a linear relationship between the dose of medroxyprogesterone and the peak serum level. The data suggest that a dose of 100 mg medroxyprogesterone orally twice daily would sustain a serum level of 40–60 ng. Data for depot medroxyprogesterone and 17α-hydroxyprogesterone caproate indicate that biweekly injections may be sufficient to sustain blood levels of 40 ng and 30 ng, respectively. It is not known what an adequate blood level should be, and it should be noted that in 1965 Anderson14 was giving 300 mg/day of oral medroxyprogesterone.
Sall and co-workers20 found that the serum level of medroxyprogesterone was consistently higher if the medication was given orally (50 mg three times daily) rather than as an intramuscular depot injection (300 mg weekly). After 10 weeks of therapy, the mean serum values were 183 ng/ml for oral administration and 73 ng/ml for intramuscular injection. Laatikainen and associates21 found no difference in blood levels during the first 24 hours after administration of medroxyprogesterone if given orally or by injection. Their values were much lower than those found by Sall and colleagues,20 and the authors pointed out that unless the same antibody and the same methodology are used, different studies are not comparable. In a long-term study, Hesselius and Johansson22 found that the plasma concentration in patients receiving 100 mg medroxyprogesterone twice daily reached a steady state, whereas patients receiving intramuscular medication, 1000 mg every week, showed gradually increasing values that leveled off after 6 months. At the end of 12 months, the intramuscular route was associated with a plasma medroxyprogesterone value of 100 ng/ml. This was about three times the value found when the medication was given orally. Thus, although a single oral dose may give a higher peak level than a single intramuscular dose, the cumulative effect of depot injection over many months may give higher sustained plasma levels. These data may be of fundamental importance in the choice of progestational medication and its route of administration.
The confusing data on the low efficacy of oral medroxyprogesterone in some recent studies may be related to the lower late blood levels found with oral administration. The oral 50-mg tablet of medroxyprogesterone has not been validated for clinical use by the US FDA. Because the only significant side effect of intramuscular 17α-hydroxyprogesterone or medroxyprogesterone is the pain of the injection, oncologists have increasingly turned to oral megestrol as the only available progestational agent in advanced or metastatic endometrial carcinoma. No data are available on serum levels for megestrol. This subject still needs to be investigated so that the question of route of progestogen administration can be settled. This issue may be significant, as megestrol has superseded the use of other progestogens, yet progestogens are being reported to be less efficacious in this disease.
MAINTENANCE DOSE OF PROGESTOGENS
The available data suggest that an adequate maintenance dose of 17α-hydroxyprogesterone caproate is 500 mg twice weekly and 400 mg twice weekly for depot medroxyprogesterone; when given orally, the dose is 200 mg daily. The usual maintenance dose of megestrol is 160 mg daily, although some oncologists give 240 mg daily. It is important to use the 40 mg tablet of megestrol.
THERAPEUTIC RESPONSE
Length of response and cessation of therapy
Most investigators advocate that therapy be continued while a response is sustained. Responses vary from a few months to many years. There is a clinical impression that if progestational treatment is stopped there may be relapse, and that some amount of drug should be continued indefinitely. Continuing treatment is in fact the usual practice. The only objection is the cost of the medication and the discomfort of the injection should injectable medication still be used.
Criteria of response
The objective response to chemotherapy is usually defined as 50% reduction in measurable tumor parameters lasting 3 months or more without progression of disease and without development of new metastases. The response should be associated with a significantly prolonged survival time, and it is usual to accept patients for assessment only if the medication has been given for a minimum period: three cycles of chemotherapy, or a 3-month administration of a progestational agent. It is generally considered that the efficacy of a drug can be addressed only if the total intended dose has been administered.
Drug effectiveness
Analysis of reports clearly categorizing response to progestational agents showed that treatment with medroxyprogesterone yielded an objective response in 52 of 151 patients (34%).17 A further 14% showed a partial response and 77 patients had no response. With megestrol acetate, the overall objective response rate was 33% in five patients, with an additional 18 patients showing a partial response. The objective response rate for medrogestone was 30% in 56 patients, and 11 of these showed a partial response. This information is summarized in Table 2. Reifenstein23 analyzed 314 cases from a cooperative study, in all of which 17α-hydroxyprogesterone caproate was used. All of the patients had stage III and stage IV endometrial carcinoma. The objective response rate was 37%. There was significant subjective response in terms of pain relief, amelioration of bleeding, increased appetite, and increased well-being. This observation has been made in common among all investigators using progestational agents. The medication was found to be safe; there was only a 0.6% incidence of allergic reaction, which was probably associated with the solvent, rather than the steroid hormone. Other described side effects were minor. There was a clear correlation between the incidence of response and the duration of therapy, and the subjective response rate increased if the initial length of treatment was more than 12 weeks.
Table 2. Therapeutic response to progestational agents
| No. patients | Objective response (%) | Partial response (%) |
17α-Hydroxyprogesterone | 314 | 30.2 | 6.8 |
Medroxyprogesterone | 151 | 34.0 | 14.0 |
Megesterol | 125 | 33.0 | 14.4 |
Medrogestone | 56 | 30.0 | 19.6 |
There have been no convincing reports of progestogens causing acceleration of disease, no death due to therapy, and no adverse effect of therapy on concomitant disease. Progestogens do not interfere with concomitant radiation therapy or chemotherapy. In fact, it has been suggested that progestogens may be radiosensitizers.
Correlation with histologic differentiation
All reports concerning progestational therapy of endometrial cancer agree that there is a high correlation between response and the degree of tumor differentiation (Table 3). The well-differentiated tumor responds more frequently than the anaplastic tumor; however, some poorly differentiated tumors may respond. It should be noted that a tumor may show histologic changes characteristic of progestational therapy but that there is no objective clinical response, and progression of spread and growth still occur.
Table 3. Therapeutic response related to tumor grade
Response | Grade 1 | Grade 3 |
Objective | 51.7% | 15.5% |
None | 48.2% | 84.4% |
(Adapted from Gowan ADT: Histologic changes induced in adenocarcinoma uteri by ethynodiol diacetate. In Brush MG et al (eds): Symposium on Endometrial Cancer. London, William Heinemann, 1973; Malkasian GD et al: Progestogen treatment of recurrent endometrial carcinoma. Am J Obstet Gynecol 110: 15, 1971; and Rosier JC, Underwood PB: Use of progestational agents in endometrial adenocarcinoma. Obstet Gynecol 44: 60, 1974)
The distribution of response to progestogens with poorly differentiated and well-differentiated tumors taken from data that make this distinction quite clear is shown in Table 3. Malkasian and colleagues24 found an objective response in 15 of 45 well-differentiated tumors, but in only three of 29 poorly differentiated tumors. Geisler16 found an objective tumor response in four of six patients with well-differentiated carcinoma, but in only one of 11 patients with poorly differentiated tumors. Rozier and Underwood25 showed that nine of 13 responses occurred in patients with well-differentiated tumors, whereas only three of 15 poorly differentiated tumors showed objective regression. These investigators also found that prolonged response was more common in well-differentiated tumors.
Podratz and co-workers26 updated the Mayo Clinic experience. Of 155 patients with metastatic disease, only ten had grade 1 tumors, of whom four had a response. Of 71 patients with histologic grade 2 tumors, 11 (15%) had a response; only one (1%) of 73 patients with histologic grade 3 tumors had a response. The length of response ranged from 3 to 95 months. Piver and associates27 also found such a decreased overall response rate. It appears that this lack of response of endometrial cancer to progestational agents reflects the greater frequency of anaplastic tumor rather than a change in the biology of the disease.
Thus, patients with well-differentiated tumors still show a 50% response to progestogens, yet it may still be worthwhile to treat patients with poorly differentiated tumors since a few will show response. We need to recognize that the response rate with nonhormonal chemotherapy in patients with poorly differentiated tumors is better than with progestogens, but the toxicity is of course greater. What seems unjustified is to treat patients with poorly differentiated endometrioid endometrial carcinomas with progestogens only when the response rate is so low.
Relation to age
There is a clear clinical impression that younger women with endometrial carcinoma will have a higher rate of response to progestational agents compared to older women. Twelve younger women (aged 33–49) had an objective response rate of 58%, while a group of older women (aged 71–86) had a response rate of 22%.23 The response rate related to age is, however, very similar to the response rates related to the grade of the tumor (see Table 3). In endometrial carcinoma, well-differentiated tumors are much more frequent among younger women, and older or postmenopausal patients have a higher frequency of anaplastic tumor. The grade of the tumor, rather than the age of the patient, therefore determines response to progestational agents. Any analysis of results of therapy in endometrial carcinoma, therefore, needs to take into consideration the grade of the tumor, and the fact that age and grade may be dependent variables should be recognized.
Relation to length of disease
All reports of progestational therapy agree that the longer a patient has had endometrial cancer, the better and more prolonged the response rate is to progestogens. Reifenstein23 showed that patients with a history of less than 36 months had a mean survival of 10 months after beginning therapy, whereas those with a longer history had a mean survival of 20 months. The patients with prolonged history almost invariably had a well-differentiated tumor.
Relation to site of disease
Early reports of progestational therapy suggested that lung and bone metastases respond more frequently than intra-abdominal disease. The collected and extensive reports of Bonte and colleagues28 and Reifenstein23 indicate, however, that there is no absolute correlation of response to tumor site and that pelvic and abdominal metastases respond as well as lung metastases.
Relation to use of radiation therapy
Several studies have stated that progestational agents are radiopotentiators. Bonte and co-workers28 gave medroxyprogesterone to a group of 20 women for 2 weeks prior to intracavitary radium application and no hormone therapy to a control group of 20 women. At hysterectomy, 5 weeks after the brachytherapy, histologically viable-appearing tumor was found in only seven of the 20 patients treated with progestogen, compared to 18 of the 20 patients in the control group. The number of cases of residual viable tumor after intracavitary radium appears rather high in this series. Mussey and Malkasian29 and Reifenstein23 also suggested that concomitant therapy with progestogen and radiation may give a better response than either alone. None of these studies was controlled, and a prospective, randomized trial is needed to elucidate this important issue. It is of interest that the addition of medroxyprogesterone to tissue cultures of endometrial adenocarcinoma has been reported to increase the susceptibility of the cells to irradiation.30
Indications
Because progestogens are nontoxic and often dramatically effective anticancer agents in endometrial carcinoma, there is a great temptation to use them for less than discriminate indications. A patient adequately treated with surgery and irradiation does not need progestogens. Hormone therapy should be given only when precise indications are present.
ABSOLUTE INDICATIONS
A patient who presents with extrapelvic metastases or who develops extrapelvic metastases after surgical treatment with or without irradiation should receive progestational agents. The loading dose should be adequate and the maintenance dose continued for at least 3 months before assuming that no response will take place. If at 8 weeks, however, there is progressive disease, other chemotherapy may be added without abandoning the gestagen.
Objective measurements of tumor should be obtained if at all possible. All patients should have a chest x-ray. With grade 3 histology, a computed tomography (CT) scan of the lung is advisable, as this may demonstrate micrometastases. Lesions discovered by CT scan after treatment is commenced, or months or years after response, will invalidate the initial assessment. As endometrial carcinoma is a cancer that spreads by way of the bloodstream, isotopic bone scanning should also be routine. Other investigations for assessing a patient with metastatic carcinoma include hemoglobin estimation, white blood cell count, platelet count, and chemical estimations of renal and hepatic function. A periodic abdominal CT scan may validate clinical assessment of palpable disease or may permit assessment of subclinical disease that is known to be present from previous surgical exploration.
General supportive measures may accompany gestagen treatment, since hormone therapy does not interfere with any other medication or treatment. This applies not only to analgesics, but also to measures aimed at controlling local symptomatic disease. Thus, localized bone metastases or symptomatic cervical lymph nodes may be treated with local palliative irradiation. In fact, localized disease should be treated with surgery and/or irradiation in preference to progestational therapy, as the chance of response is significantly higher. This applies particularly to vaginal vault recurrences, which are better treated by radiation brachytherapy, or by surgery if brachytherapy fails, than by primary progestational treatment. Progestational therapy should be reserved for disseminated or widespread disease, or localized disease refractory to surgery and irradiation.
In any given patient, therapy with progestogens in effect constitutes a therapeutic trial. The most important criterion that helps forecast whether therapy is likely to be successful is the grade of the tumor. If the tumor is well differentiated, the chance of response is 50%; if it is poorly differentiated, the response rate is 15% or less. The use of the progesterone receptor assay has demonstrated a high correlation between progesterone receptor status (positive or negative) and clinical response to gestagen. Quinn and co-workers31 summarized 94 patients with endometrial carcinoma in whom there was such a correlation: 82% of progesterone receptor-positive patients had a response, compared to only 11% of progesterone receptor-negative patients. The cutoff level of receptor content between positive and negative varied widely. Receptor presence correlated highly with well-differentiated tumors. It is of interest that estrogen receptor status also correlated highly: 71% of estrogen receptor-positive patients had a response, compared to only 5% of estrogen receptor-negative patients.
From a clinical standpoint it would seem that the real question is not whether progesterone should be withheld, but rather whether nonhormonal chemotherapy should be given in addition to gestagen right from the start in patients with more anaplastic tumors who have a poor chance of response to hormone therapy. If estrogen and progesterone receptor assays are available, they may be used as additional information in treatment planning. Gestagen treatment, however, has virtually no toxicity, and four (6%) of 69 patients who were “negative” for progesterone receptors had an objective response.31
RELATIVE INDICATIONS
There is very little evidence that progestational agents are of benefit to the patient with stage II or even stage III disease treated adequately by surgery and irradiation. In my opinion, in patients with uterine cancer that is more extensive than stage I, grade 1, it is more important to perform a thorough surgical exploration of the peritoneal contents and lymph node areas than to place patients routinely on progestogens. Well-differentiated localized residual tumor after surgery is best treated with irradiation. The argument is even more persuasive if the tumor is less than well differentiated. Suggestions that “a bit of progestogen will do some good” are based on clinical impression. It is better to give the medication when a specific indication arises.
Adjuvant progestational therapy
Progestational therapy adjuvant to surgery and irradiation was popular in some centers. It is unfortunate that reports advocating such treatment are from uncontrolled trials. Because the majority of endometrial carcinomas are confined to the uterus and therefore have a relatively high cure rate, it has been estimated that approximately 700 patients and their controls would be needed to demonstrate a significant difference. Lewis and colleagues32 attempted to analyze the effect of adjuvant progestogen therapy in a cooperative study in which 287 patients were given 500 mg medroxyprogesterone weekly for 14 weeks. There were 285 controls, and there was no difference in recurrence rate or survival in the two groups at 4 years. In a study that stratified surgically staged patients by extent of myometrial invasion, DePalo and associates33 found no advantage for medroxyprogesterone therapy among 747 patients given either placebo or hormone; however, their follow-up period was only 22 months. McDonald and colleagues34 reported on the use of hydroxyprogesterone caproate induction and medroxyprogesterone maintenance in 429 patients with stage I–III endometrial cancer of various grades randomized to receive medication or be observed; not unexpectedly, no survival benefit was demonstrated for the group receiving hormonal medication. Other studies have also lacked sufficient numbers of patients and have included mixed histologies, making it difficult to assess the effect of adjuvant progesterone.35, 36, 37
Intracavitary therapy
The uterus was one of the initial routes of progesterone administration. Progesterone in oil, 17α-hydroxyprogesterone caproate, medroxyprogesterone, and ethynodiol diacetate have been used. All induced tumor destruction and the histologic effects characteristic of progestational therapy. A very high local dose can be attained, and the progestogen appears to have a pharmacologically destructive effect, rather than a physiologic one, at these higher doses. A Foley catheter or a specially devised metal cervical screw was used to instill the hormone into the uterine cavity. DeCoster and co-workers38 described the use of an intrauterine Silastic device impregnated with the progestogen.
Intracavitary therapy has not attained widespread acceptance because of the high cure rate of the disease by hysterectomy with or without radium therapy. In the few patients in whom hysterectomy is contraindicated because of severe medical problems (e.g., coronary heart disease, heart failure, pulmonary insufficiency) and who have a well-differentiated tumor with grade 1 histology, the use of gestagen in lieu of brachytherapy still bears examining. It may also be useful for local therapy for recurrent tumor in the uterus after radiation failure when surgery is precluded.
MECHANISM OF ACTION OF PROGESTERONE
Histologic effect
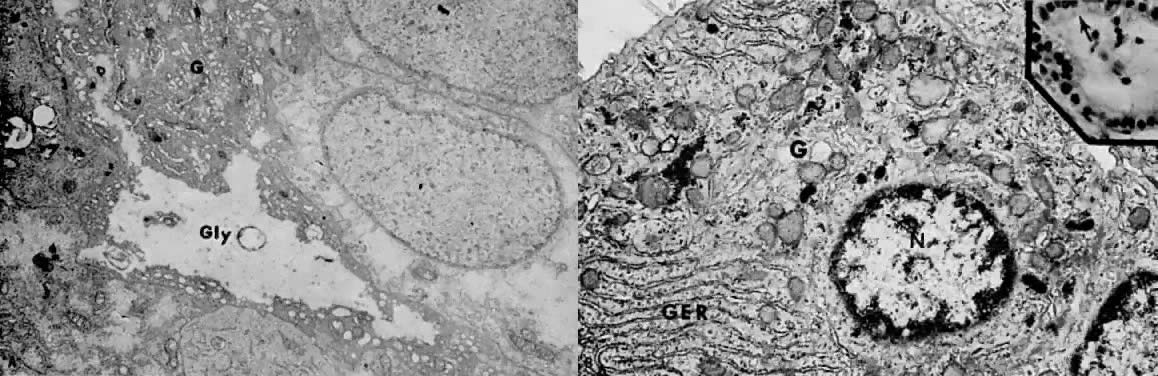
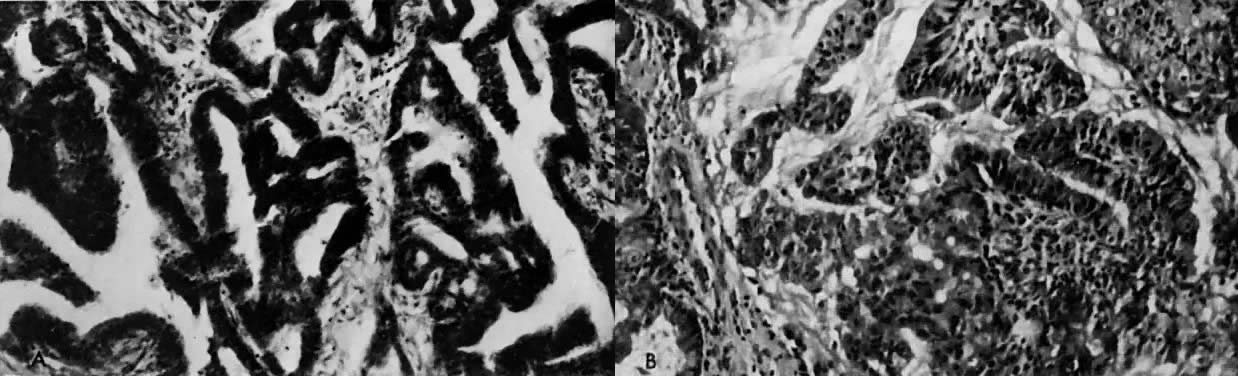
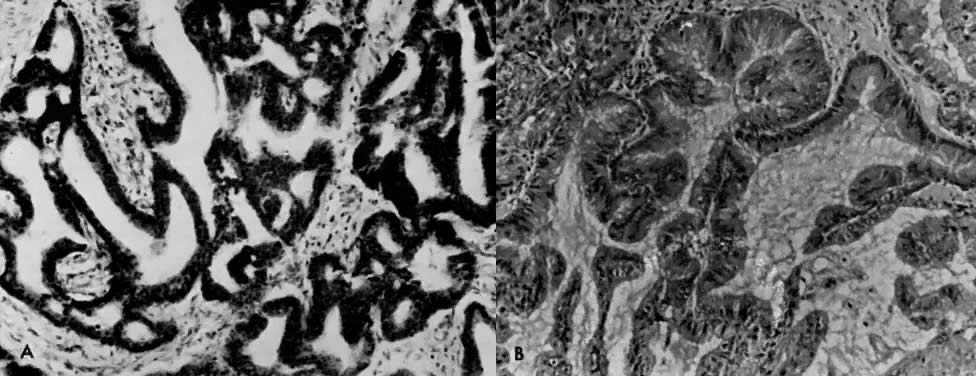
The histologic effect of progesterone in endometrial carcinoma is well described in studies of serial biopsy specimens obtained from patients in whom surgery or irradiation was contraindicated, as well as in studies in which progestogens were instilled into the uterine cavity. Progesterone causes a decrease in the atypia and pleomorphism of the glands, with a decrease in mitotic figures and better differentiation of the glands. The cytoplasm becomes more granular and eosinophilic, and secretory vacuoles appear. In well-differentiated tumors, the carcinoma is replaced by endometrial hyperplasia or by endometrial atrophy (Fig. 4).
It must be recognized that secretory vacuoles may be present in endometrial carcinoma when no progesterone has been administered. Furthermore, the neoplasm may show the histologic changes associated with progesterone, but the tumor may progress and the patient may die.
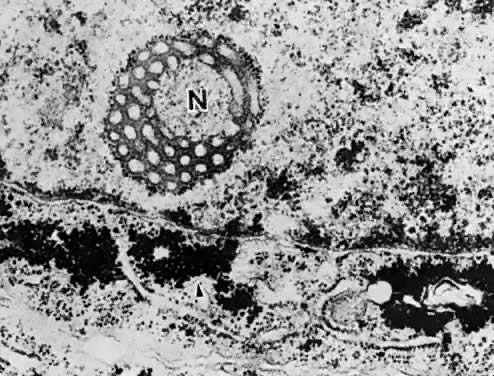
Electron microscopy is useful in differentiating endometrial hyperplasia from neoplasia (Fig. 5). Examination of endometrial hyperplasia demonstrates numerous microvilli, an active Golgi complex, and endoplasmic reticulum. In carcinoma in situ and invasive cancer, these organelles tend to be present much less frequently. Thus, light microscopy is a better means of distinguishing endometrial hyperplasia from atypia and invasive cancer, whereas electron microscopy may be a better choice for distinguishing glandular hyperplasia from atypical hyperplasia. Nevertheless, we are still completely reliant on the eye of the gynecologic pathologist to distinguish cancer from hyperplasia, as flow cytometry and other new modalities cannot distinguish between them.
The administration of progesterone in endometrial carcinoma is associated with an initial increase in endoplasmic reticulum, mitochondria, and Golgi complex, as well as with glycogen accumulation (Fig. 6A and Fig. 6B). Giant autolysosomes then appear, similar to those seen in the late luteal phase. The neoplastic cells become low cuboidal and devoid of mitotic activity. Eventually, there is complete glandular atrophy, and there may be stromal decidualization.
Organ culture studies
The addition of progesterone to organ culture of normal proliferative endometrium induces the histologic and electron microscopic characteristics of postovulatory endometrium in vivo.39 Glycogen accumulates beneath the nucleus and is secreted into the lumina of the glands. Giant mitochondria appear in association with the glycogen, and nucleolar channel systems appear in the nucleolus, as they do between days 16 and 19 of the menstrual cycle in the ovulating female (Fig. 7). All progestational agents will induce glycogen formation and giant mitochondria, but only those with an acyl group (CH3–CO) at position 17β will produce nucleolar channel systems in culture. So far, no nucleolar channel systems have been observed in endometrial carcinoma.
|
In organ cultures of endometrial carcinoma,40, 41 low-dose progestational agents produce tumor differentiation, whereas high doses produce tumor necrosis (Fig. 8). In some studies, estrogen was found to potentiate the necrotizing effect of progesterone.42
An organ culture screening study showed that 12 of 43 patients showed tissue necrosis; in an additional five patients, the culture did not survive. Thus, only 26 cultures were available for study, of which five showed necrosis with both high and low concentrations of progesterone, six showed necrosis with a high dose, and 15 showed no significant effect in culture. This incidence of progesterone sensitivity is similar to the clinical effect seen in these patients.
Although organ culture provides a good investigational tool, its clinical application has been superseded by determination of estrogen and progesterone binding. The practical lessons learned from organ culture sensitivity screening are, however, very applicable to the steroid receptor studies.
First, one must be sure that carcinomatous tissue is being tested, since the site of endometrial carcinoma is so frequently focal. It is essential to send tissue for histologic section at the same time as the tissue is sent for either organ culture or to test for estrogen and progesterone receptors. Second, the tissue used for the assay must be fresh. Curettage or even endometrial biopsy causes local tissue damage, necrosis, and infection, and healing and repair needs to take place for sampling to give valid results. At least 4 weeks should elapse before further samples are obtained. Since definitive therapy is usually implemented before this, it is suggested that samples for steroid receptor estimation or tissue culture be obtained at the time of the primary biopsy.
A mass screening program for progestogen sensitivity is now not justified. It may occasionally be useful in cases in which recurrent disease or metastases are amenable to biopsy, and this will be further discussed in relation to progesterone receptor studies.
Studies of ribonucleic acid and deoxyribonucleic acid synthesis
Studies of the effect of progesterone on RNA and DNA synthesis in endometrial carcinoma42 show that progesterone at a dose of 80 mg/ml produces a mean reduction in uptake of 39% over controls, which is a greater reduction than with DNA synthesis. Nordqvist42 performed clinical studies with the use of radioactive thymidine and uridine. Thirteen patients were treated with polyestradiol phosphate plus either 17α-hydroxyprogesterone or 19-nor-17α-hydroxyprogesterone. There was significant inhibition of DNA and RNA synthesis in ten and 12 of 32 patients, respectively. All of these patients were given estrogen as well as progestogen. There was no correlation between DNA and RNA metabolism and the histologic effect in the patients studied after 3–7 weeks of treatment.
Monolayer tissue culture studies
There are several reports of normal endometrium and endometrial carcinoma in tissue culture. Hiratsu43 reported on the successful culture of benign proliferative and secretory endometrium and showed that estradiol stimulated growth significantly at a dose of 0.1 mg/ml, but had an inhibitory effect at doses of 1 and 10 mg/ml. Progesterone, testosterone, and androstenedione displayed growth-inhibitory effects on the cells within a range concentration of 0.01–50 mg/ml. Liszczak and co-workers44 cultured normal human endometrial cells in monolayer cultures for up to 50 days by a method designed to ensure maximum harvest of glandular cells. These cells had the ultrastructure characteristics of normal endometrial epithelial cells. The addition of progesterone to the culture led to the appearance of abundant rough endoplasmic reticulum, a prominent Golgi complex, and membrane-bound electron-dense bodies, findings characteristic of secretory cells. No nucleolar canalicular systems were induced. Such a model is the tool of choice in the study of normal endometrium in relation to added hormones.
Kuramoto and associates45 described a cloned monolayer culture of endometrial carcinoma that reproduced the architecture of the original cancer when the cells were implanted into a hamster cheek pouch. Histologic studies with DNA and RNA uptake showed stimulation with estrogen and inhibition with progesterone. The addition of high-dose estrogen, androgen, or progesterone was inhibitory.
It then became feasible to perform monolayer culture studies, by which epithelial cells and stromal cells could be grown separately. They could be combined in culture, thus providing an ideal means of studying the interdependence of the epithelial and stromal elements and relating these to the effect of various hormones. Another study approach was the establishment of both normal endometrium and endometrial carcinoma in nude mice (Fig. 9). Gershwin and colleagues46 and Hayakawa and co-workers47 reported that endometrial carcinoma grows well in nude mice, and they found that fresh transplants from human tumors were established more easily compared to cells from monolayer culture.
|
By 1993, Möbus and co-workers48 were able to find reports of 28 cell lines of endometrial cancer, but most of these were from anaplastic tumors, and few were amenable to hormone manipulation. These authors added six new cell lines, only three of which produced a progesterone receptor. Nevertheless, it is likely that studies from human cells, either in vitro as monolayers or organ cultures or in tolerant mice (e.g., nude mouse, irradiated thymectomized mouse), are more likely to lead to meaningful data than study of adenocarcinoma in animal models (e.g., mouse, rat, rabbit). Although these animals are mammals, their hormonal environment is quite different from that of humans.
Estrogen and progesterone receptors
In the 1980s, gynecologists sought to place estrogen- and progesterone-receptor measurements in endometrial cancer on the same footing as it is in the management of breast cancer. This never took hold because well-differentiated endometrial cancer is cured much more often by surgery and much less often by hormone administration or hormone manipulation for all the reasons already discussed. As more high-risk endometrial carcinomas are found to be nonresponsive to progesterone, the frequency at which receptor measurements are performed as a routine of endometrial cancer management will decrease. The methods of receptor measurement are also changing.
When this chapter was revised in 1988, estrogen and progesterone receptors were being measured biochemically on tumor specimens that had an unknown amount of noncancerous endometrium, the presence of which will alter the receptor value even with meticulous histologic control. Since then, immunohistochemical assessment of steroid receptors has become significantly more reliable. The technique has the advantage that the receptor may be visualized in the cells' nuclei, and one may thus be certain that the receptor is being measured in malignant cells and not in normal cells. The concept that there is receptor in the cytosol is now obsolete. Most gynecologic oncologists now rely on histochemical measurements of both estrogen and progesterone receptor.
Current concept of steroid action
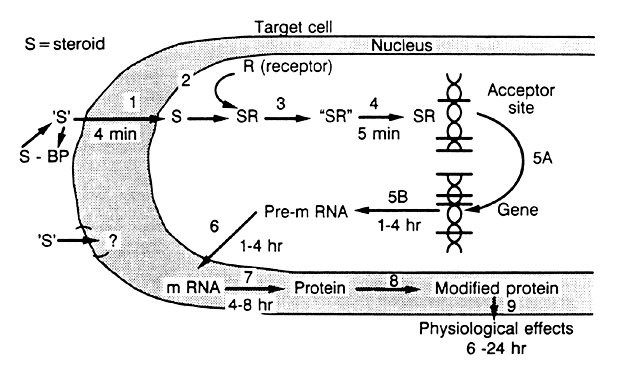
Like other steroid hormones, estrogen and progesterone circulate both as free molecules and as complexes with sex steroid-binding globulin. The specific receptors of high affinity are restricted to target tissues. Thus, estrogen receptors are present in the uterus, hypothalamus, and breast, and are absent in conditions of hormone insensitivity, such as the androgen insensitivity of testicular feminization. The free steroids diffuse in and out of all cells and are “captured” only in “target” cells by specific receptors (Fig. 10),49 which are specialized proteins in the nucleus of the target cell that form complexes with a particular steroid (see Fig. 10).49 These receptors are specific for the steroid and the target tissue. There are 10,000–60,000 such receptors in a particular cell. Each receptor binds its particular steroid with high affinity, showing an equilibrium dissociation constant (Kd) of 10-9–10-10 mol/l. The binding of the steroid molecule to the receptor causes the latter to be “activated” (step 3; see Fig. 10), and this allows the complex to attach to specific nuclear “acceptor” sites on the chromatin (step 4; see Fig. 10). This process then results in the alteration of gene transcription (step 5; see Fig. 10), resulting in changes in messenger RNA and protein synthesis. The effect of the steroid in the cell is observed 12–24 hours after steroid administration.
|
Biochemical assay methods for steroid receptors
Methods measuring the quantity of specific steroid receptors depend on their ability to form complexes with radiolabeled steroids.50 The tightly bound radiolabeled steroid can be separated from free or loosely bound ligand and counted, usually in a scintillation spectrophotometer. The major difference between the various methods is in the principle used to separate the free or loosely bound steroid from the steroid most strongly associated with the specific receptor protein. For example, with estradiol, the receptor is half saturated (Kd = approximately 10-10 mol/l at 4°C), whereas the nonspecific binding is so weak that it is difficult to measure. Estrogen is the strongest specific steroid receptor interaction known, but even the weaker association of progesterone or glucocorticoid with their respective receptors have a Kd value of less than 10-8 mol/l. The Kd value of nonspecific binding is approximately 10-5 mol/L.
The second distinction between the two types of receptor sites is the number per cell. These are thousands of specific proteins per cell, compared to millions of nonspecific proteins per cell.
The most common method of separating bound from free steroid is based on the ability of charcoal to absorb free steroid, the addition of dextran limiting any absorption of steroid receptor complexes. The removal of the free steroid by the dextran-coated charcoal leads to the dissociation of weak steroid protein complexes but has only a limited effect on higher affinity specific complexes. Polidexide (Sephadex) has been used to quantitate estrogen receptors in animal systems. It has the advantage of more complete separation of free and specific-bound steroid, but it is a less simple method than dextran-coated charcoal. Sucrose-gradient analysis was used by some investigators but is time-consuming and expensive.
Electrophoresis has been used to measure androgen receptors owing to its ability to distinguish cellular androgen receptors and serum bound globulin.
Specific actions of estrogen and progesterone on endometrium
Our present understanding of the relationship between estrogen and progesterone indicates that estrogen induces cell growth and multiplication, the formation of microvilli, and stimulates the synthesis of progesterone receptors. Progesterone reverses these processes and also inhibits the synthesis of estrogen receptors.
In humans, it appears that estradiol is the active estrogen. Labeled estradiol, but not estrone, is found bound to chromatin in human endometrium when estrone is incubated with tissue slices of endometrium. Estradiol is converted to estrone by 17β-dehydrogenase and estrone leaves the tissue, resulting in a decrease in the concentration of estradiol in the tissue. The enzymatic activity is localized in the secretory glandular epithelium. The stroma does not show estradiol 17β-dehydrogenase activity. Thus, by inducing 17β-dehydrogenase and decreasing the number of estrogen receptors, progesterone inhibits the proliferative effect of estradiol in normal endometrium.
Characteristics of the “cytosol” receptor
The estrogen receptor in the cytosol of human endometrium is a protein with a dissociation constant for estrogen of approximately 1/10–1/9 mol/l. In sucrose density gradients, it appears both in the 4S and 8S forms. Estrogen receptor binds estradiol better than estrone and estrone better than estriol. Nonsteroidal estrogens bind to estrogen receptors with high affinity, but do not bind to the sex steroid-binding globulin or to plasma.
The level of estrogen receptors is high in the proliferative phase and reaches its maximum at the time of ovulation. It is very high in hyperplastic endometrium, but low in secretory endometrium and in samples of atrophic endometrium and in patients on oral contraceptives. The number of estrogen receptors is directly related to the level of plasma estriol and inversely related to the level of plasma progesterone. The level in the endometrium is greater at the fundus than in the lower uterine segment.
The specific high-affinity progesterone receptor protein in cytosol also has a dissociation constant of 1/10–1/9 mol/l and a molecular weight of 110,000 kDa. The next best competitor after progesterone is 5α-dihydroprogesterone and deoxycorticosterone. 11-Deoxycortisol, corticosterone, and testosterone are weaker competitors, and cortisol and estradiol do not compete. In earlier studies, cortisol-binding globulin could not be differentiated from specific progesterone-binding globulin, so some reports have to be interpreted with caution.
Problems in biochemical measurement
The samples of human endometrium used for steroid receptor assay may contain progesterone or estrogen, so the receptor binding sites may be partially saturated with endogenous ligands. The receptors in the cell may therefore have binding sites that are occupied and some that are free to bind to added radiolabeled hormone. Whether all sites or only unoccupied sites are being measured depends on the incubation conditions and on which hormone is being measured. With estrogen, mainly the unoccupied cytosol sites are measured, since the estrogen receptor complexes dissociate slowly at 4°C. If the incubation is performed at higher temperatures, a total assay of occupied and unoccupied sites may be obtained because the estrogen receptor complex is stable at the higher temperature. With progesterone, the rate of response dissociation is very rapid, so an incubation at 4°C will measure both occupied and unoccupied receptor sites. Variations in technique in part explain the differences in the level of estrogen and progesterone receptors that have been reported from different laboratories.
Immunohistochemical measurement of estrogen and progesterone receptor
Monoclonal antibodies developed for the immunocytochemical localization of estrogen receptor51 and progesterone receptor52 have allowed identification of these receptors in biopsy and hysterectomy specimens from patients with endometrial carcinoma. Several studies have compared the biochemical and immunocytochemical techniques,53, 54, 55, 56 and there is general consensus that immunohistochemistry provides information that may be more accurate and reliable than the chemical techniques, even if archival tissue blocks are used. Parl and Posey57 enumerated the problems encountered with immunohistochemistry, including (1) delay in fixation, causing autolysis; and (2) impure fixative, causing alteration in receptor antigenicity. The main advantage is that one is certain that the receptor is present in tumor cells, not in adjacent nonmalignant tissue. The biochemical methods of course cannot discriminate the type of epithelial cell, and the receptor value may be inflated, particularly by the presence of hyperplastic endometrium. The concordance level between the two methods will be significantly influenced by the levels chosen as significant for each one, even if there is careful histologic control with the biochemical determination.
Role of steroid receptor assay in endometrial carcinoma
The introduction of techniques for detecting the presence of estrogen receptors and progesterone receptors and actually measuring the amount present was greeted with enthusiasm, and with the hope that the information gained would help us choose patients for hormonal therapy. Indeed, the available information indicates that receptors are present with many carcinomas; however, they may also be absent. The presence of progesterone receptors correlates with the histologic grade of the tumor, there being a high frequency of progesterone receptors among well-differentiated tumors and a low frequency among poorly differentiated tumors. The receptor for estrogen appears to be present apparently independent of the grade of the tumor.
All investigators have been in agreement that both estrogen and progesterone receptor levels are usually high in endometrial hyperplasia, with a mean progesterone-binding capacity of 514.5 ± 421.9 femtomole (fmol)/mg protein.58 All adenomatous hyperplasias studied had progesterone receptors, but 35% of mixed adenomatous and cystic hyperplasias had no progesterone receptor. Of Ehrlich and Young's59 patients, 58% had progesterone-binding globulin. Of those with grade 1 tumor, 84% had progesterone-binding protein; with grade 2 tumor, 47%; and with grade 3 tumor, 25%. The values of progesterone-binding globulin corresponding to each grade are shown in Table 4. It should be noted that Young and Ehrlich defined a significant progesterone-binding protein level as more than 50 fmol/mg protein. Pollow and associates60 and Ehrlich and Young59 agree that there are patients with well-differentiated carcinoma in whom no progesterone-binding protein is detectable, whereas a certain proportion of patients with poorly differentiated tumors have significant progesterone-binding globulin. It should also be noted that histologic serous carcinomas and clear cell carcinomas have low levels of progesterone receptor.5
Table 4. Progesterone binding globulin in endometrial carcinoma related to tumor grade
| Value | Range |
Grade 1 | 420.0 ± 584.9 | 0–2400 |
Grade 2 | 60.9 ± 54.0 | 0–196.9 |
Grade 3 | 62.9 ± 127.0 | 0–507.2 |
The values are expressed in femtomoles per milligram protein. The difference between grade 1 and grade 2 or 3 is significant (p < 0.02)
(Young PCM, Ehrlich CE: Progesterone receptors in human endometrial cancer. In Lippman M, Thompson B (eds): Steroid Receptors in the Management of Cancer. Boca Raton, FL, CRC Press, 1979, vol. 1. Copyright © The Chemical Rubber Co, CRC Press, Inc)
Progesterone-binding globulin is more frequent among well-differentiated tumors. The level of estradiol 17β-dehydrogenase is poorly correlated with tumor grade. Several studies have found that patients given exogenous progestogens show a decrease in both cytoplasmic progesterone and estrogen receptor levels.61 Pollow showed that estrogen administration increased cytosolic progesterone receptor values in relation to tumor differentiation.
Ehrlich and co-workers58 and Pollow and colleagues62 gathered sufficient data to demonstrate a correlation between clinical response and histologic grade in patients with endometrial carcinoma who are treated with progestogens. When no progesterone receptor was detectable, response was rare but could occur. Response was frequent when significant progesterone levels were measured.
One must conclude that in endometrial hyperplasia the effect of estrogen on the endometrium is associated with insufficient progesterone production and that the mechanism is probably a physiologic one. This is not as certain in the case of endometrial carcinoma, and the only evidence at present is that of Pollow and associates.62 Nevertheless, it is possible that the long-term beneficial hormonal effect of progestins in endometrial carcinoma may be associated with an exhaustion of progesterone receptors through the negative feedback mechanism similar to that demonstrated in normal endometrium.
Role of tamoxifen
Tamoxifen is a nonsteroidal antiestrogen that acts by attaching to a different area of the receptor than estradiol and may interfere with the binding of estradiol. In the absence of estradiol, it acts as an estrogen agonist. It is being extensively used as adjuvant therapy in patients with early breast cancer. There is increasing evidence, however, that tamoxifen therapy is associated with an increased incidence of endometrial cancer.63, 64, 65 Tamoxifen administration has also been shown to cause endometrial hyperplasia in previously oophorectomized women.66In vitro evidence has failed to demonstrate a stimulatory effect of tamoxifen on endometrial carcinoma cells in culture,67 and it may have an inhibitory effect.68 The effect of tamoxifen on the uterus is well summarized by Barakat.69 From 1985 to 1995, 140 cases of tamoxifen-associated uterine cancer were reported. Fisher and associates70 reported that the relative risk of endometrial cancer associated with tamoxifen was 7.5 in a study of 2843 patients with node-negative, estrogen receptor-positive breast cancer randomly assigned to tamoxifen or placebo. Not all tumors are necessarily well differentiated, and there is a significant incidence of grade 3 endometrial lesions.71 Nevertheless, the beneficial effects of tamoxifen in the management of breast cancer far outweigh the risk of endometrial cancer, especially if patients have continuing gynecologic supervision.69
Because of its antiestrogenic properties, tamoxifen has been investigated in trials analyzing response in metastatic and recurrent carcinoma of the endometrium. Swenerton and colleagues72 showed objective response in four of nine patients with recurrent endometrial carcinoma treated with tamoxifen who had previously been treated with medroxyprogesterone. Bonte and colleagues73 used tamoxifen both in patients prior to therapy and in those with recurrent or metastatic disease. They reported an overall remission rate of 50% with the use of 20 mg tamoxifen twice daily and found it effective after medroxyprogesterone had failed; however, the duration of response was brief.
Moore and co-workers74 summarized the single-agent studies in the literature and found an 8% complete response rate and a 14% partial response rate among a total of 257 patients. However, the dose of tamoxifen varied from 20 to 40 mg daily, many patients were pretreated, and a large proportion of patients had grade 2 and grade 3 tumors. As with progestins, the tumors most likely to respond are those of histologic grade 1 that are positive for estrogen and progesterone receptors. It appears, however, that tamoxifen may give short-term responses after progestogens have failed.
Tamoxifen has been administered in combination with progestogens with or without estrogen. This practice is based on the finding that tamoxifen, when bound to the estrogen receptor, may stimulate the production of progesterone receptor and therefore potentiate the efficacy of progestogen therapy.75, 76
Carlson and associates77 treated 12 patients with tamoxifen and medroxyprogesterone and obtained objective response in four, with a duration of response of 7–24 months. Rendina and colleagues,78 also showed response with tamoxifen and there was increased response when tamoxifen was administered with medroxyprogesterone (MPA). In the combined groups the response was 66% for well differentiated tumors and 31% for undifferentiated endometrial cancers. The well differentiated cancers had a "duration of response". Thrombotic and hepatic toxicity has been reported with combination hormonal therapy.69
It appears that there is no advantage to using tamoxifen rather than a gestagen as primary therapy in endometrial cancer. As with the progestogens, tamoxifen must be used with well-differentiated tumors in order to be effective as primary therapy in metastatic or recurrent disease. The case for tamoxifen in combination or sequenced with either estrogen or a progestational agent is currently unsubstantiated.
Other hormonal manipulation in endometrial carcinoma
Wall and co-workers79 treated nine patients with endometrial carcinoma with clomiphene citrate and noted a histologic response in two. Quinn and associates80 treated 11 women unresponsive to medroxyprogesterone with aminoglutethimide and noted a clinical response in one patient.
CONCLUSION
While there has been significant progress in our understanding of the physiology – particularly the receptor physiology – of estrogen, progestogens, and tamoxifen in endometrial cancer, the clinical role of hormonal therapy has become less secure. The reason for this is that advanced and metastatic disease is less frequently associated with hormone-sensitive, well-differentiated carcinomas that are rich in progesterone receptor. Such disease usually has a high-grade histology with low progesterone-receptor content and is insensitive to progestogens and tamoxifen. It is for such patients that cytotoxic chemotherapy is being increasingly used. Nevertheless, the pessimism concerning progestational therapy expressed in several reviews70 appears unjustified, as the response rate with grade 1 histology is significant and is achieved without toxicity. Because of its lack of toxicity, progestational therapy should be considered in all patients with advanced endometrial adenocarcinoma. With anaplastic tumors, cytotoxic chemotherapy should be commenced concomitant with the progestogen.
REFERENCES
Kottmeier HL: Annual report on the results of treatment in gynecologic cancer. FIGO, Stockholm, Sweden 17: 115, 1979 |
|
Chambers JT, Merino M, Kohorn EI et al: Uterine papillary serious carcinoma. Obstet Gynecol 69: 109, 1987 |
|
FIGO Annual Report on the results of treatment in gynecological cancer. Int J Gynecol Obstet 28: 189, 1989 |
|
Muss H: Chemotherapy of metastatic endometrial cancer. Semin Oncol 21: 107, 1994 |
|
Carcangiu ML, Chambers JT, Voynick IM et al: Immunohistochemical evaluation of estrogen and progesterone receptor content in 183 patients with endometrial carcinoma. Am J Clin Pathol 94: 247, 1990 |
|
Wellenbach BL, Rakoff AW: Hyperplasia of the endometrium. J Einstein Med Cent 2: 3, 1953 |
|
Kaufman RH, Abbott WP, Wall JA: The endometrium before and after wedge resection of the ovaries for Stein-Leventhal syndrome. Am J Obstet Gynecol 77: 1271, 1959 |
|
Kistner RW, Griffith CT, Craig JM: The use of progestational agents in the management of endometrial cancer. Cancer 18: 1563, 1965 |
|
Kistner RW: Histologic effects of progestins on hyperplasia and carcinoma in situ of the endometrium. Cancer 12: 1106, 1959 |
|
Kelly RM, Baker WH: The effect of 17 alpha-hydroxyprogesterone caproate on metastatic endometrial cancer. Natl Cancer Inst Monogr 9: 235, 1960 |
|
Kennedy BJ: A progestogen for treatment of advanced endometrial cancer. JAMA 184: 758, 1963 |
|
Varga A, Henricksen E: Clinical and histopathologic evaluation of the effect of 17 alpha-hydroxyprogesterone on endometrial carcinoma. Obstet Gynecol 18: 658, 1961 |
|
Wentz WB: Effect of a progestational agent on endometrial hyperplasia and endometrial cancer. Obstet Gynecol 24: 370, 1964 |
|
Anderson D: Management of advanced endometrial adenocarcinoma with medroxyprogesterone acetate. Am J Obstet Gynecol 92: 87, 1965 |
|
Thigpen T, Blessing J, DiSaia P, Ehrlich C: Acetate in advanced or recurrent carcinoma: results of therapy and correlation with estrogen and progesterone receptor levels. The Gynecologic Oncology Group Experience. Presented at First International Congress on Cancer and Hormones, Rome, 4/23/86. In Baulieu EE, Slacobelli, McGuire WW (eds): Endocrinology and Malignancy, pp 446–454. Park Ridge, NJ, The Parthenon Publishing Group, 1986 |
|
Geisler HE: The use of megestrol acetate in the treatment of advanced malignant lesions of the endometrium. Gynecol Oncol 1: 340, 1973 |
|
Kohorn EI: Gestagens and endometrial cancer. Gynecol Oncol 4: 389, 1976 |
|
Kistner RW: Chemotherapy for carcinoma of the endometrium. Obstet Gynecol Dig 26: 37, 1975 |
|
Gowan ADT: Histologic changes induced in adeno-carcinoma uteri by ethynodiol diacetate. In Brush MG, Taylor RW, Williams DC (eds): Symposium on Endometrial Cancer, pp 225–228. London, William Heinemann, 1973 |
|
Sall S, DiSaia P, Morrow CP et al: A comparison of medroxyprogesterone serum concentrations by the oral or intramuscular route in patients with persistent or recurrent endometrial carcinoma. Am J Obstet Gynecol 135: 647, 1979 |
|
Laatikainen T, Nieminen U, Adlercreutz H: Plasma medroxyprogesterone acetate levels following intramuscular or oral administration in patients with endometrial adenocarcinoma. Acta Obstet Gynecol Scand 58: 95, 1979 |
|
Hesselius I, Johansson EDB: Medroxyprogesterone acetate (MPA) plasma levels after oral and intramuscular administration in a long-term study. Acta Obstet Gynecol Scand 101 (suppl): 65, 1981 |
|
Reifenstein EC: The treatment of advanced endometrial cancer with hydroxyprogesterone caproate. Gynecol Oncol 2: 377, 1974 |
|
Malkasian GD, Decker DG, Mussey E et al: Progestogen treatment of recurrent endometrial carcinoma. Am J Obstet Gynecol 110: 15, 1971 |
|
Rozier JC, Underwood PB: Use of progestational agents in endometrial adenocarcinoma. Obstet Gynecol 44: 60, 1974 |
|
Podratz KC, O'Brien PC, Malkasian GD et al: Effects of progestational agents in treatment of endometrial carcinoma. Obstet Gynecol 66: 106, 1985 |
|
Piver MS, Barlow JJ, Lurain JR: Medroxyprogesterone acetate (Depo-Provera) vs hydroxyprogesterone caproate (Delalutin) in women with metastatic endometrial adenocarcinoma. Cancer 45: 268, 1980 |
|
Bonte J, DeCoster JM, Ide P: Radiosensitization of endometrial adenocarcinoma by means of medroxyprogesterone. Cancer 25: 907, 1970 |
|
Mussey E, Malkasian GD: Progestogen treatment of recurrent carcinoma of the endometrium. Am J Obstet Gynecol 94: 78, 1966 |
|
Huber H, Husslein P, Michalica W et al: Radiosensitizing effect of medroxyprogesterone acetate on endometrial cancer cells in vitro. Cancer 54: 999, 1984 |
|
Quinn MA, Cauchi M, Fortune D: Endometrial carcinoma: Steroid receptors and response to medroxyprogesterone acetate. Gynecol Oncol 21: 314, 1985 |
|
Lewis GC, Nelson H, Slack NH et al: Adjuvant progestogen therapy in the primary definitive treatment of endometrial cancer. Gynecol Oncol 2: 368, 1974 |
|
DePalo G, Merson M, DelVecchio M: A controlled clinical study of adjuvant medroxyprogesterone acetate (MPA) therapy in pathologic stage I endometrial carcinoma with myometrial invasion (abstr). Proc Annu Meet Am Soc Clin Oncol 4: 121, 1985 |
|
McDonald RR, Thorogood J, Mason MK: A randomized trial of progestogens in the primary treatment of endometrial carcinoma. Br J Obstet Gynaecol 95: 166, 1988 |
|
Bonte J: Hormone thérapie adjuvante par medroxyprogesterone dan le traitement de l'adénocarciome endometrial au stage I. Med Hyg 35: 4193, 1977 |
|
Kaupilla A, Gronroos M, Nieminen U: Adjuvant progestin therapy in endometrial carcinoma. Prog Cancer Res Ther 25: 219, 1983 |
|
Malkasian GD, Decker DG: Adjuvant progesterone therapy for stage I endometrial carcinoma. Int J Gynaecol 16: 48, 1978 |
|
DeCoster JM, Bonte J, Marcq A: MPA release from Silastic devices as replacement for local irradiation by radium tubes in preoperative intrauterine packing for endometrial adenocarcinoma. Gynecol Oncol 5: 189, 1977 |
|
Kohorn EI, Rice SI, Hemperly S et al: The relation of the structure of progestational steroids to nucleolar differentiation in human endometrium. J Clin Endocrinol Metab 34: 257, 1972 |
|
Nordqvist S: Hormonal Responsiveness of Human Endometrial Carcinoma: Studies in Vitro and in Vivo. Lund, Sweden, Tornblad Institute, 1969 |
|
Kohorn EI, Tchao R: The effect of hormone on endometrial carcinoma in organ culture. J Obstet Gynaecol Br Commonw 75: 1262, 1968 |
|
Nordqvist S: Aspects of the cellular action of progesterone with particular reference to endometrial carcinoma. In Brush MG, Taylor RW, Williams DC (eds): Symposium on Endometrial Cancer, pp 33–48. London, William Heinemann, 1973 |
|
Hiratsu T: In vitro cultivation of human endometrium and the influence of steroid hormones on a cell line derived from the endometrium. Kobe J Med Sci 14: 29, 1968 |
|
Liszczak TM, Richardson GS, MacLaughlin DT et al: Ultrastructure of human endometrial epithelium in monolayer culture with and without steroid hormones. In Vitro 13: 344, 1977 |
|
Kuramoto H, Tamura S, Notake Y: Establishment of a cell line of human endometrial adenocarcinoma in vitro. Am J Obstet Gynecol 114: 1012, 1972 |
|
Gershwin ME, Ideda RM, Kawakami TG et al: Immunobiology of heterotransplanted human tumors in nude mice. J Natl Cancer Inst 58: 1455, 1977 |
|
Hayakawa K, Okudaira Y, Nishiura H et al: Serially transplantable human endometrial carcinoma in nude mice. Acta Obstet Gynecol Jpn 29: 875, 1977 |
|
Möbus V, Gerharz CD, Mitze M et al: Establishment and characterization of six new human endometrial adenocarcinoma cell lines. Gynecol Oncol 48: 370, 1993 |
|
Spelsberg TC, Rories CF, Rejman JJ et al: The role of proto-oncogenes as regulatory genes in the steroid action pathway. Biol Reprod 40: 54, 1989 |
|
King RJB: Clinical relevance of steroid-receptor measurements in tumors. Cancer Treat Rev 2: 273, 1975 |
|
Greene GL, Jensen EV: Monoclonal antibodies as probes for estrogen receptor detection and characterization. J Steroid Biochem 16: 353, 1982 |
|
Press MF, Uilave JA, Greene GL: Progesterone receptor distribution in the human endometrium: Analysis using monoclonal antibodies to the human progesterone receptor. Am J Pathol 131: 112, 1988 |
|
Soper JT, Segreti EM, Novotny DB et al: Estrogen and progesterone receptor content of endometrial carcinomas: Comparison of total tissue versus cancer component analysis. Gynecol Oncol 36: 363, 1990 |
|
Marchal S, Marchal C, Hoffstetter S et al: Determination of estrogen and progesterone receptors in endometrial adenocarcinomas: Comparison between dextran-coated charcoal and immunoenzymatic methods on curettage biopsies. Anticancer Res 12: 1307, 1992 |
|
Nyholm HCJ, Nielsen AL, Lyndrup J et al: Estrogen and progesterone receptors in endometrial carcinoma: Comparison of immunohistochemical and biochemical analysis. Int J Gynecol Pathol 12: 246, 1993 |
|
Satyaswaroop PG, Mortel R: Sex steroid receptors in endometrial carcinoma. Gynecol Oncol 50: 278, 1993 |
|
Parl F, Posey Y: Discrepancies of the biochemical and immunohistochemical estrogen receptor assay in breast cancer with monoclonal antibody. Cancer 62: 342, 1986 |
|
Ehrlich CE, Cleary RE, Young PCM: The use of progesterone receptors in the management of recurrent endometrial cancer. In Brush MG, King RJB, Taylor RW (eds): Endometrial Cancer, St. Thomas' Hospital, London, pp 258–264. London, Bailliére, Tindall, 1978 |
|
Ehrlich CE, Young PCM: Progesterone receptors in human endometrial cancer. In Lippman M, Thompson B (eds): Steroid Receptors in the Management of Cancer, Vol 1, 135–139. Cleveland, CRC Press, 1979 |
|
Pollow K, Robel P, Vihko R: Steroid receptors and the human endometrium. In Richardson GS, MacLaughlin DT (eds): Hormonal Biology of Endometrial Cancer. Geneva, UICC Technical Report, 1978 |
|
Janne O, Kauppila A, Kontula K et al: Female sex steroid receptors in normal, hyperplastic and carcinomatous endometrium: The relationship to serum steroid hormones and gonadotropins and changes during medroxyprogesterone acetate administration. Am J Cancer 24: 545, 1979 |
|
Pollow K, Schmidt Gollwitzer M, Nevinny-Stickel J: Progesterone receptors in normal and neoplastic tissues, pp 313–338. In McGuire WI, Raynaud J-P, Baulieu E-E (eds): Progesterone Receptors in Normal and Neoplastic Tissues. New York, Raven Press, 1977 |
|
Gusberg SB: Tamoxifen for breast cancer: Associated endometrial cancer. Cancer 65: 1463, 1990 |
|
Fornander T, Cedermark B, Mattson A et al: Adjuvant tamoxifen in early breast cancer: Occurrence of new primary cancers. Lancet 1: 117, 1989 |
|
Malfetano JH: Tamoxifen associated endometrial carcinoma in postmenopausal breast cancer patients. Gynecol Oncol 39: 82, 1990 |
|
Cross SS, Ismail SM: Endometrial hyperplasia in an oophorectomized woman receiving tamoxifen therapy: Case report. Br J Obstet Gynaecol 97: 190, 1990 |
|
Anzai Y, Holinda CI, Kuramto H et al: Stimulatory effects of 4 hydroxytamoxifen on proliferation of human endometrial adenocarcinoma cells. Cancer Res 49: 2362, 1989 |
|
Mortel R, Levy C, Wolff JC: Female sex steroid receptors in postmenopausal endometrial carcinoma and biochemical response to anti-estrogen. Cancer Res 41: 1140, 1981 |
|
Barakat RR: The effect of tamoxifen on the endometrium. Oncology 9: 129, 1995 |
|
Fisher B, Costantino JR, Redmond CK et al: Endometrial cancer in tamoxifen-treated breast cancer patients: Findings from NSABP B-14. J Natl Cancer Inst 86: 527, 1994 |
|
Magriples U, Naftolin F, Schwartz PE et al: High grade endometrial carcinoma in tamoxifen-treated breast cancer patients. J Clin Oncol 11: 485, 1993 |
|
Swenerton KD, White GW, Boyes DA: Treatment of advanced endometrial carcinoma with tamoxifen. N Engl J Med 301: 105, 1979 |
|
Bonte J, Ide P, Billiet G et al: Tamoxifen as a possible chemotherapeutic agent in endometrial adenocarcinoma. Gynecol Oncol 11: 140, 1981 |
|
Moore TD, Phillips PH, Nerenstone SR, Cheson BD: Systemic treatment of advanced and recurrent endometrial carcinoma: Current status and future directions. J Clin Oncol 9: 1071, 1991 |
|
Robel P, Gravania A, Roger JL et al: Cytoplasmic progesterone and estradiol receptors in normal hyperplastic and carcinomatous endometria: Therapeutic implications. Am J Obstet Gynecol 141: 539, 1986 |
|
Schwartz PE, MacLusky N, Naftolin F et al: Tamoxifen-induced increase in cytosol progestin receptor levels in a case of metastatic endometrial cancer. Gynecol Oncol 16: 41, 1983 |
|
Carlson JA, Allegra JC, Day TG et al: Tamoxifen and endometrial carcinoma: Alterations in estrogen and progesterone receptors in untreated patients and combination hormonal therapy in advanced neoplasia. Am J Obstet Gynecol 149: 149, 1984 |
|
Rendina GM, Donadio C, Fabri M et al: Tamoxifen and medroxyprogesterone therapy for advanced endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol 17: 285, 1984 |
|
Wall JA, Franklin RR, Kaufman RH et al: The effect of clomiphene citrate on the endometrium. Am J Obstet Gynecol 93: 842, 1965 |
|
Quinn MA, Campbell JJ, Murray R et al: Tamoxifen and aminoglutethimide in the management of patients with advanced endometrial cancer not responsive to medroxyprogesterone. Aust NZ J Obstet Gynaecol 21: 226, 1981 |


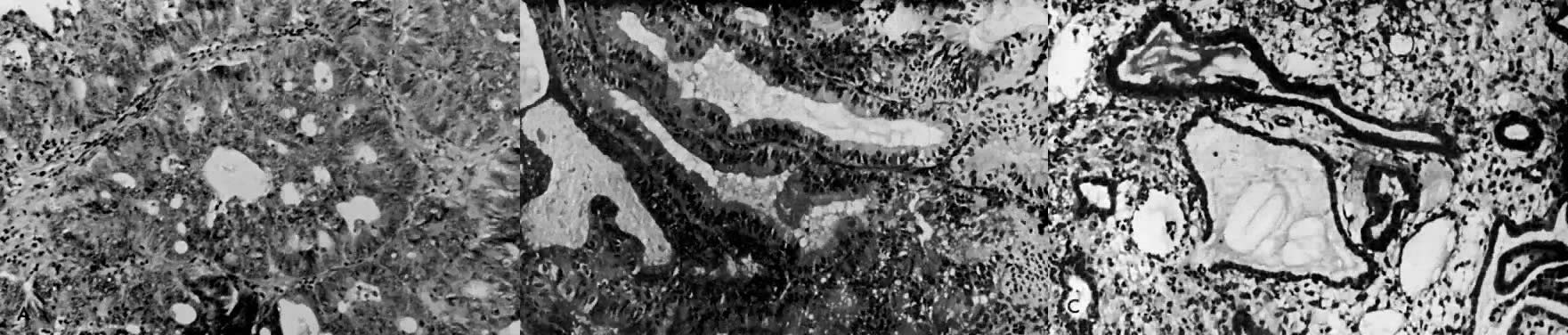
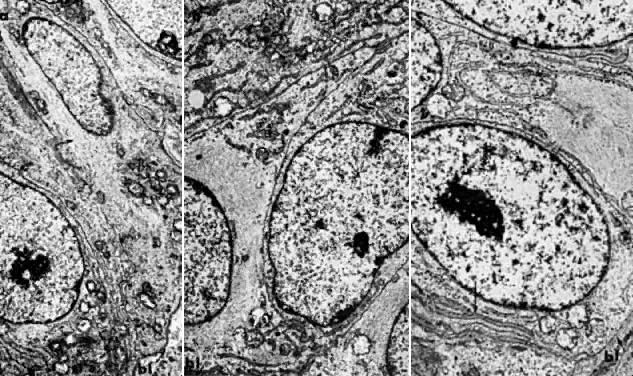
 7000). B. Carcinoma in situ of the endometrium. Note nuclear rounding, pleomorphic organelles, and interlacing bundles of microfilaments in a perinuclear location (
7000). B. Carcinoma in situ of the endometrium. Note nuclear rounding, pleomorphic organelles, and interlacing bundles of microfilaments in a perinuclear location (