Infections as a Cause of Infertility
Authors
INTRODUCTION
Approximately 35% of women with an infertility problem are afflicted with postinflammatory changes of the oviduct or surrounding peritoneum that interfere with tuboovarian function. Most of these alterations result from infection. Salpingitis occurs in an estimated 15% of reproductive-age women, and 2.5% of all women become infertile as a result of salpingitis by age 35.1 Because in most cases, especially those caused by Chlamydia trachomatis, signs and symptoms are often minimal or nonexistent, the actual percentage of women with upper genital tract infections is probably underestimated. Symptomatic, asymptomatic, or latent infections or their sequelae may also contribute to chronic inflammation of the cervix and endometrium, alterations in reproductive tract secretions, induction of immune mediators that interfere with gamete or embryo physiology, and structural disorders such as intrauterine synechiae. Infection is also a major factor in male infertility, second only to oligospermia.
Unfortunately, the impact of infectious sequelae on human reproduction continues to increase as a consequence of sexual promiscuity and the popularity of nonbarrier methods of contraception. C. trachomatis and gonorrheal infections, as well as mixed anaerobic infections, are the most prevalent causes of upper genital tract infections resulting in pelvic inflammatory disease (PID). Bacterial vaginosis, Trichomonas vaginalis, and Candida albicans are the most prevalent bacterial, protozoan, and fungal causes of lower genital tract infections. Although gonorrheal infections have been on the decline in the last decade, chlamydial infections of the male and female genital tract continue to be an increasing problem, and C. trachomatis is the major cause of tubal factor infertility.2 C. trachomatis is usually recovered three to five times more frequently than Neisseria gonorrhoeae from the reproductive tracts of infected individuals. Women are twice as likely as men to acquire gonorrhea or Chlamydia during a single act of unprotected intercourse with an infected partner. Many newly infected women have no symptoms and so do not seek medical intervention and continue to spread the infection to other sexual partners. An estimated 10% to 20% of untreated women with endocervical gonorrhea or chlamydial infection eventually develop salpingitis.3
The best hope for reducing the incidence of infertility related to infection lies in prevention and early detection and treatment of newly acquired asymptomatic or mildly symptomatic infections. The importance for the preservation of future fertility of avoiding high-risk sexual behavior and the mandatory use of condoms must be stressed. Concomitantly, there must be an increased awareness by health care providers and consumers of the need for intensive screening using the latest and most effective molecular techniques followed by early effective treatment if positive.4
Despite the current focus on sexually transmitted diseases (STDs), infertility may also follow bloodborne infections such as tuberculosis, mixed aerobic and anaerobic infections of other pelvic sites, inflammatory complications of surgical trauma, postabortal and puerperal sepsis, and appendiceal rupture. Because inflammatory factors may affect the reproductive tract at virtually every level, it is useful to follow an anatomic approach in considering the relationship of infection to infertility. Infections in the male are discussed in the context of managing the infertile couple as a reproductive unit.
UPPER GENITAL TRACT INFECTION
Endometritis
Cultures obtained at hysterectomy indicate that the endometrial cavity is normally sterile. Endometrial infections may follow procedures that alter the usual protective role of the cervix, such as cervical conization or procedures associated with the introduction of contaminated cervical mucus into the uterus. Endometrial biopsy, hysterosalpingography, and the insertion of an IUD may predispose to endometritis and ascending genital tract infection. Secondary infections of the endometrium may follow primary invasion with C. trachomatis or N. gonorrhoeae. Uterine infections are more likely to occur in postpartum women when decreased host resistance and surgical trauma act synergistically to make the uterine cavity more susceptible to infection. Factors that tip the balance in favor of bacterial invaders are prolonged labor, premature rupture of the membranes, and operative delivery. Prophylactic antibiotics appear to decrease the incidence but not the severity of infections in cesarean section patients. In managing patients with secondary infertility, it is especially important to elicit the details of past cesarean section or postpartum endometritis.
Acute endometritis, especially as observed postpartum or after abortion, is a misnomer, because the infection is unlikely to involve the endometrium alone. Usually, there is an associated inflammatory reaction of the myometrium, parametrium, and in some cases, adnexal structures. Patients with endometritis usually have a decrease in lochial flow for 12 to 24 hours before becoming febrile. It is important to establish prompt uterine drainage and to remove any retained infected tissue. The infections are almost invariably polymicrobial except in cases with β-hemolytic streptococcal endometritis in which rapid tissue invasion and bacteremia usually produce less pronounced local signs of pelvic infection. Broad-spectrum coverage for the most frequently recovered aerobic and anaerobic organisms includes the use of single extended-spectrum drugs, such as cephalosporins (cefoxitin or cefotetan) or penicillins (mezlocillin or piperacillin) or the combination of an amino glycoside and clindamycin or metronidazole. Triple antibiotic therapy with the addition of ampicillin is usually reserved for the critically ill patients, whereas a single drug or the combination of two drugs that provide activity against anaerobes is often used in less serious situations. The principles of management and antibiotic therapy of major gynecologic sepsis are discussed in detail elsewhere in these volumes.
It is generally acknowledged that the prognosis for future fertility is improved if the initial response to antibiotics is prompt. The patients requiring operative intervention for postpartum sepsis are at greater risk for developing pelvic adhesions and subsequent infertility. For most patients, endometritis after cesarean section infrequently interferes with tubal morphology and function unless a pelvic abscess develops.17 However, secondary infertility was more common among women who underwent primary cesarean section (6%) than among those who delivered vaginally (2%).18
C. trachomatis salpingitis is not uncommon in infected women after induced abortion or vaginal delivery.19 The organism presumably ascends from the cervix, usually producing mild or no symptoms 2 weeks (range, 1 to 6 weeks) postpartum. Salpingitis occurs in 15% of women with C. trachomatis who undergo an induced abortion.20 Between one fourth and two thirds of women with tubal infertility have been pregnant before becoming infertile.21 Because C. trachomatis is usually present in 5% to 10% of pregnant women, the impact of chlamydial salpingitis after pregnancy and subsequent infertility may be substantial. Identification and treatment of C. trachomatis and N. gonorrhoeae during pregnancy is recommended to reduce postdelivery salpingitis and its sequelae. Obstruction of the uterotubal junction may accompany septic abortion or streptococcal infection. As a practical matter, it is difficult to relate any particular organism causing endoparametritis to unique structural reproductive damage.
Endometritis in nonpregnant women can be classified into acute, chronic, and fibrotic stages (Table 3). Endometritis is present in 40% of women with cervicitis.12 C. trachomatis and, to a lesser extent, N. gonorrhoeae infections are closely associated with endometritis. Because many women have neither organism (although testing by the more sensitive PCR technique is not often used), it seems likely that other bacteria also cause endometritis in nonpregnant women. After the acute inflammatory process has subsided, an endometrial biopsy should be obtained to exclude persistent inflammation. Foreign bodies, retained products of conception, infected polyps, chronic salpingitis, and uterine cancer can also lead to chronic endometritis. Although the causative agents in the chronic condition may vary as indicated in Table 3, the histopathologic features are similar. The characteristic picture consists of a diffuse infiltration of plasma cells in the endometrial stroma.
TABLE 3. Classification of Endometritis
Acute
Chlamydia trachomatis
Neisseria gonorrhoeae
Bacterial
Chronic
C. trachomatis
Bacterial (nontuberculous)
Tuberculous
Nonspecific
Other (Mycoplasma, viral, toxoplasmosis, rickettsia)
Fibrotic
Intrauterine (Asherman's syndrome)
C. trachomatis infection in particular should be considered as a cause of plasma cell endometritis. The presence of plasma cells is also highly correlated with salpingitis. Moreover, women diagnosed clinically with salpingitis but found to have normal fallopian tubes at laparoscopy frequently demonstrate endometritis by biopsy.22 Bacterial vaginosis has been associated with plasma cell endometritis23 and with endometritis or salpingitis.24 The association of bacterial vaginosis with endometritis is strengthened by the finding that plasma cell endometritis is linked to the recovery of bacterial vaginosis-associated microorganisms from the endometrium23 and with the recovery of anaerobic gram-negative rods from the endometrium even after statistical adjustment for gonorrhea and chlamydial infection.25
Histologic dating of the endometrium may be inaccurate because chronic endometritis is frequently associated with a mixed proliferative and secretory endometrium or inactive cyclically dilated glands. The usual clinical presentation includes discharge, pelvic pain, and dysfunctional uterine bleeding.
In contrast to other types of endometritis, the response of the endometrium to tuberculosis is much more specific. The typical lesion is the noncaseating granuloma composed of epithelial cells, giant cells, and peripheral lymphocytes. Genital tuberculosis is rare, but it should be considered when the endometrium shows signs of inflammation. It is nearly always secondary to a focus elsewhere in the body.
Many of the agents implicated in chronic endometritis have also been implicated in spontaneous abortion, including C. trachomatis, U. urealyticum, toxoplasmosis, cytomegalic inclusion virus, Rickettsia, and Listeria monocytogenes. Women with serologic evidence of C. trachomatis infection had a significantly higher occurrence of spontaneous abortion than other women.26 In one study, chlamydial infection was the second most frequent cause for recurrent fetal losses.27 However, systematic studies for toxoplasmosis, Listeria, and U. urealyticum have not provided convincing data that these organisms are common causes of recurrent abortion.
There is increasing evidence that an endometritis can interfere with implantation of the embryo or that spermatozoa are removed more quickly from the uterine cavity in the presence of a chronic inflammatory reaction. In laboratory animals, a single intrauterine injection of glycogen induces a marked leukocytic response and effectively terminates pregnancy before and during the implantation period. Transfer of viable leukocytes to the uterine lumen during early pregnancy causes a marked reduction in fertility. Inflammatory cells and their products have been shown to be toxic to preimplantation embryos in vitro.28
Although still controversial, a large number of studies indicate an adverse effect of prior chlamydial infection (as determined by positive chlamydial serology or heat shock protein-60 [HSP60] antibodies) on in vitro fertilization (IVF) outcome.16,29 Because pregnancy normally induces a TH2 (antibody-dominated) immune response, it has been postulated that an embryo toxic effect or a disruption of endometrial receptivity occurs as a result of the induction of a TH1 (cell-mediated immunitydominated) immune response. Endometrial infection may induce macrophage activation and proinflammatory cytokine production. The latter mechanism is supported by other studies that demonstrate that inflammatory hydrosalpinges have an adverse effect on endometrial receptivity, which in some cases may be overcome by surgical treatment of the hydrosalpinges.30 Proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and other bioactive substances present in hydrosalpinx fluid reflux into the uterine cavity, thereby altering endometrial stomal and epithelial cell integrin expression that interferes with the “window of implantation.”
Traumatic damage to the endometrium may cause hypomenorrhea, amenorrhea, and intrauterine adhesions (i.e. signs of Asherman's syndrome). The extent of intrauterine adhesions correlates with the degree of menstrual insufficiency. The adhesions are sequelae of uterine trauma, almost always related to pregnancy. It is likely that infection plays a contributory role in their pathogenesis. Intrauterine adhesions may develop with a tuberculous endometritis, lending further support to the idea that Asherman's syndrome has an infectious basis. Fertility is severely impaired in this entity and may be caused by interference with implantation or to changes in endometrial metabolism. In the event of conception, potential complications include abortion, premature delivery, and problems with separation of the placenta. The diagnosis of intrauterine adhesions depends on hysterosalpingography or hysteroscopy. The prognosis for this syndrome with reference to fertility varies with the severity of the adhesions. In Valle's study of 47 patients with severe intrauterine adhesions, only 55% conceived, and less than one third of the patients had term pregnancies.31
Whenever possible, the cause of chronic endometritis should be determined. C. trachomatis and N. gonorrhoeae should be sought, and treatment should be given, as discussed in the following section on PID. The bacterial origin for non-STD organisms is difficult to prove, because endometrial cultures taken by the transcervical route are contaminated with cervical organisms. The tissue diagnosis of chronic nonspecific endometritis is best made during the follicular phase to avoid the normal inflammatory changes that occur premenstrually. Conversely, if tuberculosis is being considered, the granulomas are best recognized on days 24 to 26 of the cycle or within 12 hours after the onset of menstruation. The diagnosis of tuberculous endometritis may be aided by creating a pseudopregnancy without menses for 2 to 3 months, followed by a thorough curettage. The curettings are divided into two portions, one for histologic examination and one for culture. If these are positive for Mycobacterium tuberculosis, prolonged treatment with antituberculous agents is necessary, and the prognosis for fertility is poor.
Nonspecific chronic endometritis can be selflimited and is not uniformly influenced by therapy, but it may respond to curettage and cyclic estrogen and progestin therapy. Conjugated estrogens (2.5 mg/day for 30 days) with medroxyprogesterone acetate (10 mg/day for the last 10 days) and doxycycline (200 mg/day for an entire cycle) are recommended. A posttreatment biopsy is useful to determine whether therapy has been helpful.
Treatment of Asherman's syndrome is primarily surgical. In some cases, cervical and isthmic adhesions respond to transcervical dilatation and lysis. Adhesions can be resected with a hysteroscope under direct vision. In more severe instances, the dangers of perforating the bladder or uterus are best avoided by approaching the adhesions with a transfundal hysterotomy. If the vaginal approach is chosen, it is useful to be prepared for diagnostic laparoscopy in the event of a uterine perforation. An IUD is left in situ for 6 weeks postoperatively to prevent apposition of raw surfaces. The patient receives broad-spectrum antibiotics during this time and is maintained on large doses of conjugated estrogens and progestin cyclically for 2 months.32
Pelvic Inflammatory Disease
PID is a common but vaguely defined complex of signs and symptoms resulting from the spread of pathogenic microorganisms from the vagina and endocervix to the uterus, body of the endometrium, and fallopian tubes. It is a common complication of STDs and has reached epidemic proportions in the United States. Of the estimated 1 million women who annually develop PID, an average of 200,000 enter hospitals each year. According to statistics from the Centers for Disease Control and Prevention, the cost of PID measured in lost earnings and money spent for health services was estimated at $4.2 billion in 1990.33. The long-term consequences of PID include chronic pelvic pain, infertility, and ectopic pregnancies that are increased several-fold.
The best data on involuntary infertility after salpingitis are found in large Swedish studies,1,34 in which the initial diagnosis was confirmed by laparoscopy. Tubal infertility occurs in approximately 11% of women who have one episode, in 23% of women who have two episodes, and in 54% of women who have three or more episodes of salpingitis (Table 4).
TABLE 4. Factors Influencing the Frequency of Tubal Occlusion After Salpingitis
Clinical Findings | Tubal Occlusion |
Degree of acute inflammation at laparoscopy* |
|
Mild | 6% |
Moderate | 13% |
Severe | 30% |
Number of episodes of salpingitis* |
|
One | 11% |
Two | 23% |
Three or more | 54% |
Type of salpingitis† |
|
Gonococcal | 9% |
Nongonococccal | 16% |
* Westrom L: Incidence, prevalence and trends of acute pelvic inflammatory disease and the consequences of industrialized countries. Am J Obstet Gynecol 135:880, 1980.
† Westrom L: Effects of acute pelvic inflammatory disease on infertility. Am J Obstet Gynecol 121:707, 1975.
Acute salpingitis with or without oophoritis often coexists with various degrees of pelvic peritonitis. Infertility results from tubal occlusion, peritubal adhesions, or adhesions encasing the ovary in any combination. Tubal infertility is directly related to a number of factors present during the initial episode of salpingitis, which include (besides the number of episodes) the initial severity of tubal inflammation, the organisms responsible, and the occurrence of a subsequent ectopic pregnancy. The best predictor of subsequent infertility is the degree of tubal inflammation observed through the laparoscope during the acute phase (Table 4). The estimation of severity was based on direct observation of the tube and not on the severity of clinical symptoms and signs such as pain, fever, tenderness, or leukocytosis. Tubal infertility was subsequently found in 6% of women with mild, 13% of women with moderate, and 30% of women with severe tubal changes. Women with a pelvic abscess have had the highest (85% to 90%) rate of subsequent infertility.35
Approximately one half of the women with an ectopic pregnancy have grossly visible tubal damage or a partial occlusion of the tubes. About 7% to 10% of pregnancies that occur after an episode of salpingitis are in an ectopic location, and women with salpingitis have a 10-fold higher rate of ectopic pregnancy than does the general population. Ectopic pregnancy provides a poor prognosis for fertility. Approximately 40% of women who have had an ectopic pregnancy are not able to achieve an intrauterine pregnancy subsequently.36
To establish the diagnosis of salpingitis, other diseases, such as acute appendicitis, endometriosis, ovarian cysts, ectopic pregnancy, urinary tract infection, and gastrointestinal disease, must be excluded. The clinical diagnosis of acute salpingitis is confirmed by laparoscopy in fewer than two thirds of the patients. In the remaining patients, one fifth have normal pelvic findings, and other diagnoses are established in the others.37 The combination of lower abdominal discomfort with pain on motion of the cervix and bilateral adnexal tenderness was present in most patients who had salpingitis, but these findings were also common in the other women. Salpingitis is usually bilateral, but an 8% incidence of unilateral disease is reported; this manifestation may be more common in women using IUDs.38
Prompt recognition and vigorous treatment reduce subsequent severe complications of salpingo-oophoritis, such as generalized pelvic peritonitis, abscess formation, and adnexal destruction. It deserves reemphasis that salpingitis often produces minimal clinical signs. Approximately 60% to 80% of women with acute salpingitis have a normal temperature or no white blood cell elevation. This finding correlates with the observation that most women with tubal infertility have never been treated for a recognized episode of salpingitis. Epidemiologic studies support the concept of silent PID wherein a strong link exists between serum antibodies to C. trachomatis and tubal factor infertility or ectopic pregnancy in patients without a history of clinical PID.16 There seems to be no correlation between traditional indicators of severe clinical infection (e.g. tenderness, fever, leukocytosis) and the degree of tubal damage.
Physicians should be willing to treat women with mild symptoms for salpingitis. If the patients with mild symptoms had only cervicitis or cervicitisendometritis and not salpingitis, prompt treatment before the onset of salpingitis would have a major impact on preventing tubal occlusion. Inadequate treatment may predispose the patient to recurrent pelvic infection with the sequelae of hydrosalpinx, infertility, ectopic pregnancy, and chronic pelvic pain. So-called chronic salpingitis is often caused by indolent infection in patients who have received suboptimal antimicrobial therapy or to recurrent infection. Failure to use doxycycline or azithromycin to inhibit C. trachomatis may contribute to chronic salpingitis.39 Recurrent PID is a distinctly common event; the timing of recurrences, however, suggests that many are attributable to reinfection rather than relapse.
A population-based study of fertility in women with human immunodeficiency virus type 1 (HIV-1) infection in Uganda demonstrated that fertility is greatly reduced in HIV-1-infected women because of a lower rate of conception and increased rates of miscarriage and stillbirth.40 Numerous epidemiologic studies have demonstrated that there is an synergy among bacterial and viral STDs. Bacterial STDs have been implicated in the enhancement of HIV transmission. Conversely, the immunosuppression caused by HIV worsens the clinical course of other STDs. The low prevalence and incidence of pregnancy among HIV-infected women could reflect preexisting tubal factor infertility and higher clinical and subclinical fetal losses resulting from HIV-1 infection.
Salpingitis caused by M. tuberculosis, parasites, or fungi is uncommon in developed countries. The incidence of genital tuberculosis is higher in Europe, Israel, and South America, where it may be present in 5% to 10% of women seeking help in infertility clinics. In the United States and Australia, an incidence of less than 1% is reported. Nontuberculous salpingitis can be divided into gonococcal, chlamydial, and nongonococcal-nonchlamydial disease based on the results of endocervical or peritoneal fluid cultures.
GONOCOCCAL INFECTION.
When endocervical cultures are routinely employed, N. gonorrhoeae is recovered from approximately 30% of untreated patients with acute salpingitis. The frequency of gonococcal disease varies with the socioeconomic status of the population studied. In Swedish populations, the gonococcus was isolated in 10% to 30% of patients, whereas at an American city hospital, N. gonorrhoeae was recovered from most of the indigent women seen.1,41 Gonococcal PID is still a major cause of infertility in women in developing Asian and African countries.42
The recovery of N. gonorrhoeae from tubal or peritoneal fluid in acute salpingitis patients with endocervical gonorrhea ranges from 6% to 70%.38 Approximately one third of patients have N. gonorrhoeae as a sole isolate, one third have N. gonorrhoeae plus a mixture of aerobic and anaerobic bacteria, and one third have a mixture of aerobic and anaerobic bacteria in the cul-de-sac only.41 Aerobic and anaerobic streptococci and Bacteroides species constitute most of the nongonococcal isolates. The variable correlation between positive endocervical gonococcal cultures and specimens from peritoneal fluid has several possible explanations. Gonococci that invade the upper genital tract have different auxotrophic types and are less susceptible to antibiotics than are gonococci from uncomplicated anogenital gonorrhea.41 Although N. gonorrhoeae preferentially infects nonciliated tubal cells, the gonococcal toxin can destroy the cilia of adjacent cells. Not only is the organism difficult to isolate from pus, but the recovery of N. gonorrhoeae depends on the stage of infection. The gonococcus is most frequently isolated within 2 days of the onset of symptoms and is rarely isolated if symptoms are present for 7 or more days.38 Most symptomatic gonococcal PID cases have their onset during or just after the menses. These observations are consistent with the view that the gonococcus initiates the infection and, if the infection is not promptly treated, sets the stage for a mixed aerobic-anaerobic infection, involving pathogens that originate in the cervix and vagina.
CHLAMYDIAL INFECTION.
C. trachomatis is an intracellular bacterium that proliferates in columnar epithelial cells, where it remains protected from host immune defenses by a cell membrane. It takes a longer time for C. trachomatis to divide (24 to 48 hours) than for classic bacteria (1 to 4 hours). There is a characteristically long time between infection and the onset of symptoms among women with C. trachomatis, and only mild symptoms usually occur. Widespread or systemic symptoms are unusual, although infection of the endosalpinx can produce generalized peritonitis by contiguous spread, including perihepatitis (Fitz-Hugh-Curtis syndrome).
C. trachomatis causes the same spectrum of disease (e.g. urethritis, cervicitis, endometritis, salpingitis) as the gonococcus. C. trachomatis causes salpingitis more frequently than the gonococcus. The importance of chlamydiae has been recognized as women with mild symptoms or asymptomatic women have been included for study. The lower rate of C. trachomatis isolation in earlier studies may have been related to relatively mild symptoms and signs caused by chlamydiae compared with gonococci or the lack of a sensitive detection assay. It is apparent, however, that the degree of acute tubal damage among women with chlamydial infection equals or exceeds that observed with gonococcal infection.43 Women with chlamydial infection may have gonorrhea and vice versa.
C. trachomatis is inhibited in vitro by doxycycline and azithromycin but not by cephalosporins. Women with salpingitis should be treated with tetracyclines or other antibiotics that inhibit C. trachomatis, because cephalosporin therapy alone does not eradicate C. trachomatis.44
Chlamydia appears to be a particularly important organism in infertility. There are multiple published reports in which women with tubal infertility have a 25% to 70% higher incidence of C. trachomatis antibody than do infertile women with normal tubes.45 In the United States, C. trachomatis infections are now clearly the leading cause of tubal infertility.
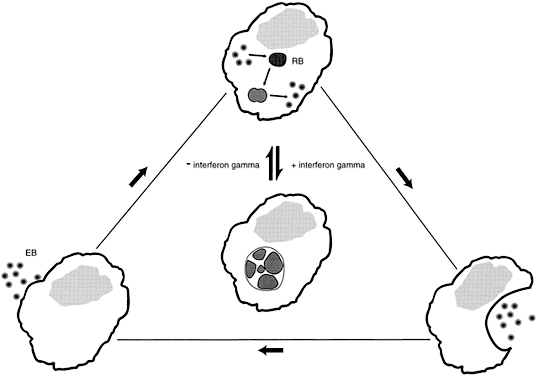
Women with asymptomatic C. trachomatis infections are less likely to seek medical attention than are women with genital tract symptoms. The undetected C. trachomatis are able to ascend from the lower to the upper genital tract, evade the host's immune response and persist for long periods of time.46 The mechanisms leading to chlamydial persistence and subsequent damage to the fallopian tubes have only begun to be elucidated. Experiments in vitro have established that interferon-γ (IFN-γ) produced in response to the chlamydial infection, blocks the intracellular life cycle of this organism, and results in the formation of large aberrant reticulate bodies. However, once IFN-γ is removed, as would occur when an extracellular chlamydial infection is cleared, the aberrant forms revert to normal reticulate bodies, and the typical chlamydial life cycle resumes (Fig. 1). The reticulate bodies differentiate into elementary bodies, the infected cell lyses, and neighboring epithelial cells are infected.47 A similar intracellular chlamydial persistence may occur after treatment with some antibiotics.48 Each cycle of chlamydial growth and inhibition damages the fallopian tube epithelia by an immunologic mechanism, resulting in an increasing extent of fibrosis and eventual tubal occlusion.49
In an in vitro fallopian tube organ culture, C. trachomatis does not cause any visible damage.50 It has become increasingly evident that the immune response to a C. trachomatis infection, not the infection per se, induces fallopian tube occlusion. A single antigen, the HSP60, has been implicated in initiating a proinflammatory immune response after a C. trachomatis upper genital tract infection. HSP60 is a highly conserved protein present in organisms ranging from bacteria to man. The amino acid sequence of the chlamydial and human HSP60s have almost a 50% homology.51 This protein functions as an intracellular chaperone, aiding protein assembly and transport. Under conditions of cell stress, such as an increase in temperature or exposure to free oxygen or nitrogen radicals, HSP60 gene transcription greatly increases in an attempt to prevent protein denaturation and maximize cell survival. In a quiescent but persistent chlamydial infection, synthesis of the major structural antigens ceases or is greatly reduced; however, synthesis of HSP60 is increased.47 Microbial HSP60 is a potent inducer of proinflammatory cytokines. In guinea pigs52 and monkeys53 previously sensitized to Chlamydia, introduction of purified chlamydial HSP60 initiated a localized inflammatory response. A number of investigations have demonstrated a correlation between immunity to the C. trachomatis HSP60 and recurrent episodes of salpingitis, tubal occlusion, and ectopic pregnancy.54,55,56,57 In women with a recent chlamydial cervical infection, immunity to chlamydial HSP60 is rarely observed.56 This suggests that repeated infections or chlamydial persistence in the upper genital tract is needed for sufficient HSP60 to be released to initiate an immune response in the host.
The homology between the chlamydial and human HSP60s also suggests that immune sensitization to conserved HSP60 epitopes may result in autoimmunity to human HSP60. Evidence of sensitization to HSP60 epitopes shared between C. trachomatis and humans has been reported.58,59 In women sensitized to conserved HSP60 epitopes, expression of human HSP60 in the fallopian tubes (in response to cell damage or past infection by other microorganisms) reactivates HSP60-sensitized lymphocytes and induces an inflammatory response. This may explain the sometimes puzzling observation of tubal inflammation in the apparent absence of infection.
Women with tubal factor infertility seek to become pregnant by assisted reproductive technology. However, evidence suggests that sensitization to HSP60 may also interfere with reproductive success after IVF.16,60 The early-stage embryo61 and epithelial cells in the decidua62 express HSP60. A murine hybridoma specific for HSP60 also was shown to react with the surface of human and mouse trophoblasts.63 HSP60 expression during pregnancy may reactivate HSP60-sensitized lymphocytes. The resulting proinflammatory immune response may directly interfere with embryo development or may disturb the balance of immune regulatory mechanisms needed to prevent rejection of the semiallogeneic embryo. Women undergoing IVF who had cervical IgA antibodies to chlamydial HSP60 had an increased rate of transient implantation after embryo transfer and a significantly poorer outcome than did antibody-negative women.16 Further analysis revealed that cervical immunity to a shared human HSP60 epitope and C. trachomatis was similarly correlated with IVF failure60 (Table 5). Circulating systemic humoral immunity to human HSP60 has also been associated with a history of spontaneous abortion.60 An association between IVF failure, humoral immunity to C. trachomatis, and expression of human HSP60 in ovarian follicle fluid has been reported.64
TABLE 5. Chlamydia trachomatis Infection and in Vitro Fertilization (IVF) Outcome
IVF Outcome | No. of Patients | No. (%) with Anti-Ct Cervical IgA |
Not pregnant | 99 | 17(17.2) |
Biochemical pregnancy | 21 | 4(19.0) |
Spontaneous abortion | 10 | 3(30.0) |
Live birth | 68 | 1(1.5) |
*Endocervical samples obtained at the time of oocyte aspiration were assayed for IgA antibodies to C. trachomatis by ELISA (Savyon Diagnostics).
Adapted from Witkin et al. Unsuspected Chlamydia trachomatis infection and in vitro fertilization outcome. Am J Obstet Gynecol 171:1208, 1994.
NONGONOCOCCAL-NONCHLAMYDIAL INFECTION.
Nongonococcal-nonchlamydial salpingitis may also arise de novo as a primary infection. Approximately 25% of women with PID have a nongonococcal-nonchlamydial cause.65 Patients with nongonococcal PID have the onset of pain distributed evenly throughout the cycle and less frequently associated with menses. There is less fever, vaginal discharge, and liver tenderness than with gonococcal PID. Despite these differences, the clinical presentation does not adequately distinguish between the two, and reliance on culture is necessary. Except for the presence of N. gonorrhoeae or C. trachomatis, no difference in vaginal or cervical flora exists between patients with gonococcal or chlamydial and nongonococcal-nonchlamydial salpingitis. As shown in Table 1, the cervix and vagina of healthy women contain an abundance of aerobic and anaerobic microorganisms. There may be a critical number of organisms needed to overwhelm local host defense mechanisms in the cervix, allowing an infection to ascend to the upper genital tract. There is probably a continuum from bacterial vaginosis to endometritis and salpingitis, because women with bacterial vaginosis are significantly more likely to be diagnosed with PID.10 The substantial isolation rate of bacteria other than gonococci or C. trachomatis from tubal fluid of these PID patients has shown that bacterial vaginosis organisms can cause acute salpingitis without antecedent chlamydial or gonococcal infection.66 Peritoneal or tubal cultures have yielded a mixed aerobic and anaerobic flora in 35% to 50% of patients, anaerobes alone in 15%, and aerobes alone in approximately 30% to 40% of patients. Between 4% and 17% of women with PID have had M. hominis, and 2% to 20%, have had U. urealyticum recovered from the fallopian tubes.66 Genital mycoplasmas probably play an infrequent role in PID, based on isolation rates, serologic data, and the observation that they produce little or no damage in human oviductal tissue cultures.67
PREDISPOSING FACTORS.
Previous gonorrhea, use of an IUD, frequent douching, and uterine instrumentation predispose to the development of nongonococcal PID.68 It is possible that unrecognized tubal damage impairs normal defense mechanisms even in the absence of clinically overt PID. When patients who have had PID subsequently acquire gonorrhea, more than one third develop acute onset PID, in contrast to the 10% to 17% rate in general.3
The use of an IUD is associated with approximately a threefold to fivefold increased risk of PID, which appears to exist for as long as the IUD is in place.21 The IUD may be a greater risk factor in nongonococcal than in gonococcal PID and is associated with an increased frequency of adnexal masses.21 Several reports have indicated the possible association between pelvic infection caused by Actinomyces israelii, an anaerobic gram-positive bacterium, and the use of an IUD.69 The use of any type of tail in an IUD provides a potential route for infection to the uterine cavity. Oral contraceptives may decrease the risk of developing PID, although they have less protective effect than barrier contraceptives. It is logical that women who have used IUDs suffer more tubal infertility and that women who used oral contraceptives have less infertility than women who have used neither method.21,70
Hysterosalpingography is commonly used in a complete infertility investigation. The introduction of water-soluble contrast media has eliminated the complications of oil embolism and has reduced the risk of granuloma formation, but inflammatory reactions continue to be serious complications of this procedure. The frequency of serious infection after hysterosalpingography varies from 0.3% to 3.1% of patients.71 It is possible that these episodes are caused by reactivation of preexisting disease rather than a de novo infection. High-risk patients for post-hysterosalpingography infections include those with prior pelvic infection or prior adnexal tenderness, a mass, or dilated fallopian tubes. Antibiotic prophylaxis with doxycycline (100 mg twice daily for a total of 7 days) reduces the incidence of post-hysterosalpingography infections.72
Pathogenesis
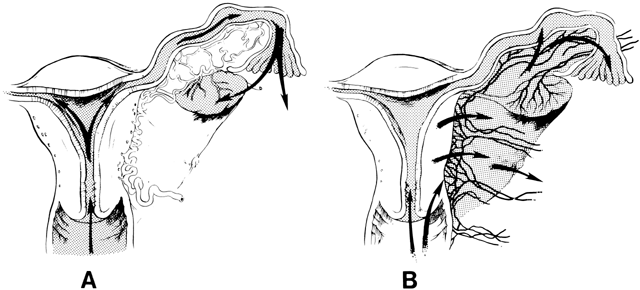
The pathways of spread of genital tract infections are shown in Figure 2. In gonococcal and chlamydial salpingitis, the microorganisms ascend by surface extension from the lower genital tract through the cervical canal by way of the endometrium to the fallopian tubes (Fig. 2A). Microscopically, the endosalpinx is inflamed and edematous. There can be adhesion of the mucosal folds, destruction of cilia, occlusion of the infundibulum, and production of a pyosalpinx. The gonococcal infection may spread beyond the endosalpinx, with possible focal abscess formation and perisalpingitis. In some cases of nongonococcal salpingitis, particularly with M. hominis,73 the pathogens may enter through lesions in the cervix or endometrium and spread to the parametria and tubes through lymphatics and blood vessels (Fig. 2B). The inflammatory swelling that affects the parametria and the tubes is more pronounced than in gonococcal salpingitis, but the endosalpinx is usually intact.
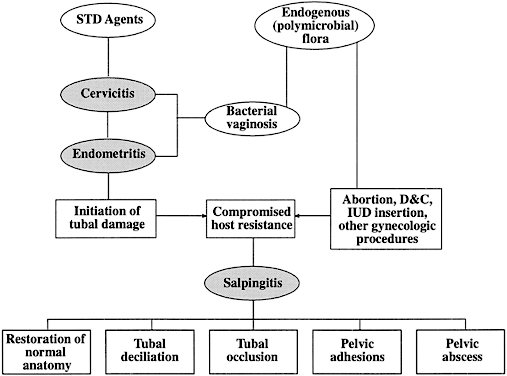
The sequelae of PID that are responsible for infertility include chronic interstitial salpingitis, hydrosalpinx, salpingitis isthmica nodosa, and periadnexal adhesions. Infertility may also occur because of abnormal secretory, ciliary, and peristaltic function of the fallopian tube. The postulated interrelationships of STDs and endogenous organisms in the pathogenesis of tubal infertility secondary to PID are depicted in Figure 3.74
Therapeutic Considerations
Early recognition and proper treatment of upper genital tract infection are mandatory to prevent permanent damage to the female reproductive tract and subsequent infertility. There is controversy over the issue of outpatient versus inpatient treatment of patients with acute salpingitis. For economic and logistical reasons, most women are treated on an outpatient basis. The decision for hospitalization is usually based on the clinical severity of the illness, although criteria vary. It seems reasonable to treat major pathogens such as N. gonorrhoeae and C. trachomatis in every patient. An antibiotic regimen that takes into account the polymicrobial nature of the cause of acute salpingitis must be used. However, after treatment with different antibiotics, similar infertility rates have been found.75 This could be interpreted to indicate that the ideal antibiotic has not been found or, more likely, that most tubal damage occurs before the patient presents for treatment. Women treated after 3 or more days of symptoms had significantly more infertility than those treated earlier.76 Better recognition and treatment of cervicitis and endometritis before salpingitis develops is even more important in the prevention of infertility than the treatment of salpingitis per se. Recommended treatment schedules for uncomplicated salpingitis are shown in Table 6.
TABLE 6. Recommended Therapy for Salpingitis
Parenteral Regimen A
Cefotetan 2 g, IV every 12 hours,
or
Cefoxitin, 2 g, IV every 6 hours,
plus
Doxycycline, 100 mg, IV or orally every 12 hours
Parenteral Regimen B
Clindamycin, 900 mg, IV every 8 hours,
plus
Gentamicin loading dose IV or IM (2 mg/kg of body weight), followed by a maintenance dose (1.5 mg/kg) every 8 hours. Single daily dosing may be substituted.
Regimen A
Ofloxacin, 400 mg, orally twice each day for 14 days,
plus
Metronidazole, 500 mg, orally twice each day for 14 days.
Regimen B
Ceftriaxone, 250 mg, IM once,
or
Cefoxitin, 2 g, IM plus Probenecid, 1 g, orally in a single dose concurrently
once,
or
Other parenteral third-generation cephalosporin (e.g., ceftizoxime, cefotaxime),
plus
Doxycycline, 100 mg, orally twice each day for 14 days. (Include this regimen with one of the above regimens.)
Centers for Disease Control and Prevention: 1998 Guidelines for treatment of sexually transmitted diseases. MMWR Morb Mortal Wkly Rep 1998;47(RR1):82–82.
For outpatients, all women with suspected PID should have an initial parenteral antibiotic to inhibit N. gonorrhoeae. In many areas with high rates of penicillin-resistant gonorrhea, cefoxitin or ceftriaxone should be given. However, single-agent therapy is not appropriate for PID. Tetracyclines or doxycycline given alone no longer reliably inhibits N. gonorrhoeae. Tetracyclines, however, should be given for 10 to 14 days to inhibit C. trachomatis. Patients with suspected abscesses or severe illness that may indicate the presence of organisms other than gonococci or chlamydiae should be hospitalized. Recommended treatment regimens inhibit not only N. gonorrhoeae and C. trachomatis but also a wide variety of aerobic and anaerobic bacteria. For instance, parenteral clindamycin is effective against C. trachomatis and anaerobes. One review indicates that few hospitalized women receive the recommended antibiotic regimens.39
The concomitant use of steroids with antibiotics has been thought to reduce the sequelae of salpingitis, but in a prospective study, Falk77 could show no beneficial effect as judged by hysterosalpingography findings or subsequent laparotomy. Prevention of PID recurrence and its adverse effects on fertility also requires treatment of asymptomatic male sexual partners.
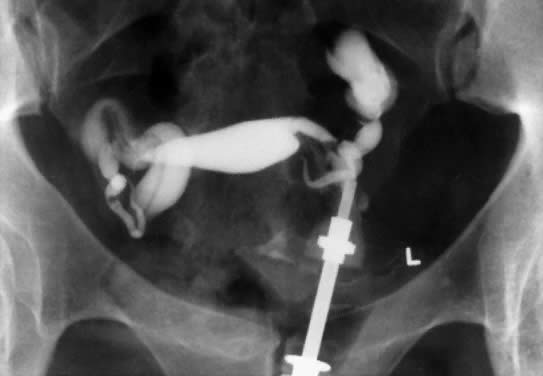
In patients with postinflammatory tubal disease, pregnancy outcome has been correlated with the presence or absence of fallopian tube rugae on hysterosalpingograms (Fig. 4). Pregnancy occurred in 61% of patients with moderate to excellent rugal patterns, whereas only 7% of patients with no demonstrable rugae conceived postoperatively.78 Laparoscopic and salpingoscopic evaluation of the endosalpinx provides another means to assess prognosis for fertility.79 However, visualization of the tubal mucosa by salpingoscopy provides even more reliable data on which to classify and score the extent of tubal disease.
Management of Tubal Infertility
Today and in the foreseeable future, assisted reproductive technologies (ART), endoscopic surgery, and microsurgery have an important place in the management of infertility that results from tubal disease. There are some tubal causes of infertility for which surgery can offer little or no chance of success, such as after severe bilateral hydrosalpinx, multisite tubal obstruction, or in patients with extensive and dense pelvic adhesions. At the other end of the spectrum are patients who can achieve a 50% to 65% intrauterine pregnancy rate after microsurgical or laparoscopic adhesiolysis when the fimbriae are spared from disease and a male factor is not encountered.79 In choosing between IVF and tubal surgery, the physician must compare success rates (which can are best defined by the birth of a live baby) and take into account the patient's age, presence of a male subfertility factor, the personal priorities of the couple, and the availability of expertise.
Differentiating the cause of tubal occlusion by history and ancillary tests (e.g. chlamydial serology) can contribute to the assessment of prognosis. Severe male factor combined with tubal disease in the female partner is an indication for advanced laboratory techniques in assisted reproduction such as intracytoplasmic sperm injection.
In patients with mild tubal disease (stage I hydrosalpinx) the prognosis is good with term pregnancy rates of 39% to 59% and ectopic pregnancy rates of 4% to 10% after microsurgical neosalpingostomy.79 Patients with moderate disease (stage II hydrosalpinx) make up about one third of the total and have an intermediate prognosis for a term pregnancy, but the risk of ectopic pregnancy is at least 10%.
The surgical prognosis for pregnancy is uniformly poor in patients with flat tubal mucosa or a fibrotic and thick-walled hydrosalpinx (stage III or IV); IVF is advised for this group of patients. However, patients with large hydrosalpinges can benefit from prophylactic salpingectomy before undergoing IVF to improve implantation rates and to reduce the likelihood for ectopic pregnancy.30,80
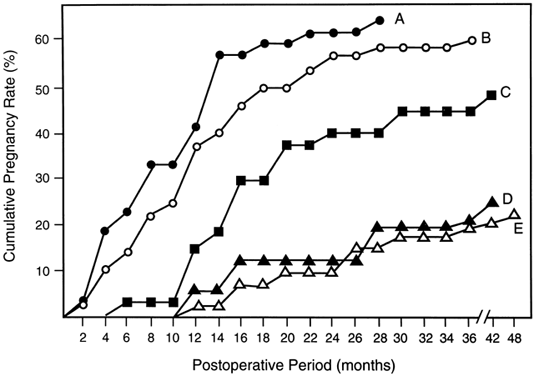
It is widely acknowledged that pregnancy outcome after tubal surgery is time dependent81 (Fig. 5). Most physicians advise their patients to opt for IVF at 12 to 18 months after unsuccessful surgery. In contrast to salpingoneostomy, the cumulative pregnancy rate increases rapidly after fimbrioplasty (i.e. deagglutination of visible fimbriae) and results in intrauterine pregnancy rates of 60% or better (Fig. 5). Although less common than distal fallopian tube disease, proximal tubal obstruction (PTO) occurs in 10% to 20% of hysterosalpingographies performed to evaluate infertility.82 The diagnostic and therapeutic options for managing PTO have been expanded by the introduction of fluoroscopic or hysteroscopic fallopian tube catheterization, which results in tubal patency in about 85% of patients with PTO.82 Microsurgical resection and tubal cornual anastomosis is the preferred surgical option for women with persistent occlusive disease in the proximal oviduct not opened by transcervical catheterization.82 A review of the world literature indicates a mean intrauterine pregnancy rate of 58% after tubocornual anastomosis, with an ectopic pregnancy rate of 4%.83 It is reasonable to expect a continued improvement in pregnancy outcome for IVF procedures (reported in the United States for the year 1995 as 22.5% delivery rate per oocyte retrieval).84 Data from large U.S. centers indicate a 40% to 70% cumulative delivery rate after three IVF cycles in younger women with tubal disease and without associated male-factor infertility.85,86 These results compare very favorably with the best outcomes after tubal reconstructive surgery.
INFECTIONS AND MALE INFERTILITY
Acute and chronic genital tract infections are well-known causes of infertility in men (Table 6). Episodes of acute orchitis or epididymitis may result in permanent damage to the testis or to obstruction in the efferent ejaculatory ducts. C. trachomatis causes approximately 50% of epididymitis in sexually active men under age 35. Unilateral epididymal obstruction is seldom diagnosed, and its effect on fertility is largely unknown. However, 80% of men with unilateral ductal obstruction have antibodies to sperm, a potential cause of male infertility.87 Azoospermia on semen analysis requires testicular biopsy and radiographic studies of the vas, because infection-induced obstruction can be surgically corrected.
Most men do not develop antibodies to their own spermatozoa because the male genital tract is essentially a closed tube, and sperm are isolated from the immune system. Genital tract infections, even those without symptoms, can weaken this barrier, leading to sperm leakage and the influx of immunologically competent cells. Genital tract infections are a major cause of antisperm antibody formation in men.88,89 Similarly, genital tract infections and antisperm antibody formation in men can lead to immune-mediated infertility in women.90,91
Mumps orchitis is the most common testicular infection resulting in damage to the germinal epithelium. Systemic infections, whether bacterial or viral, may also cause depression of sperm production for variable periods. Between 2 and 6 months may be required for normal seminal cytology to reappear after a severe febrile illness.
Urethral stricture is an occasional complication of untreated gonorrhea. Although the stricture does not in itself interfere with sperm motility, it may cause recurring urinary tract infection or prostatitis and epididymitis. The mechanisms by which infection can influence semen parameters are outlined in Table 7.
TABLE 7. Male Genital Tract Infections That May Cause Infertility
Orchitis: mumps, tuberculosis, syphilis, pancreatitis
Epididymitis: gonorrhea, tuberculosis, chlamydiae, ureaplasmas,
Pseudomonas, coliform, and other bacterial infections
Seminal vesculitis: tuberculosis, trichomoniasis, other bacteria
Urethritis: gonorrhea, chlamydiae, ureaplasmas, trichomoniasis
The fertility of a couple may be impaired if the man has a chronic bacterial prostatitis. Chronic prostatitis is presumed to be caused by a pathogenic organism and in most cases is associated with leukocytes in the semen. The prevalence of leukocytospermia among male infertility patients is about 10% to 20%.92 Clinical evidence for prostatitis is found in a large number of asymptomatic infertile men with leukocytospermia and abnormal semen parameters.93 Long-term treatment with an appropriate antibiotic has been shown to normalize or improve the semen analysis in a substantial number of men.94 Nevertheless, the relation between leukocytospermia, microorganisms in the male genital tract, abnormal sperm function, and infertility is not always clear-cut.
Most semen specimens from fertile men contain leukocytes (median concentration, 1.4 to 4.6 × 104/ml).95 According to World Health Organization criteria, leukocytospermia is defined as 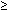 106 white blood cells/1 ml of semen.96 Granulocytes are the most prevalent type (50% to 60%), followed by macrophages (20% to 30%) and TH1 lymphocytes (2% to 5%).92,97 Peroxidase staining and immunocytology are necessary to distinguish immature germ cells from white blood cells in the semen sample and to classify the various white blood cell types. The significance of leukocytospermia may ultimately depend on the composition and activation of the white cell population and the site and cause of the leukocytic infiltration, none of which can be deduced from a simple count of leukocyte numbers in the ejaculate. Some men exhibit transient episodes of leukocytospermia (possibly related to smoking, alcohol, or marijuana consumption) that resolve spontaneously.97 The semen analysis must be combined with relevant clinical information, including rectal examination, ultrasonography, bacterial cultures, and serology, to confirm the existence of infection or inflammation in the male accessory glands.
106 white blood cells/1 ml of semen.96 Granulocytes are the most prevalent type (50% to 60%), followed by macrophages (20% to 30%) and TH1 lymphocytes (2% to 5%).92,97 Peroxidase staining and immunocytology are necessary to distinguish immature germ cells from white blood cells in the semen sample and to classify the various white blood cell types. The significance of leukocytospermia may ultimately depend on the composition and activation of the white cell population and the site and cause of the leukocytic infiltration, none of which can be deduced from a simple count of leukocyte numbers in the ejaculate. Some men exhibit transient episodes of leukocytospermia (possibly related to smoking, alcohol, or marijuana consumption) that resolve spontaneously.97 The semen analysis must be combined with relevant clinical information, including rectal examination, ultrasonography, bacterial cultures, and serology, to confirm the existence of infection or inflammation in the male accessory glands.
Split ejaculate studies in men with pyospermia indicate that the epididymis, testes, prostrate, and seminal vesicles can be the source of white cells.98 Normally, most white blood cells appear to originate in the epididymis, because vasectomized males show a reduced level of white cells in semen.92,99 However, pyospermic samples show low citric acid levels pointing to prostatic inflammation as a major source of leukocytes in the semen. In the event of a low-grade orchitis, inflammatory changes in the seminiferous tubules would be expected to disrupt normal spermatogenesis. Similarly, exposure of spermatozoa to the damaging effects of leukocytes in the inflamed epididymis would be prolonged in comparison to exposure in the ejaculate.
The preponderance of evidence supports a connection between leukocytospermia and abnormal sperm function but the causal relationship to microorganisms is less clearly established. In one study, approximately 80% of pyospermic samples were culture negative, albeit antibodies to C. trachomatis were present in 25%.92 However, a high percentage of positive bacterial isolates has been reported in semen from asymptomatic men attending an infertility clinic, but this may represent skin or urethral contamination.100 The urine must be sterile if significance is to be attributed to positive cultures of semen or prostatic secretions. When quantitative bacteriology is performed, no difference in fertility rates is observed between negative cultures and cultures containing less than 10,000 colony-forming units/ml. Conversely the presence in semen of counts more than 10,000 colony-forming units/ml had a negative effect on IVF pregnancy rates when E. coli, Proteus, or S. aureus organisms were isolated.101 Although Enterococcus is commonly cultured from semen, it had no effect on pregnancy rates. Because E. coli, Proteus spp., and S. aureus may have an adverse effect on male infertility, treatment is usually recommended.101
Some authorities consider trimethoprim-sulfamethoxazole an effective drug for the treatment of chronic prostatitis. A 12-week treatment period is twice as effective as a 2-week course. Other microbials, namely the quinolines (e.g. ofloxacin) and macrolides (e.g. erythromycin, azithromycin), are more likely to penetrate prostatic tissue in optimal therapeutic concentrations. Ofloxacin, a fluorinated carboxyl quinoline, appears to be safe and does not affect sperm parameters at high concentrations, nor does it appear to be mutagenic.102 Doxycycline (100 mg twice daily for 14 days) provides adequate therapy for nongonococcal urethritis, especially for Chlamydia-positive urethritis, but it does not achieve optimal concentrations in the accessory sex glands.98
More research is needed in this area as the pathogen detection methods used have been less than optimal and generally have not included modern molecular techniques. Moreover, the possibility that leukocytospermia may be caused by viral infections of the genital tract (including cytomegalovirus, herpes simplex, human papilloma, Epstein-Barr, hepatitis B, and HIV) has not been looked at systematically.
The adverse effects on sperm function of genital tract infection derive largely from the inflammatory cells.92 Leukocytes release cytokines, proteases, and free radicals, which significantly inhibit motility and the fertilizing capacity of spermatozoa in the hamster ovum penetration test and in IVF.95,103 The maximal damage by reactive oxygen species from activated granulocytes probably occurs in the testes or epididymis because of long sperm-leukocyte contact time and to the absence of seminal plasma protection. However, an impairment in glandular secretion as a byproduct of infection could also diminish the antioxidant effect of seminal plasma on leukocyte action. The degree of seminal plasma protection of spermatozoa against oxidative damage is highly variable among individuals, which explains why some men show no impairment of sperm function despite significant leukocytospermia.104 There is also substantial evidence that infection contributes to the development of sperm antibodies.89 Sperm antibodies have been detected in 48% of men with culture-positive asymptomatic infections, 47% of men with a history of urethritis or prostatitis, and in only 5% of men with no infection and a normal semen analysis. The presence of IgA antibodies was associated with reduced fertility.88
High concentrations of sperm antibodies can interfere with fertility by several mechanisms. Antibodies on sperm heads or tails may cause sperm to agglutinate. Tail-bound antibodies also interfere with sperm motility. Antibodies anywhere on spermatozoa can lead to sperm phagocytosis through binding to Fc or complement receptors on phagocytic cells. Similarly, antibody-bound sperm react with cervical mucus leading to sperm immobilization and expulsion from the female genital tract. Antibodies on sperm can interfere with sperm binding and penetration of the oocyte.105
The incidence of vaginitis and salpingitis is higher among women whose husbands have a history of genitourinary infection. There is evidence that C. trachomatis adheres to spermatozoa and penetrates the sperm head.106 The potential for transmission to the female genital tract is clear.
The relationship of past exposure to C. trachomatis to male fertility was examined in men from infertile couples whose wives had no known infertility factors. In this population, the frequency of C. trachomatis antibody was low (6%), but it was associated with a history of nonspecific urethritis and with sperm agglutinating antibodies in the serum.107
Similar to the situation in women, C. trachomatis infection of the male genital tract is often asymptomatic and therefore may persist for a long time. There also is an association between detection of anti-chlamydial IgA in semen of men with no history of symptomatic genital tract infection and the expression of HSP60 in semen.89 There have been few studies on the fertility consequences of a male genital tract C. trachomatis infection. Asymptomatic chlamydial colonization of the male urethra and prostrate gland have been described.108 Asymptomatic chlamydial urethral infections may become symptomatic if not promptly treated.109 One study using PCR analysis of semen specimens suggested that an asymptomatic unsuspected C. trachomatis male genital tract infection may be the cause of previously unexplained infertility.110
Asymptomatic male genital tract C. trachomatis infection has been associated with the formation of antisperm antibodies. Detection in semen of C. trachomatis by PCR or by anti-chlamydial IgA immunoassay correlated with antisperm antibodies on the surface of ejaculated sperm and with circulating antisperm antibodies in the female partner in several studies110 but not in some others.111 Anti-chlamydial IgA was detected with a significantly higher prevalence in semen than in serum while the reverse was true for anti-chlamydial IgG. IgA antibodies on the surface of ejaculated sperm and in maternal sera have been associated with infertility and with IVF failure.112,113
The mechanism leading to antisperm antibody formation in men after a C. trachomatis genital tract infection remains to be elucidated. The inflammation associated with this infection may compromise the barrier isolating sperm from immunocompetent cells resulting in the induction of antisperm immunity. Studies have also demonstrated that T lymphocytes that possess the γδ form of the T-cell antigen receptor (γδ T cells) are concentrated in the human male genital tract and that their numbers are greatly increased in association with a C. trachomatis infection.113 A large fraction of γδ T cells are specifically activated by HSP60.114 In response to a persistent asymptomatic chlamydial infection and HSP60 production, γδ T cells may be induced in the male genital tract; γδ T cells are capable of releasing proinflammatory cytokines and could therefore initiate an antisperm immune response within the genital tract. Conversely, some γδ T cells have been shown to inhibit the release of immunoglobulins from activated B lymphocytes.115 Although γδ T cells are markers and possible mediators of infection in the male genital tract, together with HSP60, they may also play a role in downregulating proinflammatory immune responses and antisperm immunity at this location.116.
GENITAL MYCOPLASMAS AND UNEXPLAINED INFERTILITY
Mycoplasmas share characteristics of bacteria (they reproduce on cell-free media) and viruses (they have no cell wall and are 100 to 300 μm in diameter). Two species of mycoplasmas have been commonly isolated from the female and male reproductive tracts: M. hominis and the heterogeneous group known collectively as T mycoplasma (so named for their characteristic “tiny” colonies). A distinctive property of the T strains is their ability to hydrolyze urea, and they have been named U. urealyticum. A third species, Mycoplasma genitalium has been isolated from the urethra of men and is a cause of urethritis.117
Genital mycoplasmas are rarely isolated from prepubertal girls and boys. After puberty, colonization with genital mycoplasmas occurs primarily through sexual contact. Sexually immature young women with no history of sexual contact have a negligible rate of colonization with ureaplasmas, whereas 37% of women who have had intercourse with a single partner and 75% of those who have had intercourse with three or more partners are colonized.118 Colonization with M. hominis is less prevalent but follows the same general pattern. Genital mycoplasmas can be isolated from the external cervical os, vagina, and distal urethra and from semen. The proximal urethra, bladder, and upper reproductive tracts are normally free of mycoplasma. The vagina is the site most likely to yield a positive culture for genital mycoplasmas.
M. hominis and U. urealyticum colonization of the genital tract persists if no treatment is instituted. Penicillin and other antibiotics that inhibit cell wall synthesis and sulfonamides do not inhibit genital mycoplasmas. M. hominis and U. urealyticum are sensitive to tetracycline or their analogues, which are the drugs of choice. M. hominis is sensitive to lincomycin but relatively resistant to erythromycin. U. urealyticum is sensitive to erythromycin. Both genital mycoplasmas may also be sensitive to chloramphenicol, spectinomycin, and gentamicin, and M. hominis is sensitive to clindamycin.
Genital mycoplasmas may be of etiologic importance in nonspecific urethritis, cervicitis, and vaginitis; some cases of acute salpingitis; fever after abortions; chorioamnionitis; and puerperal infections. The evidence is weak that mycoplasma cause fetal wastage and low birth weight.
The role of genital mycoplasmas in infertility is unresolved. Cultures from the lower genital tract of healthy women recovered M. hominis and U. urealyticum in 16% to 20% and 43% to 57% of subjects, respectively.118,119 These percentages may be underestimates, because culture techniques are less than 100% sensitive. In a study of culturenegative women undergoing IVF, subsequent lower genital tract samples analyzed by PCR for M. hominis and U. urealyticum were identified as positive in 2% and 17% of patients, respectively.120 Controlled studies have not demonstrated a convincing difference in isolation rates between fertile couples and couples with long-standing infertility.121 Cassell and coworkers recovered M. hominis from 6% and U. urealyticum from only 1% of the endometria of infertile women.122
M. hominis in the vagina is associated with bacterial vaginosis.123 However, the specific role, if any, of M. hominis in the induction of vaginal pathology is unclear. Its presence in the lower genital tract per se does not seem to influence fertility outcome. In the female upper genital tract, M. hominis has been isolated from women with endometritis or salpingitis. Whether this organism, alone or in combination with other microbes, contributes to fallopian tube occlusion is unclear. The higher frequency of antibodies to M. hominis in infertile women with a history of pelvic infection than in other women suggests that this organism may contribute to tubal pathology.124
Like M. hominis, U. urealyticum is occasionally isolated from the fallopian tubes of women with pelvic infections, but its role in disease remains uncertain in large part because it is prevalent in the lower genital tract of healthy fertile women. In vitro studies with fallopian tube explant systems have suggested that mycoplasmas may be commensals rather than pathogens in acute PID. Nonetheless, it has been shown by scanning electron microscopy that M. hominis induces pathologic swelling in fallopian tube ciliated cells in tissue culture.125 In vitro systems preclude study of a potential host immune response that may contribute to the pathogenesis of salpingitis.
The low virulence of these organisms and the multifactorial nature of infertility have contributed to uncertainty regarding a causal relationship. Most therapeutic studies have not controlled for the number of sexual partners or for other organisms, such as C. trachomatis, that contribute to infertility. Uncertainties about the pathogenic role of mycoplasmas might be reduced if certain parameters of infertility could be related to quantitative data on mycoplasma colonization, to the extent of tissue invasion by the organisms (e.g. by serologic testing) or to specific strains of M. hominis and U. urealyticum, of which 12 are already recognized. Serotype 3 has been isolated predominantly from infertile women, whereas serotype 6 is the predominant isolate among fertile women.126
Chimpanzees and other subhuman primates can be infected with human genital mycoplasmas. The development of a suitable animal model may provide new insights into the role of ureaplasmas in human infertility. In the Grivet monkey model, M. hominis produces a parametritis rather than acute salpingitis, which could explain the infrequent recovery of mycoplasmas from human fallopian tubes.73 Several studies have demonstrated an association between U. urealyticum and spontaneous abortion.127 One study has examined the presence of different U. urealyticum biovars in 254 women by PCR.128 One biovar, T960, was associated with PID and spontaneous abortion. Further studies on additional populations are needed to confirm these initial findings.
Although both organisms inhibit sperm penetration of denuded hamster oocytes,129 U. urealyticum did not interfere with fertilization of hamster,130 mouse,131 or human132 oocytes. Swenson and coworkers found a significant improvement in the motility (i.e. speed of forward progression and percent of motile cells) of spermatozoa in ejaculates of infertile men after the eradication of U. urealyticum from their semen.133 M. hominis and U. urealyticum may interfere with sperm function in vitro, but the extent that these interactions contribute to infertility or to IVF failure remains unclear.
FERTILITY PRESERVATION BY EARLY DIAGNOSIS OF SEXUALLY TRANSMITTED DISEASES
After C. trachomatis or another pathogen ascends to the fallopian tubes and establishes a persistent infection, infertility can be induced by at least two mechanisms: fallopian tube occlusion and immune rejection of the embryo. The best prevention is to detect and treat early-stage asymptomatic and symptomatic infections. This can be achieved by the screening of all sexually active reproductive age women and by educating clinicians and patients on the importance of this testing. A woman should be made aware that every time she has unprotected sexual intercourse with a new partner she risks compromising her future fertility. Reduction in the prevalence of C. trachomatis has followed widespread screening programs in Sweden134 and selective screening in Wisconsin135 and Region X of the United States.136 In the latter areas, a 50% reduction in the prevalence of C. trachomatis was achieved after screening. A 60% reduction in salpingitis prevalence was observed in a population subjected to partial screening compared with a population in which routine screening for C. trachomatis was not employed.4
The diagnosis of STDs has been greatly aided by the introduction of gene amplification technology for the identification of microorganisms. By means of the PCR and use of oligonucleotide primer pairs specific for the microbe of interest, the microbial DNA in a lower genital tract sample can be amplified up to 1 million-fold in several hours. This approach is many times faster than the time required to grow the microbe in an in vitro system and avoids the technical problems associated with cultivation. PCR is also much more sensitive than nonamplification antigen detection or DNA hybridization techniques. Several studies have demonstrated that coupled with the sensitivity of gene amplification technology, STD organisms such as C. trachomatis,137 T. vaginalis,138 and N. gonorrhoeae139 can be detected in samples obtained from the vaginal introitus, and there is no longer a requirement for a speculum examination or trained personnel to obtain samples for STD testing. Using PCR, women can obtain their own introital specimens in privacy. Detection of STDs in urine samples has also been achieved,140 but sensitive STD detection in this case requires prompt processing of the samples. Application of new technologic advances in specimen collection and STD identification, coupled with increased awareness of the need for preventative screening, offers the best hope of reducing the incidence of infection-related infertility. There is less hope for the early development of chlamydial or gonococcal vaccines, in part because it has been difficult to elicit a sustained protective immune response in the genital tract mucosa.
REFERENCES
Westrom LV: Sexually transmitted diseases and infertility. Sex Transm Dis 21: 532– 537, 1994 |
|
Cates W Jr, Wasserheit JN: Genital chlamydia infections: Epidemiology and reproductive sequelae. Am J Obstet Gynecol 164: 1771, 1991 |
|
Eschenbach DA: Infertility caused by infection. Contemp Obstet Gynecol 32: 29– 46, 1987 |
|
Scholes D, Stergachis A, Heidrich FE et al: Prevention of pelvic inflammatory disease by screening for cervical chlamydia infection. N Engl J Med 334: 1362– 1366, 1996 |
|
Chow AW, Malkasian KL, Marshall JR et al: The bacteriology of acute pelvic inflammatory disease. Am J Obstet Gynecol 122: 876, 1975 |
|
Devrick FC, Dahlberg G: Male genital tract infections and sperm viability. In Hafez ES (ed): Human Semen and Fertility Regulation in Men. St Louis: CV Mosby, 1976 |
|
Spiegel CA, Amsel R, Eschenbach DA et al: Anaerobic bacteria in nonspecific vaginitis. N Engl J Med 303: 601, 1980 |
|
Paavonen J, Teisala K, Heinonen PK et al: Microbiological and histopathological findings in acute pelvic inflammatory disease. Br J Obstet Gynecol 94: 454– 460, 1987 |
|
Soper DE, Brockwell NJ, Dalton HP, Johnson D: Observations concerning the microbial etiology of acute salpingitis. Am J Obstet Gynecol 170: 1008– 1017, 1994 |
|
Eschenbach DA, Hillier S, Critchlow C et al: Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol 158: 819– 828, 1988 |
|
Brunham RC, Paavonen J, Stevens CE et al: Mucopurulent cervicitis: The ignored counterpart in women of urethritis in men. N Engl J Med 311: 1, 1984 |
|
Paavonen J, Kiviat NB, Brunham RC et al: Prevalence and manifestations of endometritis among women with cervicitis. Am J Obstet Gynecol 152: 280– 286, 1985 |
|
Centers for Disease Control and Prevention. 1998 Guidelines for Treatment of sexually transmitted diseases. MMWR Morb Mortal Wkly Rep 47(RR-1):70–79, 1998 |
|
Chernesky M, Lee H, Schachter J et al: Diagnosis of a Chlamydia trachomatis urethral infection in symptomatic and asymptomatic men by testing first-void urine in a ligase chain reaction assay. J Infect Dis 170: 1308– 1311, 1994 |
|
Witkin SS: Circulating antibodies to Chlamydia trachomatis in women: Relationship to antisperm and antichlamydial antibodies in semen of male partners. Hum Reprod 11: 1635, 1996 |
|
Witkin SS, Sultan KM, Neal GS et al: Unsuspected Chlamydia trachomatis infection and in vitro fertilization outcome. Am J Obstet Gynecol 171: 1208– 1214, 1994 |
|
Hurry DJ, Larsen B, Charles D: Effects of postcesarean section febrile morbidity on subsequent fertility. Obstet Gynecol 64: 256, 1984 |
|
Lasala AP, Berkeley AS: Primary cesarean section and subsequent fertility. Am J Obstet Gynecol 157: 379, 1987 |
|
Hoyme VB, Kiviat N, Eschenbach DA: The microbiology and treatment of late postpartum endometritis. Obstet Gynecol 67: 229, 1986 |
|
Osser S, Persson K: Postabortal pelvic infections associated with Chlamydia trachomatis and the influence of humoral antibody. Am J Obstet Gynecol 150: 699, 1984 |
|
Cramer DW, Schiff I, Schoenbaum SC et al: Tubal infertility and the intrauterine device. N Engl J Med 312: 941, 1985 |
|
Kiviat MB, Wolner-Hanssen P, Eschenbach DA et al: Endometrial histopathology in patients with culture-proven upper genital tract infection and laparoscopically acute salpingitis. Am J Surg Pathol 14: 167– 175, 1990 |
|
Korn AP, Bolan G, Padian N et al: Plasma cell endometritis in women with symptomatic bacterial vaginosis. Obstet Gynecol 85: 387– 390, 1995 |
|
Peipert JF, Montagno AB, Cooper AS et al: Bacterial vaginosis as a risk factor for upper genital tract infection. Am J Obstet Gynecol 177: 1184– 1187, 1997 |
|
Hillier SL, Kiviat NB, Hawes SE et al: Role of bacterial vaginosis-associated microorganisms in endometriosis. Am J Obstet Gynecol 175: 435– 441, 1996 |
|
Witkin SS, Ledger WJ: Antibodies to Chlamydia trachomatis in sera of women with recurrent spontaneous abortions. Am J Obstet Gynecol 167: 135– 149, 1992 |
|
Bustos-Lopez HH, Barron-Vallyo J, Garcia-Malvaez B et al: Use of a diagnostic prospective algorithm for patients with recurrent miscarriage. Gynecol Obstet Mex 63: 96– 101, 1995 |
|
Anderson DJ, Alexander NJ: Induction of uterine leukocytosis and its effect on pregnancy in rats. Biol Reprod 21: 1143, 1979 |
|
Askienazi-Ellbhan M: Immune consequences of Chlamydia infections in pregnancy and in vitro fertilization outcome. Infect Dis Obstet Gynecol 4: 143– 148, 1996 |
|
Meyer WR, Castelbaum AJ, Somkuti S et al: Hydrosalpinges adversely affect markers of endometrial receptivity. Hum Reprod 12: 1393– 1398, 1997 |
|
Valle RF, Sciarra JJ: Intrauterine adhesions: Hysteroscopic diagnosis, classification, treatment and reproductive outcome. Am J Obstet Gynecol 148: 1459– 1470, 1988 |
|
Corson SL: Operative hysteroscopy for infertility. Clin Obstet Gynecol 35: 229– 241, 1992 |
|
Washington AE, Katz P: Cost and payment source for pelvic inflammatory disease.Trends and projections 1983 through 2000. JAMA 266: 2565– 2569, 1991 |
|
Westrom L: Effects of acute pelvic inflammatory disease on infertility. Am J Obstet Gynecol 121: 707, 1975 |
|
Landers DV, Sweet RL: Tubo-ovarian abscess: Contemporary approach to management. Rev Infect Dis 5: 176, 1983 |
|
Pisarska MD, Carson SA, Buster JE: Ectopic pregnancy. Lancet 351: 1115– 1120, 1998 |
|
Eschenbach DA, Wolner-Hanssen P, Hawes SE et al: Acute pelvic inflammatory disease: Association of clinical and laboratory findings with laparoscopic findings. Obstet Gynecol 89: 184– 192, 1997 |
|
Sweet RL: Diagnosis and treatment of acute salpingitis. J Reprod Med 19: 21, 1977 |
|
Grimes DA, Blount JH, Patrick J et al: Antibiotic treatment of pelvic inflammatory disease: Trends among private physicians in the United States: 1966 through 1983. JAMA 256: 3223, 1986 |
|
Gray RH, Waiver MJ, Serwadda D et al: Population-based study of fertility in women with HIV-1 infection in Uganda. Lancet 351: 98– 103, 1998 |
|
Holmes KK, Eschenbach DA, Knapp JS: Salpingitis: Overview of etiology and epidemiology. Am J Obstet Gynecol 138: 893, 1980 |
|
Paavonen J: Immunopathogenesis of PID and infertility—What do we know and what shall we do. J Br Fertil Soc 1: 42– 45, 1996 |
|
Svensson L, Mardh PA, Westrom L: Infertility after acute salpingitis with special reference to Chlamydia trachomatis. Fertil Steril 40: 322, 1983 |
|
Sweet RL, Schachter J, Robbie MO: Failure of beta-lactam antibiotics to eradicate Chlamydia trachomatis in the endometrium despite apparent clinical cure of acute salpingitis. JAMA 250: 2641, 1983 |
|
Jones RB, Ardery BR, Jui SL, Cleary RE: Correlation between serum antichlamydial antibodies and tubal factor as a cause of infertility. Fertil Steril 38: 553, 1982 |
|
McCormack WM, Alpert S, McComb DE et al: Fifteen-month follow-up study of women infected with Chlamydia trachomatis. N Engl J Med 300: 123, 1979 |
|
Beatty WL, Byrne GI, Morrison RP: Morphologic and antigenic characterization of interferon-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA 90: 3998, 1993 |
|
Beatty WL, Byrne GI, Morrison RP: Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trend Microbiol 2: 94, 1994 |
|
Witkin SS: Immune pathogenesis of asymptomatic Chlamydia trachomatis infections in the female genital tract. Infect Dis Obstet Gynecol 3: 169, 1995 |
|
Cooper ME, Rapp J, Jeffery-Wiseman C et al: Chlamydia trachomatis infection of human fallopian tube organ cultures. J Gen Microbiol 136: 1109, 1990 |
|
Morrison RP, Manning DS, Caldwell HD: Immunology of Chlamydia trachomatis infections: Immunoprotective and immunopathogenetic responses. In Quinn TC (ed): Sexually Transmitted Diseases, p 57. New York: Raven Press, 1992 |
|
Morrison RP, Belland RJ, Lyng D, Caldwell HD: Chlamydial disease pathogenesis: The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med 170: 1271, 1989 |
|
Patton DL, Sweeney YT, Kuo CC: Demonstration of delayed hypersensitivity in Chlamydia trachomatis salpingitis in monkeys: A pathogenic mechanism of tubal damage. J Infect Dis 169: 680, 1994 |
|
Wager EA, Schachter J, Bavoil P, Stephens RS: Differential human serologic response to two 60,000 molecular weight Chlamydia trachomatis antigens. J Infect Dis 162: 922, 1990 |
|
Brunham RC, Peeling R, Maclean I et al: Chlamydia trachomatis associated ectopic pregnancy: Serologic and histologic correlates. J Infect Dis 165: 1076, 1992 |
|
Witkin SS, Jeremias J, Toth M, Ledger WJ: Cell-mediated immune response to the recombinant 57-kDa heat-shock protein of Chlamydia trachomatis in women with salpingitis. Am J Obstet Gynecol 171: 455, 1994 |
|
Toye B, Laferriere C, Claman P et al: Association between antibody to the chlamydial heat shock protein and tubal infertility. J Infect Dis 168: 1236, 1993 |
|
Witkin SS, Jeremias J, Toth M, Ledger WJ: Proliferative response to conserved epitopes of the Chlamydia trachomatis and human 60-kilodalton heat shock proteins by lymphocytes from women with salpingitis. Am J Obstet Gynecol 171: 455, 1994 |
|
Yi Y, Zhong G, Brunham RC: Continuous B-cell epitopes in Chlamydia trachomatis heat shock protein 60. Infect Immun 61: 1117, 1993 |
|
Witkin SS, Jeremias J, Neuer A et al: Immune recognition of the 60kD heat shock protein: Implications for subsequent fertility. Infect Dis Obstet Gynecol 4: 152, 1996 |
|
Bensuade O, Morange M: Spontaneous high expression of heat-shock proteins in mouse embryonal cells and ectoderm from day 8 mouse embryo. EMBO J 2: 173, 1983 |
|
Mincheva-Nilsson L, Baranov V, Yeung MM et al: Immunomorphologic studies of human decidua-associated lymphoid cells in normal early pregnancy. J Immunol 152: 2020, 1994 |
|
Heybourne K, Fu YX, Nelson A et al: Recognition of trophoblasts by T cells. J Immunol 153: 2918, 1994 |
|
Neuer A, Lam KN, Tiller FW et al: Humoral immune response to membrane components of Chlamydia trachomatis and expression of human 60 kDa heat shock protein in follicular fluid of in vitro fertilization patients. Hum Reprod 12: 101, 1997 |
|
Eschenbach DA, Buchanan TM, Pollock HM et al: Polymicrobial etiology of acute pelvic inflammatory disease. N Engl J Med 293: 166, 1975 |
|
Sweet SL, Mills J, Hadley WK et al: Use of laparoscopy to determine the microbiologic etiology of acute salpingitis. Am J Obstet Gynecol 13: 68, 1979 |
|
Taylor-Robinson D: T-mycoplasmas and infertility. Nature 248: 267, 1974 |
|
Baird DD, Weinberg CR, Voist LF et al: Vaginal douching and reduced fertility. Am J Public Health 86: 844– 850, 1996 |
|
Hager WO, Majmudar B: Pelvic actinomycosis in women using intrauterine contraceptive devices. Am J Obstet Gynecol 133: 60, 1979 |
|
Eschenbach DA, Harnisch JP, Holmes KK: Pathogenesis of acute pelvic inflammatory disease: Role of contraception and other risk factors. Am J Obstet Gynecol 128: 838, 1977 |
|
Stumpf PG, March CM: Febrile morbidity following hysterosalpingography: Identification of risk factors and recommendation for prophylaxis. Fertil Steril 33: 487, 1980 |
|
Pittaway DE, Winfield AC, Maxson W et al: Prevention of acute pelvic inflammatory disease after hysterosalpingography: Efficacy of doxycycline prophylaxis. Am J Obstet Gynecol 147: 623, 1983 |
|
Miller BR, Freundt EA, Black FT et al: Experimental infection of the genital tract of female grivet monkeys for Mycoplasma hominis. Infect Immunol 20: 248, 1978 |
|
Sweet RL, Gibbs RS: Infectious Diseases of the Female Genital Tract, p 399. 3rd ed. Baltimore: Williams & Wilkins, 1995 |
|
Westrom L, Iosif S, Svensson L et al: Infertility after acute salpingitis: Results of treatment with different antibiotics. Curr Ther Res 26 (Suppl 6): 752, 1979 |
|
Hillis SD, Joesoef R, Marchbanks PA et al: Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol 168: 1503– 1509, 1993 |
|
Falk V: Treatment of acute non-tuberculous salpingitis with antibiotics alone and in combination with glucocorticoids. Acta Obstet Gynecol Scand 44 (Suppl 6): 5, 1965 |
|
Young PE, Egan JE, Barlow JJ et al: Reconstructive surgery for infertility at the Boston Hospital for Women. Am J Obstet Gynecol 108: 1092, 1970 |
|
Novy MJ: Tubal surgery or IVF: Making the best choice in the 1990s. Int J Fertil 40: 292– 297, 1995 |
|
Anderson AN, Lindhard A, Loft A et al: The infertile patient with hydrosalpinges—IVF with or without salpingectomy? Hum Reprod 11: 2081– 2084, 1996 |
|
Donnez J, Casanas-Rouz F: Prognostic factors of fimbrial microsurgery. Fertil Steril 46: 200– 204, 1986 |
|
Novy MJ, Thurmond AS: Proximal tubal obstruction. In Schlaff WD, Rock JA (eds): Decision Making in Reproductive Endocrinology, pp 477–487. Boston: Blackwell Scientific Publications, 1993 |
|
Marana R, Quagliarello J: Proximal tubal occlusion: Microsurgery versus IVF—A review. Int J Fertil 33: 338– 340, 1988 |
|
Assisted reproductive technology in the United States and Canada: 1995 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry, Birmingham, Alabama. Fertil Steril 69:389–398, 1998 |
|
Neumann PJ, Gharib SD, Weinstein MC: The cost of a successful delivery with in vitro fertilization. N Engl J Med 331: 239– 243, 1994 |
|
Benadiva CA, Kligman I, Davis O, Rosenwaks Z: In vitro fertilization versus tubal surgery: Is pelvic reconstructive surgery obsolete? Fertil Steril 64: 1051– 1061, 1995 |
|
Hendry WF, Parslow JM, Stredouska J et al: The diagnosis of unilateral testicular obstruction in subfertile males. Br J Urol 54: 774, 1982 |
|
Witkin SS, Toth A: Relationship between genital tract infections, sperm antibodies in seminal fluid and infertility. Fertil Steril 40: 805– 808, 1983 |
|
Witkin SS, Kligman I, Bongiovanni AM: Relationship between an asymptomatic male genital tract exposure to Chlamydia trachomatis and an autoimmune response to spermatozoa. Hum Reprod 10: 2952– 2955, 1995 |
|
Witkin SS: Production of interferon gamma by lymphocytes exposed to antibody-coated spermatozoa: A mechanism for sperm antibody production in females. Fertil Steril 50: 498– 502, 1988 |
|
Witkin SS, Chaudhry A: Circulating interferon-γ in women sensitized to sperm: New mechanisms of infertility. Fertil Steril 52: 867– 869, 1989 |
|
Wolff H: The biologic significance of white blood cells in semen. Fertil Steril 63: 1143– 1157, 1995 |
|
Branigan EF, Muller CH: Efficacy of treatment and recurrence rate of leukocytospermia in infertile men with prostatitis. Fertil Steril 62: 580– 584, 1994 |
|
Grimarellou H, Tympanidis K, Bitos NA et al: Infertility and chronic prostatitis. Andrologia 16: 417– 422, 1984 |
|
Aitken RJ, Baker HWG: Seminal leukocytes: Passengers, terrorists or good Samaritans? Hum Reprod 10: 1736– 1739, 1995 |
|
World Health Organization. WHO Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. Cambridge, UK: Cambridge University Press. 1992 |
|
Yanushpolsky EH, Politch JA, Hill JA et al: Antibiotic therapy and leukocytospermia: A prospective randomized controlled study. Fertil Steril 63: 142– 147, 1995 |
|
Purvis K, Christiansen E: The impact of infection on sperm quality. J Br Fertil Soc 1: 31– 41, 1995 |
|
Witkin SS, Goldstein M: Reduced levels of T suppressor/cytotoxic lymphocytes in semen from vasectomized men: Relationship to sperm autoantibodies. J Reprod Immunol 14: 283– 290, 1988 |
|
Hillier SL, Rabe LK, Muller CH et al: Relationship of bacteriologic characteristics to semen indices in men attending an infertility clinic. Obstet Gynecol 75: 800– 804, 1990 |
|
Shalika S, Dugan K, Smith RD et al: The effect of positive semen bacterial and Ureaplasma cultures on in-vitro fertilization success. Hum Reprod 12: 2789– 2792, 1997 |
|
Erhart B, Chan PJ, Patton WC, King A: Oflaxacin: the next generation of antibiotic in sperm and embryo cultures for assisted reproductive technologies. Fertil Steril 69: 246– 251, 1997 |
|
Hill JA, Haimovici F, Politch JA et al: Effects of soluble products of activated lymphocytes and macrophages (lymphokines and monokines) on human sperm motion parameters. Fertil Steril 47: 460– 465, 1987 |
|
Kovalski NN, deLamirandi E, Gagnon C: Reactive oxygen species generated by human neutrophils inhibit sperm motility: Protective effect of seminal plasma and scavengers. Fertil Steril 58: 809– 816, 1992 |
|
Witkin SS, Viti D, David SS et al: Relation between antisperm antibodies and the rate of fertilization of human oocytes in vitro. J Assist Reprod Gen 9: 9– 13, 1993 |
|
Erbengi T: Ultrastructural observation on the entry of Chlamydia trachomatis into human spermatozoa. Hum Reprod 8: 416– 421, 1993 |
|
Close CE, Wang SP, Roberts PL et al: The relationship of infection with Chlamydia trachomatis to the parameters of male fertility and sperm autoimmunity. Fertil Steril 48: 880, 1987 |
|
Dan M, Samra Z, Siegel YI et al: Isolation of Chlamydia trachomatis from prostatic tissue of patients undergoing transurethral prostatectomy. Infection 19: 162, 1991 |
|
Rietmeijer CA, Judson FN, Van Hensbroek MB: Unsuspected Chlamydia trachomatis infection in heterosexual men attending a sexually transmitted disease clinic: Evaluation of risk factors and screening methods. Sex Transm Dis 18: 28, 1991 |
|
Witkin SS, Jeremias J, Grifo JA, Ledger WJ: Detection of Chlamydia trachomatis in semen by the polymerase chain reaction in male members of infertile couples. Am J Obstet Gynecol 168: 1457, 1993 |
|
Eggert-Kruse W, Rohr G, Demirakca T et al: Hum Reproduction 12:1464–1475, 1997 |
|
Clarke GN, Lopata A, McBain JC et al: Effect of sperm antibodies in males on human in vitro fertilization (IVF). Am J Reprod Immunol 8: 62, 1985 |
|
Munoz MG, Witkin SS: Autoimmunity to spermatozoa, asymptomatic Chlamydia trachomatis genital tract infection and T lymphocytes in seminal fluid from the male partners of couples with unexplained infertility. Hum Reprod 10: 1070, 1995 |
|
O'Brien RL, Fu YX, Crabfill R, Born W: Heat shock protein hsp60-reactive T cells: A large, diversified Tlymphocyte subset with highly focused specificity. Proc Natl Acad Sci USA 89: 4348, 1992 |
|
Hacker G, Adam D, Wagner H: Interaction between T cells and cells regulating IgG production. Immunology 84: 105, 1995 |
|
Witkin SS, Jeremias J, Bongiovanni AM, Munoz MG: Immune regulation in the male genital tract. Infect Dis Obstet Gynecol 4: 131, 1996 |
|
Taylor-Robinson D: Infections due to species of Mycoplasma and Ureaplasma: An update. Clin Infect Dis 23: 671, 1996 |
|
McCormack WM, Rosner B, Alpert S et al: Vaginal colonization with Mycoplasma hominis and Ureaplasma urealyticum. Sex Transm Dis 13: 67, 1986 |
|
Kovacs GT, Wescott M, Rusden J et al: Microbiological profile of the cervix in 1,000 sexually active women. Aust N Z J Obstet Gynecol 28: 216, 1988 |
|
Witkin SS, Kligman I, Grifo JA, Rosenwaks Z: Ureaplasma urealyticum and Mycoplasma hominis detected by the polymerase chain reaction in the cervices of women undergoing in vitro fertilization: Prevalence and consequences. J Assist Reprod Genet 12: 610, 1995 |
|
Gump DW, Moore M, Askikaga T: Lack of association between genital mycoplasmas and infertility. N Engl J Med 310: 937, 1984 |
|
Cassell GH, Younger JB, Brown MB et al: Microbiologic study of infertile women at the time of diagnostic laparoscopy: Association of Ureaplasma urealyticum with a defined subpopulation. N Engl J Med 308: 502, 1983 |
|
Rosenstein IJ, Morgan DJ, Sheehan M et al: Bacterial vaginosis in pregnancy: Distribution of bacterial species in different Gram stain categories of the vaginal flora. J Med Microbiol 44: 1, 1996 |
|
Moller BR, Taylor-Robinson D, Furr PM et al: Serological evidence that chlamydiae and mycoplasmas are involved in infertility of women. J Reprod Fertil 73: 237, 1985 |
|
Mardh PA, Westrom L, van Mecklenburg C et al: Studies on ciliated epithelia of the human genital tract.I. Swelling of the cilia of fallopian tube epithelium in organ cultures infected with Mycoplasma hominis. Br J Vener Dis 52: 52, 1976 |
|
Cracea E, Botez D, Constantinescu S et al: Ureaplasma urealyticum serotypes isolated from cases of female sterility. Zentralbl Bakteriol Mikrobiol Hyg 252: 535, 1982 |
|
Quinn PA, Shewchuk AB, Shuber J et al: Serologic evidence of Ureaplasma urealyticum infection in women with spontaneous pregnancy loss. Am J Obstet Gynecol 145: 245, 1983 |
|
Abele-Horn M, Wolff C, Dressel P et al: Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetrics patients, and gynecological patients with pelvic inflammatory disease. J Clin Microbiol 35: 1199, 1997 |
|
Rose BI, Scott B: Sperm motility, morphology, hyperactivation, and ionophore-induced acrosome reactions after overnight incubation with mycoplasmas. Fertil Steril 61: 341, 1994 |
|
Shalhoub D, Abdel-Latif A, Fredericks CM et al: Physiological integrity of human sperm in the presence of Ureaplasma urealyticum. Arch Andol 6: 75, 1986 |
|
Riedel HH, Langenbucher H, Mettler L: Significance of sperm bacteriology for the in vitro fertilization of human and mouse oocytes. J Reprod Med 31: 605, 1986 |
|
Montagut JM, Lepretre S, Degoy J, Rousseau M: Ureaplasma in semen and IVF. Hum Reprod 6: 727, 1991 |
|
Swenson CE, Toth A, O'Leary WM: Ureaplasma urealyticum and human infertility: The effect of antibiotic therapy on semen quality. Fertil Steril 31: 660, 1979 |
|
Herrmann B, Egger M: Genital Chlamydia trachomatis (Ct) infections in Uppsala County, Sweden 1985-1993: declining rates for how much longer? Sex Transm Dis 22: 253, 1995 |
|
Addiss DG, Vaughn ML, Ludka D et al: Decreased prevalence of Chlamydia trachomatis infection associated with a selective screening program in family planning clinics in Wisconsin. Sex Transm Dis 20: 24, 1992 |
|
Britten TF, DeLisle S, Fine D: STDs and family planning clinics: A regional program for Chlamydia control that works. Am J Gynecol Health 6: 24, 1992 |
|
Witkin SS, Inglis SR, Polaneczky M: Detection of Chlamydia trachomatis and Trichomatis vaginalis by polymerase chain reaction in introital specimens from pregnant women. Am J Obstet Gynecol 175: 165, 1996 |
|
Heine RP, Wiesenfeld HC, Sweet RL, Witkin SS: Polymerase chain reaction analysis of distal vaginal specimens: A less invasive strategy for detection of Trichomonas vaginalis. Clin Infect Dis 24: 985, 1997 |
|
Hook EW, Ching SF, Stephens J et al: Diagnosis of Neisseria gonorrhoeae in women by using the ligase chain reaction on patient-obtained vaginal swabs. J Clin Microbiol 35: 2129, 1997 |
|
Oh MK, Richey CM, Pate MS et al: High prevalence of Chlamydia trachomatis infections in adolescent females not having pelvic examinations: utility of PCR-based urine screening in urban adolescent clinic setting. J Adolesc Health 21: 80, 1997 |