Infectious Vaginitis
Authors
INTRODUCTION
Vaginal discharge is mainly composed of water with electrolytes, microorganisms, epithelial cells, and organic compounds such as fatty acids, proteins, and carbohydrates.19 Vaginal fluid is largely derived from serum transudate in vaginal beds that seeps from capillaries through intercellular channels. Smaller amounts of fluid are derived from Bartholin's glands, cervix, endometrium, and fallopian tubes. Cellular elements represent sloughed cells from cervical columnar and vaginal squamous epithelium. White blood cells are present only in small numbers, among women without vaginitis.20
Estrogen and the pH are two important factors that influence the types of bacteria present in the vaginal flora. Vaginal lactic acid content provides an acidic pH of less than 4.5 in adult women. Lactic acid is produced from the metabolism of Lactobacillus and by vaginal epithelial cells through the breakdown of glycogen. The low pH favors the growth of acidophilic organisms such as Lactobacillus, but it inhibits the growth of most other bacteria. Lactobacillus appears central in limiting the growth of other bacteria.6Lactobacillus are also capable of producing H2O2,21 which inhibits the growth of bacteria that do not contain catalase. The combination of a halide ion such as chloride present in abundance in the vagina with peroxidase, present in endometrial and vaginal fluid,22 and H2O2 produced by certain strains of Lactobacillus forms a potent inhibiting system for certain bacteria in the vagina23 and of HIV and other virus in vitro.9
Normal Vaginal Microorganisms
Between 5 and 10 microorganisms can be recovered form the vagina of women, and most of the focus has been on the number of bacteria recovered. Most studies have not controlled for the presence of BV or “intermediate” flora based on Gram stain,24 and depending on the population, a sizable and wide range of individuals have those vaginal conditions.25
Table 1 describes the frequency and concentration of microorganisms recovered by culture from the vagina of pregnant women, stratified by Gram-stain criteria.24Lactobacillus species was recovered from 96% of the normal group.23 Based on the mean log concentration, Lactobacillus was present at a concentration 10 times greater, at log 107 levels, than the concentration of the next microorganism, Gardnerella vaginalis, present at log 106 levels in about one half the patients.23Lactobacillus was present at a concentration of 90 to 100 times greater than the next most numerous microorganisms, Enterococcus species and Ureaplasma urealyticum. For one half of the women without G. vaginalis, Lactobacillus species accounted for 98% or more of the actual number of microorganisms, and in those with G. vaginalis, Lactobacillus accounted for about 90% of the number of microorganisms in the vagina of those with normal Gram-stain results and Lactobacillus-dominant flora. From these results, it is possible to appreciate the total dominance of Lactobacillus species in the vagina of women with normal vaginal flora. In this normal flora, only 1% to 5% of the concentration consists of potentially pathogenic aerobic bacteria and mycoplasmas such as Staphylococcus aureus, group B streptococci, E. coli, or some of the potentially pathogenic anaerobes.
TABLE 1. Frequency and Logarithmic Concentration of Selected Microorganisms in the Vagina Stratified by Gram-Stain Pattern
| Frequency (Logarithm Concentration) | |||
| Normal | Intermediate | BV |
|
Microorganism | (n = 85) | (n = 47) | (n = 39) | P |
value |
|
|
|
|
Total Lactobacillus | 96 (107.0) | 85 (106.6) | 67 (106.3) | <0.001 |
H2O2-positive Lactobacillus | 61 (107.2) | 40 (106.5) | 5 | <0.001 |
Gardnerella vaginalis | 46 (106.0) | 79 (106.5) | 92 (107.7) | <0.001 |
Group B streptococci | 15 (104.2) | 17 (104.1) | 21 (105.8) | 0.2 |
Escherichia coli | 17 (104.1) | 15 (103.0) | 21 (104.6) | 0.6 |
Mycoplasma hominis | 15 (103.5) | 38 (104.8) | 61 (105.2) | <0.001 |
Ureaplasma urealyticum | 78 (105) | 91 (105) | 92 (105) | 0.01 |
Anaerobic gram-negative rod | 91 (104.3) | 89 (105.0) | 100 (106.0) | 0.1 |
>105 CFU/ml | 33 | 45 | 62 | 0.002 |
Prevotella bivia disiens | 61 (104.1) | 68 (104.6) | 77 (105.5) | 0.1 |
>105 CFU/ml | 15 | 21 | 39 | 0.005 |
Bacteroides ureolyticus | 36 (103) | 40 (103.5) | 59 (104.0) | 0.01 |
Mobiluncus sp. | 5 | 13 | 28 | 0.004 |
Fusobacterium nucleatum | 8 (103.3) | 19 (102.9) | 21 (103.8) | 0.05 |
Peptostreptococcus sp. | 91 (108.2) | 91 (104.9) | 90 (105.3) | 0.7 |
>105 CFU/ml | 26 | 47 | 59 | 0.002 |
Candida albicans | 31 | 17 | 21 | 0.4 |
CFU/ml, colony forming unit per milliliter of vaginal fluid; BV, bacterial vaginosis.
Data from Hillier SL, Krohn MA, Rabe LK et al. The normal vaginal flora, H2O2-producing lactobacilli and bacterial vaginosis in pregnant women. Clin Infect Dis 1993;16(Suppl 4):S273.
The prevalence of Lactobacillus is significantly decreased in BV cases in which Lactobacillus species are no longer the dominant microorganisms. In BV, the concentration of G. vaginalis (present in 92% of patients) is found at a mean log concentration of 107.7.23 In BV, the prevalence of Lactobacillus decreases, H2O2-positive Lactobacillus virtually disappears, and the concentration of anaerobic gram-negative rods and Prevotella species increase. With BV, Lactobacillus comprises 1% or less of the number of bacteria present (Table 1).
Women in this report with intermediate flora had a similar prevalence and concentration of Lactobacillus and G. vaginalis, and about 10% of the remaining microorganism consisted of Enterococcus, U. urealyticum, and anaerobic gram-negative rods.23 A surgical procedure contaminated with an overwhelming predominance of nonpathogenic Lactobacillus would be expected to cause considerably less infection than ones in which higher concentrations of pathogenic bacteria exist.
Mechanisms of Vaginal Infection
When the complex balance of microorganisms changes, potentially pathogenic endogenous microorganisms that are part of the normal flora, such as Candida albicans in cases of candidiasis and G. vaginalis and anaerobic bacteria in cases of BV, proliferate to a concentration that causes symptoms. Little is known about factors that contribute to the overgrowth of normal flora. Pathogenic exogenous sexually transmitted microorganisms such as Trichomonas vaginalis, N. gonorrhoeae, and C. trachomatis can also cause infection.
DIAGNOSIS
Diagnosis of vaginitis cannot be based solely on the presence or absence of symptoms. A wide range of symptoms occurs among women with vaginitis that provides great overlap with the symptoms that occur in women with no infection or vaginitis. Physical and laboratory parameters and not symptoms must be used to establish a diagnosis of vaginitis by physicians. Except for certain individuals with typical and well-spaced symptoms of candidiasis, self-diagnosis is even more inaccurate.3
The diagnosis of vaginitis is largely based on microscopic criteria. The specificity is virtually 100% when trichomonads, clue cells, or hyphae are detected. However, the sensitivity of identifying any of these three microscopic features by wet mount is only about 80% under ideal circumstances26 and is much lower with recurrent candidiasis and with inexperienced microscopists. Syndromal diagnosis and treatment is inaccurate and not acceptable in any modern medical setting. If a diagnosis cannot be established with certainty, selected cultures and a repeat examination should be performed several days later, at least for symptomatic women with suspected vaginitis to make a specific diagnosis and for symptomatic women with a presumed normal discharge to further exclude vaginitis. Two examinations with normal findings theoretically raise the diagnostic accuracy in both categories to 96% (80% + [0.80 × 20%]). The use of a repeat examination reduces the physician's tendency to exclude vaginitis after one “normal examination” for symptomatic women with infection and can provide reassurance for symptomatic women with normal findings on both examinations that no infection is present.
PHYSICAL EXAMINATION
Vulvar Appearance
The vulva should be inspected for the geographic erythema or fissures that can occur with candidiasis and contact dermatitis, for the white epithelium of lichen sclerosis, for hypertrophic epithelia of neurodermatitis, and for other lesions (e.g. warts, cysts, cancer). A thin vaginal discharge is often present at the introitus of women with trichomoniasis or BV. Patients with excessive vulvar tenderness should be scrutinized for vestibulitis, particularly if they have pain with penetration during intercourse. In vestibulitis, the vestibular area at 4 and 8 o'clock just external to the hymeneal ring is typically red and tender to even slight touch.27
Appearance of Vaginal Discharge
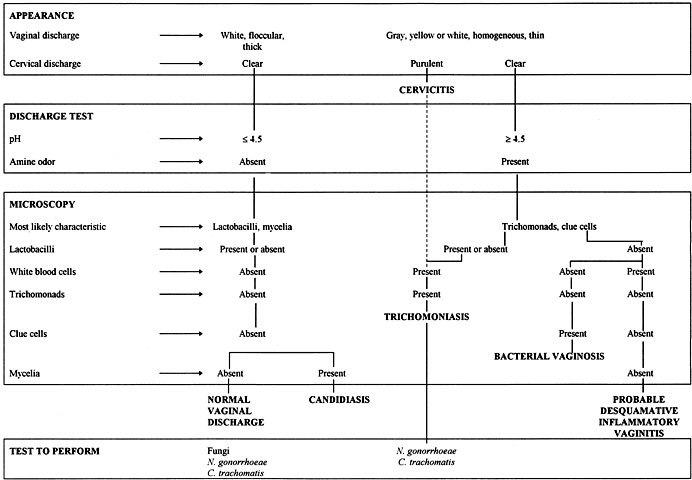
Normal vaginal discharge is usually white and clumpy, and it pools in the vagina. In contrast, the discharge from BV is gray and homogeneous (i.e. watery appearance of skim milk), and it is present on the anterior and lateral vaginal walls.20 Candida can cause a “cottage cheese” plaque on the vaginal wall,4 and trichomoniasis often causes a purulent discharge.28 The characteristics of vaginal discharge are sufficiently different to be helpful in an initial classification attempt (Fig. 1). However, a specific diagnosis usually cannot be made solely on the appearance of the discharge, because most patients do not have a “typical” appearance.
Cervical Appearance
During the early estrogen-dominant phase of the menstrual cycle, a clear mucous endocervical discharge is normal. In the later progesterone phase of the cycle, cervical mucus is thick, scant, or not visible. Vaginal discharge on the ectocervix needs to be wiped from the cervical portio with a cotton swab to examine for a purulent appearance from vaginal discharge. The presence of a purulent discharge in the endocervical canal should prompt a diagnosis of cervicitis. N. gonorrhoeae and C. trachomatis are present in about one half of the women with cervicitis.18 With cervicitis, endocervical bleeding may occur from swabbing the cervix because of excessive friability of the columnar epithelium.
Office Analysis
pH
Simple office analysis of the vaginal discharge is helpful and inexpensive. Results allow the placement of patients in one of the two major diagnostic categories of normal discharge/candidiasis if the pH is normal or BV/trichomoniasis/desquamative vaginitis if the pH is elevated (Fig. 1). The pH paper should have a range between 4 and 6. The pH should be tested by placing a drop of the vaginal discharge on pH paper or rubbing the paper on the vaginal wall. Cervical mucus must be avoided, because it has a basic pH. A normal pH virtually excludes BV.
AMINE ODOR.
A drop of 10% potassium hydroxide (KOH) mixed with normal vaginal fluid on a glass slide does not produce an odor.29 A fishy trimethylamine odor occurs in women with BV and in many women with trichomoniasis. The fish odor is caused by the volatilization of mostly trimethylamine, which is a byproduct of anaerobic metabolism.
Microscopic Analysis
An approximately 1:4 ratio of vaginal discharge to normal saline is mixed on a glass slide and covered with a coverslip to make a saline wet mount. A larger 2:1 ratio of vaginal discharge to 10% KOH is mixed, the amine odor is smelled, and the sample is covered with a coverslip to make the KOH wet mount. The microscopic examination should be performed within a few minutes of preparing the slide. Based on the previous appearance and chemical determinations, the most likely characteristic to be identified by microscopy is sought (Fig. 1). The most likely features in patients with a pH 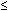 4.5 and no amine odor is Lactobacillus, an indication of normal flora or hyphae in patients with candidiasis. In contrast, the microscopists should search for clue cells, trichomonads, small bacteria morphotypes, and white blood cells (WBCs) in patients with a pH >4.5 and an amine odor. Systematic analysis of several microscopic features is the key to an accurate diagnosis.
4.5 and no amine odor is Lactobacillus, an indication of normal flora or hyphae in patients with candidiasis. In contrast, the microscopists should search for clue cells, trichomonads, small bacteria morphotypes, and white blood cells (WBCs) in patients with a pH >4.5 and an amine odor. Systematic analysis of several microscopic features is the key to an accurate diagnosis.
WHITE BLOOD CELLS.
A few WBCs can be present in the vagina as a result of physiologic cervical discharge, particularly premenstrually, but the number normally does not exceed the number of vaginal epithelial cells. A large number of WBCs suggests trichomoniasis, cervicitis, or occasionally candidiasis. Women with desquamative inflammatory vaginitis (DIV) also have a large number of WBCs.
LACTOBACILLUS MORPHOTYPES.
The discharge of women with candidiasis or a normal discharge usually contains a predominance of large rods, which are gram positive if stained. These large rods can be seen on wet mount preparation and represent lactobacilli. They are usually decreased in number or absent in patients with BV, trichomonal infection, or DIV.
SHORT BACTERIA MORPHOTYPES.
In contrast to patients with normal Lactobacillus flora, those with BV, trichomonal infection, and DIV usually have a predominance of cocci or small coccobacillary form and none or only a few Lactobacillus morphotypes. These small bacteria are particularly numerous in BV.
TRICHOMONADS.
The trichomonad is a motile, flagellated microorganisms that is slightly larger than a WBC. Fully motile trichomonads are easily identified by their characteristic undulating swimming motion. However, WBCs often inhibit their mobility, and in about 20% of women with trichomoniasis, motile trichomonads are not observed under low 100x power, but the beating flagella can be detected on stationary trichomonads under the 400x objective. About one half of women with trichomoniasis on culture have too few trichomonads to be detected by direct microscopy. Fortunately, most of these patients are asymptomatic.
CLUE CELLS.
The clue cell is a vaginal epithelial cell to which such a large number of bacteria attach that the cell border is obscured and has a serrated appearance. Clue cells are most objectively identified by observing the absence of a straight cell border through the 400x objective. In women with BV, 5% to 50% of the vaginal epithelial cells are clue cells.
HYPHAE.
Hyphae are identified on a 10% KOH wet mount. Hyphae have a characteristic branching appearance that can usually be identified in the 100x objective. The entire surface of the coverslip should be scanned, because even in symptomatic women, hyphae may be clumped in only one area of the slide. Buds from yeast can also be identified by experienced microscopists.
GRAM STAIN.
Vaginal Gram stains can be used in place of the wet mount to detect WBCs, predominant bacterial flora, and yeast forms. The Gram stain is not useful for detecting trichomonads. Patients with BV have a predominance of small gram-negative bacillus flora (e.g. Gardnerella spp., anaerobes) and a relative absence of large gram-positive bacillus (e.g. Lactobacillus morphotypes). A Gram stain is useful to identify normal, intermediate, and BV flora.24 The Gram stain is more sensitive than the wet mount to identify Candida.
Vaginal Cultures
Vaginal bacterial cultures are of limited benefit to diagnose vaginitis. Cultures should be used only in specific circumstances. Cervical tests for N. gonorrhoeae and C. trachomatis should be obtained for any woman with a purulent cervical exudate. Gonorrheal and chlamydial infections are also common among women with trichomoniasis. DNA detection tests (i.e. polymerase chain reaction or ligase chain reaction) are the most sensitive and available.30
Vaginal cultures for Candida organisms are useful for women with suspected candidiasis but normal KOH preparation results. Candida cultures should be obtained from women without hyphae on the KOH smear who have pruritus, an erythematous vulvar rash, vulvar fissures, or white vulvar plaques and from those unresponsive to antifungal medication.
Trichomonas cultures on Diamond's media can be obtained in cases of a purulent vaginal discharge when repeated microscopic examinations fail to identify the organism. Wet mounts identify only 50% to 70% of asymptomatic women with trichomoniasis.31 However, these cultures are not readily available in many clinical settings.
Vaginal cultures for G. vaginalis, other normal vaginal flora bacteria, or genital mycoplasmas are of almost no benefit to diagnose vaginitis. G. vaginalis are isolated from about 50% of asymptomatic women without vaginitis so their presence correlates poorly with vaginitis and BV.
PHYSIOLOGIC VAGINAL DISCHARGE
About 10% of women with complaints of a vaginal discharge have a physiologic increase in the amount of normal cervical mucus or normal vaginal fluid exudate. Women with a physiologic discharge usually have no vulvar abnormality and a white floccular (clumpy) vaginal discharge. The discharge is very thick, and it tends to pool in the inferior portion of the vagina. The vaginal and cervical epithelial surfaces are a normal pink color. The pH of normal discharge is usually less than 4.5, and there is no amine odor when KOH is applied (Fig. 1). The most striking microscopic finding is an abundance of vaginal epithelial cells and large rods representing normal gram-positive lactobacilli (Fig. 1). No clue cells, trichomonads, or mycelia are seen, and only a few WBCs and short rod bacteria are present on microscopy.
Cervical mucus can cause a large amount of discharge, particularly in women with a large surface area of cervical columnar epithelium. Such women typically have excessive discharge midcycle. Examination of the cervix at midcycle reveals a large amount of clear cervical mucus and usually a large area of columnar epithelium. Gram stain of the cervical discharge reveals only an occasional WBC. The microscopic appearance of vaginal discharge in these women is that of the physiologic vaginal discharge. Cervical gonococcal and chlamydial testing should be obtained to exclude their presence.
Women with a physiologic discharge should be reassured that the discharge is normal and that therapy is not needed. Symptomatic patients who still suspect they have infectious vaginitis should be reexamined in 1 to 2 weeks. Long-term use of tampons should be avoided to prevent vaginal ulceration. Although the practice should generally be discouraged, some women insist on douching, in which case, mild vinegar solutions or water should be used. However, the repeated drying effect of multiple douches may increase the amount of discharge, and douching with commercial preparations may cause abnormal shifts in vaginal flora.32 Douching should be actively discouraged because of its link with salpingitis.33 Antimicrobial regimens should not be administered to women with a physiologic vaginal discharge because of their ineffectiveness, propensity to cause candidiasis, cost, and most importantly because their failure to eliminate symptoms creates unneeded concern. Cryocautery, laser cautery, electrical cautery, and silver nitrate application treatment of a normal columnar epithelium usually are unnecessary for an excessive normal cervical discharge.
CANDIDIASIS
Candida albicans causes 80% to 90% of vaginal fungal infections, and other Candida species and Torulopsis cause the remainder.34 These saprophytic fungi can be isolated in small numbers from 5% to 20% of asymptomatic women. Symptoms usually only result when these organisms proliferate to large numbers.
Epidemiology
The true incidence of vulvovaginal candidiasis is unknown. Women who carry C. albicans in the genital tract can be asymptomatic or have symptoms from severe inflammation. Symptoms reflect the host immune response. Diagnosis without the benefit of microscopy or culture indicates that one half of women diagnosed with candidiasis instead have other conditions.1 By college age, about one half of women have one physician-diagnosed episode of candidiasis.35 Candidiasis increases after menarche, in part related to initiation of sexual activity.36,37 The rate of candidiasis is increased during pregnancy. Postmenopausal women given estrogen replacement can have candidiasis.
The frequency of intercourse is related to candidiasis.36,37 The number of sexual partners36 and oral-genital contact37 do not increase the rate of candidiasis. Candidiasis appears weakly related to high-dose estrogen contraceptive pills.38 Sexual intercourse with the use of nonoxynol-9 spermicide appears to increase colonization with Candida.39 Douching with commercial products appears to temporarily alter vaginal flora32 and intermittently has been associated with recurrent Candida infection.36,37
Antibiotics have been strongly related to candidiasis,40 and this association may be especially important for women with recurrent infection.41 It is not clear whether antibiotics kill bacteria such as Lactobacillus that may inhibit the growth of Candida or use other mechanisms.6
Diet may play little in the role of candidiasis.42 Hygiene practices, including frequent bowel movements, wipe direction after bowel movement, type of menstrual protection, underclothing fabric, and tight clothing appear to play no role in candidiasis.37 Immunosuppression from HIV infection is associated with C. torulopsis infection34 and increased rates of esophageal and perhaps vaginal C. albicans infection. Uncontrolled diabetes is associated with candidiasis, particularly with unresponsive infections.
Diagnosis
Recurrence of typical severe symptoms such as pruritus and vulvar irritation often represent candidiasis, but atypical or minimal symptoms are incorrectly self-diagnosed in about one half of cases.43 Besides the finding of a low pH and the microscopic findings previously discussed, cultures are necessary for women with suspected candidiasis for whom the microscopic results for Candida are negative. Among culture-positive women with vulvar symptoms of external dysuria, pruritus, swelling, or redness, the KOH wet mount was positive for only about 60%, leaving 30% of women undiagnosed by KOH.4
Uncomplicated candidiasis refers to sporadic infrequent episodes with mild to moderate symptoms in a normal, nonpregnant woman. Virtually all uncomplicated infections are caused by C. albicans. Complicated candidiasis refers to recurrent infection, infection with severe symptoms or infection in women who are pregnant, diabetic, or immunosuppressed. Many cases of complicated infection are caused by non-albicans species.
Treatment
INFREQUENT, UNCOMPLICATED ACUTE INFECTION.
Topical azole therapy remains the first choice to treat infrequent acute candidiasis. Azoles are fungistatic by their inhibition of ergosterol (and membrane) synthesis. Candida organisms are killed by the host lymphocytes through cell-mediated immune mechanisms. Only a limited fungicidal effect can be achieved by a high concentration of azoles that produce direct membrane damage. Topical azoles are effective, well tolerated, and relatively inexpensive. The products available include buconazole (Femstat), clotrimazole (Gyne-Lotrimin, Mycelex), miconazole (Monistat), and terconazole (Terazol). A wide range of doses in cream and suppository forms are available (Table 2). For some preparations, the treatment interval has been increased to twice daily or the dose has been increased from 100 to 200 mg while the length of medication was reduced concurrently from 7 to 3 days. Cure rates for the 3-day course have been equal to longer courses for uncomplicated candidiasis. Short-term (7- to 30-day) cure rates with topical azoles used for 3 to 7 days are usually 80% to more than 90%. There is no suggestion that the cure rates differ between various different azoles or between the suppository and the cream form.
TABLE 2. Recommended Azole Regimens for the Treatment of Vulvovaginal Candidiasis
Azole | Intravaginal Dose | Duration |
Butaconazole (Femstat) | 2% cream hs | 3 days |
Clotrimazole (Gyne-Lotrimin, Mycelex) | 100-mg tablet bid | 3 days |
| 100-mg tablet hs | 7 days |
| 1% cream hs | 7 days |
Miconazole (Monistat) | 200-mg suppositories hs | 3 days |
| 100-ms suppositories hs | 7 days |
Terconazole (Terazole) | 80-mg suppositories hs | 3 days |
| 0.4% cream hs | 7 days |
Fluconazole | 150-mg tablet | 1 dose |
I do not recommend single-dose therapy, because clinical experience suggests that 1-day courses are less effective than published reports indicate. This may be explained in part by the tendency to include women with mild symptoms of short duration and no prior candidiasis in the studies. Usually, only short-term (7- to 30-day) clinical cure rates are reported, and higher mycologic failure rates occur at 30 days when patients are given a single dose compared with a longer course of therapy.44 However, it is not certain that the increased rate of Candida recovery after treatment is related to increased rates of subsequent clinical candidiasis, and the duration of therapy may be of less importance in uncomplicated sporadic infection than in cases of complicated infection.
The oral azole fluconazole makes oral medication a safe choice for a vaginal candidiasis. A one-time 150-mg oral dose is effective for patients with mild to moderate symptoms.45 This drug has become popular for patients because vaginal cream is not used and for physicians because short-term use is without liver toxicity, a problem that prevented widespread use of ketoconazole. However, the dose often needs to be repeated in 4 to 5 days for very symptomatic patients because of high failure rates that otherwise exceed failure rates of local therapy.45 Physicians need to be aware of drug interaction between fluconazole and antihistamine drugs that prolong the QTc interval, oral hypoglycemics, Coumadin, and other drugs (consult a drug reference).46 Among nonimmunosuppressed patients, widespread use probably does not lead to resistance.
Topical polyene therapy consisting of nystatin has been largely replaced by topical azole treatment. Nystatin is well tolerated and inexpensive, but cure rates of 50% to 80% are lower than those for azoles. Nystatin is a second-line choice in treating uncomplicated candidiasis.
Boric acid capsules (600 mg or boric acid powder in a 0 size gelatin capsules) inserted twice daily for 14 days provides clinical cure rates similar to the topical azoles.47 The boron ion has not been detected in blood,48 and boric acid is inexpensive and well tolerated. However, boric acid can produce esophageal ulcers if inadvertently swallowed, and the preparation should be stored in bottles with childproof caps, kept in a locked medicine cabinet, and used with caution when small children are in the household. Because of unproven fetal safety, boric acid should not be used in pregnancy. Gentian violet treatment is effective for the treatment of candidiasis, but the staining of clothing and skin limits its use to the rare case unresponsive to other medication. Potassium sorbate and povidone iodine have limited effectiveness against candidiasis.
Local Candida therapy is usually well tolerated, and reactions are unusual. If increased vaginal irritation occurs with use, the medication should immediately stop and changed to a different preparation. Most of these irritations result from reactions to “inactive” compounds in the cream vehicle.
RAPIDLY RECURRENT INFECTION.
Patients with return of symptoms a few days after completing a course of medication usually have taken only a short course or been noncompliant. Many times, a longer course or different preparation suffices. Resistance of Candida to antifungal medication is uncommon, and some patients with continual symptoms have other diseases. Patients with a rapid recurrence need to have candidiasis documented by a KOH wet mount, culture, or both. Patients without objective evidence of Candida but with persistent symptoms should be scrutinized for vaginitis or vulvitis caused by other conditions. Neurodermatitis, lichen planus, lichen sclerosis, burning vulvar syndrome, and minor vestibular gland inflammation should be considered.
The small number of patients with drug resistance usually has a reduction of symptoms while on therapy but a rapid recurrence of symptoms after medication is stopped. C. albicans is almost uniformly sensitive to all azoles. However, resistance to azoles is more common among unusual fungi such as Candida glabrata, Candida tropicalis, or other non-albicans Candida species,49 and possible resistance should be considered if these fungi are present. Terconazole treatment may be tried because it has greater activity than other azoles against C. glabrata and C. tropicalis. Patients can be switched to the nonazole drugs, nystatin or boric acid. Boric acid is more effective than fluconazole for treatment of non-albicans vulvovaginal candidiasis.49,50 Gentian violet therapy may also be of benefit. In rare instances, none of the topical medications is effective in eliminating vaginal fungi, and nystatin or boric acid need to be given every 2 to 3 days indefinitely to suppress rather than eliminate resistant fungi.
Treatment failures have been so infrequent for candidiasis that systematic studies have not been performed to determine the causes of treatment failure. The follow-up period has been too short (30 days or less) to accurately assess the frequency of failures. Compliance failure, pseudofailure due to a reaction to vehicle, and drug resistance have been mentioned. Two other prominent theories of treatment failure require discussion.
The first theory is that recurrent vaginal candidiasis results from gastrointestinal tract Candida. Candida organisms are found in higher rates in the oral cavity and gastrointestinal tract of patients with candidiasis than controls.51 However, most patients carry Candida in the gastrointestinal tract without developing vaginal candidiasis, and in longitudinal studies, vaginal candidiasis has not been related to gastrointestinal Candida. Oral nystatin therapy has provided mixed results. Oral nystatin has not had a dramatic effect, although a slight statistically significant reduction in subsequent Candida vulvovaginitis occurred in the group receiving oral nystatin compared with placebo.52 Eating 4 ounces of lactobacilli-containing yogurt twice daily has also provided a modest reduction in recurrence.53
The second theory involves sexual transmission of recurrent candidiasis. Male sexual partners of patients with vaginal candidiasis have higher rates of genital colonization than controls.54 Sexual transmission probably does occur for the small number (estimated to be 10% to 15%) of males who have Candida balanitis concurrent with their partner's vaginal candidiasis. However, only 20% of male sexual partners are colonized with Candida, and treatment of males has not decreased vaginal candidiasis for the female. Most males appear passively colonized by the female rather than play a major role in recurrent vaginal candidiasis.
ADJUNCTIVE TREATMENT.
Temporary reduction of vulvar symptoms occurs with a sitz bath followed by superdrying of the skin with a hairdryer using the low heat setting. Topical azole creams can also be prescribed for direct vulvar use, but little information is available on its effectiveness.
A diet to reduce high carbohydrate levels has been of some benefit for a subset of patients who developed candidiasis shortly after ingesting an unusually high amount of sugar. Among diabetics, candidiasis treatment is usually not successful until glucose levels are controlled. The avoidance of foods made from yeast has been popularized, but scientific documentation of effectiveness is lacking. Yeast organisms in food are not the same species that cause vaginitis. The yeast that cause vaginitis are so prevalent in the environment and on the skin that is difficult to believe that a yeast-free diet influences vaginal candidiasis. Cessation of antibiotic therapy can be considered, when possible, in a small number of patients on chronic antimicrobials. Cessation of oral contraceptive therapy is controversial and probably of limited benefit. Abandonment of tight, poorly ventilated clothing is probably of little benefit.
CHRONIC RECURRENT INFECTION.
A significant subset of patients have been identified with chronic or frequently recurrent (at least four annual) episodes of vaginal candidiasis. These patients accumulate to represent a significant number of patients with vaginal candidiasis attending certain clinics.
It is not clear whether patients with chronic recurrent candidiasis have a limited immunologic defect in which lymphocytes do not kill Candida organisms in the vagina or other reasons for recurrence. Recurrence is not related to unusual or drug-resistant strains of Candida, and few patients are diabetic or taking oral contraceptives, immunosuppressive drugs, or antibiotics. Women with frequent recurrences of vaginal candidiasis or candidiasis that do not respond to treatment should be considered for HIV testing. However, few women with recurrent candidiasis have HIV or any other commonly recognized factor that predisposes patients to candidiasis.
Symptomatic disease often is difficult to manage, but an important breakthrough was chronic suppressive therapy similar to that used for urinary tract infection. The initial study used a therapeutic dose of 400 mg of oral ketoconazole for 14 days, followed by a maintenance dose for 6 months.55 Potential liver toxicity and expense limits ketoconazole use, and the 14-day regimen now should consist of fluconazole given every 4 days or vaginal azole. In a controlled study, ketoconazole produced a recurrence rate at 6 months of 70% for those on placebo and 5% for those on daily ketoconazole.55 Practical maintenance regimens include biweekly or weekly topical azole administration.56 Similar results are achieved using oral itraconazole or fluconazole as maintenance or topical boric acid.57 Recurrence of vaginal candidiasis may resume at the same rates as before suppression when maintenance therapy ceases.55
TREATMENT IN PREGNANCY.
Systemic absorption of azoles or nystatin from the vagina is so limited that both can be safely used during all trimesters of pregnancy. Boric acid, fluconazole, itraconazole, and ketoconazole should not be used during pregnancy. Compared with nonpregnant women, candidiasis in pregnancy is more resistant to treatment, is more likely to relapse, and responds better to 7- or even 14-day therapy compared with 1- to 3-day therapy.
TRICHOMONIASIS
T. vaginalis is a ubiquitous, sexually transmitted, anaerobic parasite. T. vaginalis has been associated with vaginitis, atypical cytologic smears, and other sexually transmitted infection. Up to one half of women with gonorrhea also have trichomoniasis.58 Inflammation caused by Trichomonas may increase the transmission of HIV twofold to threefold.59 An estimated 200 million people are infected worldwide. There has been a gradual decline in the number of people treated for trichomoniasis in the United States from the 1970s,60 perhaps related to metronidazole treatment of BV. However, only 50% of women with trichomonads have symptoms.61
T. vaginalis is exclusively transmitted by sexual intercourse. The organism exists in the vagina, urethra, bladder, and Bartholin and Skene glands. Infection is most common in young, sexually active women, particularly with a new partner.7 Although trichomonal infection is unusual in college students, it was recovered from more than 12% of pregnant urban, mostly low socioeconomic women.62 More than two thirds of females and up to 60% of males have trichomoniasis when their sexual partner was found infected.63,64 A significant number of men carry the organism for a prolonged time, although some men are cured without treatment. Male contacts are particularly likely to harbor trichomonads if they are examined within 6 days of their last exposure, but 33% of male contacts carried trichomonads 6 to more than 60 days after the last exposure.65 The organism was present in 14% to 60% of male contacts of women with trichomoniasis in 19 studies, which confirms sexual transmission and the need to treat male partners.66
T. vaginalis elicits a cellular and humoral immune response. However, these responses do not protect against repeated infection, which are common. The polymorphonuclear leukocyte response to T. vaginalis is often intense and is related to the number of organisms.67
Diagnosis
Symptomatic women characteristically complain of a profuse, malodorous, and often uncomfortable vaginal discharge that causes internal and external dysuria.28 A sense of vulvar-vaginal fullness and lower abdominal tenderness may also be present. Symptoms typically exacerbate around menses.
On examination, the vulva may be slightly erythematous and edematous. The vulva and vagina may be covered with a green or yellow, purulent-appearing, frothy, and odorous discharge.28 The classic discharge occurs in only about one third of women. Small areas of subepithelial hyperemia of the vagina and cervix usually require colposcopy for identification, but the areas are occasionally identified on the cervical epithelium (i.e. strawberry cervix) by the naked eye. This finding is specific for trichomoniasis.28,67
Simultaneous sexually transmitted infection is common in women with trichomoniasis. Concomitant cervical mucous and uterine and abdominal tenderness are frequently found in women with trichomoniasis, and if these signs are present, the women need to be tested for other infections such as gonorrhea and chlamydia. However, T. vaginalis is infrequently recovered from the fallopian tubes of women with salpingitis, and it does not appear to cause upper genital tract infection in nonpregnant women. An exception may exist during pregnancy, because an increased risk of preterm delivery occurred in women with trichomoniasis at 23 to 26 weeks of pregnancy independent of other genital infection.68
The diagnosis of trichomoniasis is based on laboratory testing. Women with trichomoniasis often have an elevated vaginal pH, amine odor, and colonization with Gardnerella and Bacteroides species.25 In clinical practice, infection is usually demonstrated by finding motile trichomonads in vaginal discharge by saline wet mount microscopy.31 Various stains for trichomonads on a slide preparation are generally less sensitive than the wet mount, and although the polymerase chain reaction method is more sensitive than the wet mount,69 it is too expensive for routine use. Reports of trichomonads on Papanicolaou (Pap) smears should prompt confirmation with wet mount or culture because of a high false-positive rate with Pap smear identification.31 Cultures should be limited to women with unidentified chronic symptoms and a negative wet mount result. Cultures identify 50% more women with trichomoniasis than wet mount alone.31 About one half of the women with trichomoniasis have a low concentration of organisms, but fortunately, most of these women have few or no symptoms. Cultures are useful in research settings, and Diamond's media performs better than other media.70
Treatment
Metronidazole, a 5'-nitroimdiazole, is the only effective drug approved for the treatment of trichomoniasis in the United States. Oral therapy provides adequate levels of medication for vaginal, periurethral, urethral, and bladder infection. T. vaginalis contains ferredoxins, low redox potential proteins that play a major role in the metabolism of anaerobic microorganisms such as T. vaginalis. The 5'-nitroimidazoles require reduction of the nitro group to kill, which oxidizes DNA, causing damage of the DNA and subsequent cell death. Aerobic conditions interfere with this reduction process and reduces metronidazole effectiveness.71T. vaginalis is usually sensitive to metronidazole at a less than 1 μg/ml concentration under anaerobic conditions but at higher concentrations under aerobic conditions.71 Metronidazole in the single 2-g dose produces peak serum levels of 40 μg/ml. The serum half-life of metronidazole is about 8 hours.72 Treatment results usually reflect in vitro susceptibility,73 and the unusual patient who fails therapy with standard doses of metronidazole have increased resistance to metronidazole in vitro.74
Recommended treatment consists of 2 g of metronidazole given as a stat one-time dose or 500 mg of metronidazole given twice daily for 7 days (Table 3). A 250-g, three-times-daily regimen for 7 days has been most frequently studied but the half-life of metronidazole supports the use of the 500-mg-dose, twice-daily regimen. Because the stat and the 7-day regimens are equally effective for the treatment of uncomplicated trichomoniasis, the stat dose is preferred because of increased compliance over the 7-day regimen. Cure rates higher than 95% are usually reported with either regimen,75 particularly when male sexual partners also receive treatment.76
TABLE 3. Recommended Regimens to Treat Trichomoniasis
Uncomplicated Infection | Metronidazole Dose | Administration | Duration* |
Recommended | 2 g | Oral | Single dose |
Alternative, or for a repetitive failure | 500 mg | Oral | bid for 7 days |
Documented treatment failure | 1 g | Oral | bid for 7---14 days |
| plus 500 mg | Vaginal | bid for 7---14 days |
*Sexual partner should be concomitantly treated.
Nausea, the most common side effect, occurs in about 10% of patients after a single 2-g dose.75 A metallic taste, headache, dizziness, and dark urine also occur with metronidazole use. Patients should be advised to not drink alcohol for 24 hours after the last metronidazole dose because a disulfiram-like effect that produces nausea when alcohol is concomitantly ingested. Metronidazole may prolong the thrombin time of Coumadin users.77
Mutagens have been identified in the urine of women taking metronidazole. Metronidazole is carcinogenic in animals,78 and it must be considered a weak carcinogen capable of producing DNA damage, which has produced concern of increased rates of cancer among former metronidazole users. Two relatively small retrospective studies showed no relation between metronidazole and cancer.79,80 Rates of some cancers were slightly increased among metronidazole users compared with controls, although with relative risks less than 2. However, the studies are too small to detect an effect at less that a twofold increased risk, and weak carcinogens often are associated with risk ratios lower than 2. Data are also incomplete because of short follow-up compared with up to a 30-year latency period of clinical cancer. Physicians must remain concerned about the potential carcinogenic effect of metronidazole.
SEXUAL PARTNER THERAPY.
Concomitant male sexual partner therapy is recommended to prevent reinfection. Males may often resist therapy because they are usually asymptomatic. Female cure rates are increased by 10% to 25% when the male is also treated. A 97% cure rate has been reported with the 2-g and the 7-day regimen when incarcerated women were treated without reexposure to male partners.81,82 Male therapy also reduces spread of trichomonal infection to other females.
TREATMENT OF ASYMPTOMATIC WOMEN.
Asymptomatic women who are found to have trichomoniasis should be offered therapy to reduce sexual transmission. Therapy should also be given to patients with atypical inflammatory Pap smears and T. vaginalis.
TREATMENT FAILURE.
A possible compliance failure should be sought for patients who initially received the 7-day regimen. Because patients with persistent trichomoniasis may also have reinfection, they and their partners should be retreated with the 7-day regimen. Cure rates after retreatment remain high with standard treatment regimens.
Clinical resistance of T. vaginalis to metronidazole is reported in a small number of patients even after two to three times the usual dose of drug.74 A general correlation exists between in vitro susceptibilities and clinical cure rates, but clinical and laboratory efficacy do not correlate as well for T. vaginalis as for bacterial susceptibility testing. Nevertheless, the mean in vitro susceptibility of T. vaginalis from women resistant to standard metronidazole therapy was about eight times higher than the susceptibility of standard isolates.74
Patients who fail to respond to the usual doses of metronidazole, have been compliant, and have not been reexposed to male partners should be treated with an increased dose and duration of drug. These cases have been infrequent, and a uniform treatment regimen has not been established. One regimen I found to be helpful is given in Table 3. In general, patients who failed standard therapy responded to a 2-g daily oral dose of metronidazole given with a 1000-mg intravaginal dose of metronidazole over 7 to 14 days. Most patients have considerable nausea when given 3 g or more of metronidazole daily, but they are usually able to complete treatment. Intravenous administration has been used for clinically resistant cases,83 but this regimen is expensive, toxic, and probably unnecessary because oral metronidazole is well absorbed. Infectious disease specialists should be consulted when high doses of metronidazole are required. Administration of 4 to 6 g or more of metronidazole daily has been associated with seizures and a potential of disabling peripheral neuropathy.84
Tinidazole, with a longer half-life than metronidazole, has also been used to treat resistant trichomoniasis85 at the usual dose for twice the standard duration. However, tinidazole is not available in the United States. Topical clotrimazole has been reported to treat trichomoniasis,86 but clinical experience suggests a low cure rate. Other preparations to consider for patients with resistant trichomoniasis or patients allergic to metronidazole include topical 6% paramycin87 and nonoxynol-9.88
TREATMENT IN PREGNANCY.
Treatment of trichomoniasis with metronidazole in pregnancy is controversial. Despite an association of T. vaginalis with prematurity,68 there is little evidence that the microorganism ascends into the uterus or placenta. Treatment during pregnancy is justified for symptoms but not to reduce prematurity. Metronidazole has been administered to a large number of pregnant women, and for a modest number with outcome data, metronidazole has not been associated with a recognized teratogenic or other adverse neonatal effects.89 However, metronidazole has a mutagenic and carcinogenic potential, and although the magnitude of these effects is not certain, there is reason to use metronidazole judiciously during pregnancy.
Several modifications of therapy are possible during pregnancy. Treatment should be avoided in the first trimester, when the neonate is most susceptible to the effects of drugs, and reserved for patients with moderate to severe symptoms in the second and third trimester. Clotrimazole may temporarily reduce symptoms, although cure is unusual. Asymptomatic or minimally symptomatic patients should not receive treatment during pregnancy, but they can receive a 2-g dose of metronidazole on the day of delivery. Levels of drug in breast milk are equal to serum levels, and breast-feeding can be delayed for 24 hours to limit levels present in breast milk.
BACTERIAL VAGINOSIS
BV is the most common cause of vaginal infection. BV appears to cause upper genital tract infection, which contrasts with other forms of vaginitis, and this trait further confirms the importance of BV. The cause of BV is far from clear. BV could be caused by an overgrowth of certain bacteria in the vagina, but it could also be caused by the failure of Lactobacillus to provide an inhibitory control over other vaginal flora. The end result is an overgrowth of potentially virulent microorganisms in the vagina that causes the increased vaginal discharge and odor and an increase in upper genital tract infection.
BV is common in most populations, but the prevalence of BV is population dependent. It appears to be particularly common in sexually transmitted disease (STD) clinics, as opposed to family planning or antenatal clinics; among women with complaints of vaginal discharge; in lower socioeconomic (particularly African-American) groups; and in subSahara Africa. The prevalence of BV ranges from a low of 4% in asymptomatic university students to 15% to 25% among unselected patients attending gynecology clinics and 30% to 60% in STD populations.90 In Uganda, 51% of almost 5000 rural women had BV.91 During pregnancy, the prevalence of BV is about 12% to 20%,14,92 and these numbers provide the range of BV in young sexually active U.S. women.
Epidemiology
The role of sexual transmission for the development of BV is unclear. In the original studies, inoculation of infected vaginal discharge from women with BV into volunteers produced the disease,16 but inoculation of G. vaginalis grown in the laboratory did not produce BV.93G. vaginalis is commonly isolated from the urethra of male sexual partners of women with BV.16,94 In prospective cohort studies, a new or multiple sexual partner increased the risk of developing BV.7,95,96 Concordance of BV in lesbian couples further suggests sexual transmission.97 However, meager data exist on other male urethral flora in sexual contracts of women with BV, and the microorganisms transmitted have not been identified. Treatment of male sexual partners of women with BV has had no impact on the subsequent development of BV in the woman.98,99,100
It is possible that some other indirect microbial mechanism involved with sexual intercourse leads to BV. For example, inhibition of Lactobacillus by semen or other bacteria not directly involved in BV during intercourse could also produce BV by removing the controlling influence of Lactobacillus. The absence of H2O2-producing Lactobacillus and no Lactobacillus organisms was a factor in the acquisition of BV.7 Other factors that disturb vaginal flora such as intrauterine device use95,101 and vaginal douching7 appear to increase the risk of BV. Recent antibiotic use,7,95 contraceptive use,7 and age have not been related to BV, although hormonal factors may play a role, because BV affects mainly reproductive-age women.102
Bacteriology
Normal vaginal flora consists predominately of Lactobacillus species that make up about 90%% to 95% of the total bacterial count.23Lactobacillus is present at concentrations of 105 to 108 colony-forming units/ml in women with a normal vaginal Gram stain,23 and most women with a Lactobacillus dominant flora have H2O2-producing Lactobacillus.21,23 In contrast to the women with a Lactobacillus-dominant flora, one third of women with BV have no Lactobacillus, and the remainder have lower concentrations of usually non-H2O2-producing Lactobacillus.21,23 Women with BV have an increase in the prevalence and concentration of G. vaginalis, selected gram-negative rod anaerobic bacteria, Mobiluncus species, and Mycoplasma hominis.23,101,103,104 In women with BV, the concentration of G. vaginalis is increased by 20- to 100-fold and the gram-negative anaerobes and M. hominis by 20- to 100-fold compared with those with a Lactobacillus-dominant flora.23,105 In concert with the high concentrations of G. vaginalis and anaerobes, Lactobacillus disappears or the concentration of Lactobacillus drops about 10-fold in BV.23,105 This increase in concentration of potentially virulent flora probably explains why BV is related to upper genital infection. In other settings, infection is directly related to the concentration of virulent microorganisms. The ability of these bacteria to invade in the setting of BV is probably potentiated by the production of several molecules through bacterial metabolism, including sialidase106 and protease, which can increase invasive properties, the ability to inhibit immune resistance by succinate,107 and the cleavage of secretory IgA by some strains of G. vaginalis108 and probably by other mechanisms.
Most women with a Lactobacillus-dominant flora have lactobacilli that produce H2O2, which can inhibit many bacteria, particularly catalase-negative bacteria that do not have the enzyme that detoxifies H2O2. The combined presence of H2O2 with a halide ion such as chloride and peroxidase produces even greater inhibition of bacteria and virus. Chloride from serum transudate and peroxidase are present in the vagina,22 and the production of H2O2 by Lactobacillus provides the third component for a relatively potent system that can kill microorganisms. In concentrations that are present in the vagina, the combined three components of this system inhibit bacteria and virus, including HIV in vitro.9
In contrast, women with BV usually have no H2O2-producing Lactobacillus and lack the inhibitory effect on other flora. The flora in BV is complex and perhaps acts in concert. For example, the inoculation of G. vaginalis or Mobiluncus sp. alone into the vagina did not cause signs of vaginitis in the grivet monkey model, but placing both of these bacteria in the vagina at the same time produced vaginal discharge.109 Treatment of BV with metronidazole, a drug that does not inhibit M. hominis, drops the prevalence of M. hominis back to levels observed in women without BV.110
The characteristic odor present in BV appears to be from the production of amines by the bacterial metabolism present in BV. Placement of KOH on vaginal discharge volatilizes the amines off their protein attachment at the alkaline pH. Women often complain of increase in the odor with intercourse when seminal fluid produces a similar temporary alkalinization of vaginal fluid. The amines found include trimethylamine, which probably produces most of the characteristic odor, and putrescine and cadaverine.111
Large increases in the concentration of succinate and other organic acids occur with BV.33 Succinate inhibits the chemotactic response of WBCs.107 Increased levels of endotoxin, sialidase, and mucinase could further enhance the ability of bacteria to invade through the cervix into the upper genital tract.106,112,113
Diagnosis
Women with BV complain of a vaginal odor and increased vaginal discharge.20 Pruritus and vulvar irritation are not symptoms of BV. Symptoms and signs are especially variable in BV, and the diagnosis can only be established by laboratory testing.
Clinical criteria for diagnosing BV include three of the following: pH higher than 4.5, homogeneous (skim milk) appearance, amine odor with the addition of KOH, and clue cells by microscopy.29 A normal pH 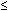 4.5 is a good negative predictor of BV, because a normal pH is found in less than 3% of women with BV.20 Clue cells alone are highly predictive of BV.114 Gram stain diagnosis of BV using a low number of Lactobacillus morphotypes (gram-positive rods) and a high number of G. vaginalis and anaerobic morphotypes (small gram-negative rods) also predicts BV.24 BV can be detected on a PAP smear115 and by a combination of pH and amines (FemCard), but other tests for BV are not clinically useful. Vaginal cultures, particularly for G. vaginalis, are especially misleading because of the 40% of women without BV who have G. vaginalis in the vagina.116
4.5 is a good negative predictor of BV, because a normal pH is found in less than 3% of women with BV.20 Clue cells alone are highly predictive of BV.114 Gram stain diagnosis of BV using a low number of Lactobacillus morphotypes (gram-positive rods) and a high number of G. vaginalis and anaerobic morphotypes (small gram-negative rods) also predicts BV.24 BV can be detected on a PAP smear115 and by a combination of pH and amines (FemCard), but other tests for BV are not clinically useful. Vaginal cultures, particularly for G. vaginalis, are especially misleading because of the 40% of women without BV who have G. vaginalis in the vagina.116
Complications
A high concentration of potentially virulent anaerobic bacteria may be expected to cause other infections. BV has been associated with a variety of upper genital tract infections (Table 4).
TABLE 4. Complications Associated With Bacterial Vaginosis
Complication | Approximate Relative Risk |
Preterm delivery | 1.5–2.0 |
Amniotic fluid infection | 2.0–2.5 |
Chorioamnionitis | 3 |
Histologic chorioamnionitis | 2.5 |
Postpartum endometritis |
|
After cesarean section | 6 |
After vaginal delivery | 2 |
After abdominal hysterectomy | 3.5 |
Postabortion Pelvic Inflammatory Disease | 2.5–3 |
PRETERM DELIVERY.
BV has been detected in 12% to 22% of about 16,000 pregnant women who participated in studies that examined the relation of BV with preterm delivery.92 The cohorts of pregnant patients came from a variety of socioeconomic backgrounds and countries. BV was more common than the combined numbers for gonorrheal, chlamydial, and urinary tract infections in a study that examined for each, and in this population, the population attributable risk of BV for preterm delivery was 6%.14 BV was consistently related to preterm delivery and low birth weight in these studies.92 BV was present in 25% to 35% of the group that delivered preterm, compared with 10% to 18% of those who delivered at term and the relative risk of preterm delivery for the BV group was 1.5 to 2.3 relative to those without BV.92
In the largest of these studies, which involved more than 11,000 patients, potential confounding variables were also examined. After controlling for demographic factors, smoking, prior preterm delivery, and the recovery of other potential pathogenic microbes from the vagina and cervix, BV remained associated with preterm delivery (RR = 1.6, 95% CI, 1.2–2.0).14 Women with BV who received antibiotics effective against BV had the same preterm delivery rate as those without BV, suggesting that treatment of BV may reduce preterm delivery.
BV can be expected to cause amniotic fluid infection if it is causally related to preterm delivery. BV is associated with an increased risk of amniotic fluid infection,117 chorioamnionic infection,118 and histologic chorioamnionitis.118 Bacteria associated with BV make up about one half of the isolates from amniotic fluid of women in preterm labor with intact membranes.119 At term, clinical amniotic fluid infection with fever in labor was 1.5 times more common in patients with than without BV.120
In two randomized, double-blind treatment trials of women at high risk for preterm delivery, treatment of BV with oral metronidazole led to a reduced rate of preterm delivery.121,122 In contrast, treatment of women at low risk for preterm delivery has not reduce preterm delivery,123,124 and additional studies are needed.
POSTPARTUM ENDOMETRITIS.
The relative risk of postpartum endometritis (PPE) after cesarean section among those with BV was 5.8 times as common as in those with a Lactobacillus-dominant flora.125 This high risk of PPE occurred after controlling for the duration of membrane rupture (which was adjusted for the duration of labor), suggesting that BV was related to PPE independent of the most important obstetric factor for such infection. About 60% of the bacteria recovered from the endometrium of women with PPE are bacteria associated with BV.126 BV is also associated with a twofold increased risk of PPE after vaginal delivery.127
VAGINAL CUFF CELLULITIS.
Vaginal cuff cellulitis occurs when vaginal bacteria contaminate the operative field and infect the serum-filled space between the vaginal cuff and peritoneum after hysterectomy. Vaginal cuff cellulitis occurs about four times more frequently among patients with BV than those with a normal Lactobacillus-dominant flora after abdominal hysterectomy.11,128 The attributable risk of BV in the development of cuff cellulitis was 69% in one study.11 A randomized treatment trial has not been done for patients with BV undergoing hysterectomy, but a single 2-g dose of tinidazole 12 hours before surgery significantly reduced postoperative cuff cellulitis after vaginal129 and abdominal hysterectomy.130 For practical purposes, women should be screened and treated for BV before surgery.
POSTABORTION PELVIC INFLAMMATORY DISEASE.
Spontaneous pelvic inflammatory disease (PID) is often caused by gonorrhea or chlamydial infections, and PID after induced abortion is related to Chlamydia.131 However, PID after induced abortion is also related to BV. Postabortion PID occurred significantly more commonly among those with than without BV (RR = 2.3).10 In a follow-up study, patients undergoing induced abortion with BV were invited to participate in a randomized, double-blind trial of placebo or metronidazole. Placebo-treated patients had three times the rate of postabortion PID as patients treated with metronidazole,132 which suggests that treatment should be given to all patients with BV undergoing induced abortion.
SPONTANEOUS PELVIC INFLAMMATORY DISEASE.
The role of BV in spontaneous PID is less certain. Randomly selected STD clinic patients with BV were more likely than those without BV to have adnexal tenderness even after controlling for gonorrheal and chlamydial infections, suggesting that BV might be related the PID.20 Among women sampled because of suspected PID, plasma cell endometritis was present in 65%.133 In this study, endometritis was significantly associated with the recovery of N. gonorrhoeae and C. trachomatis from the endometrium and with the recovery of anaerobic gram-negative rods from the endometrium (OR = 2.6).133 Because BV alone was not associated with endometritis, it may not be an risk factor for endometritis independent of gonorrheal and chlamydial infections, but the presence of anaerobic gram-negative rods in the endometrium may cause plasma cell endometritis independent of the other two STD bacteria. Many bacteria recovered from the tubes and especially from abscesses of women with PID are those associated with BV.134 However, the rate of symptomatic PID among women with BV may be low compared with the attack rate of PID in women with gonorrheal or chlamydial infections.
BV could be a factor in plasma cell endometritis. Among women with vaginal discharge or pelvic pain in an STD clinic, plasma cell endometritis was present in 45% of those with BV, compared with 5% of controls without BV.135 In another report, plasma cell endometritis was present in 40% of women with BV alone (without gonorrheal or chlamydial infections), compared with 13% of controls without infection.136 These combined data do not make a strong case for the treatment of BV in asymptomatic nonpregnant women to prevent spontaneous PID.
HUMAN IMMUNODEFICIENCY VIRUS INFECTION.
In vitro, H2O2-producing Lactobacillus, particularly together with a halide ion and peroxidase, can kill HIV.9 Women with BV generally lack H2O2-producing Lactobacillus23 and therefore could be at increased risk for the acquisition of HIV infection if exposed. Two cross-sectional studies found a higher rate of BV among women with HIV infection than those with a Lactobacillus-dominant flora.91,137 In a longitudinal study of Malawi women, BV diagnosed during pregnancy was associated with a significant increase in HIV seroconversion before (OR = 3.7) and after delivery (OR = 3.5).15 BV was weakly related to the HIV seroconversion rate of sex workers in Kenya.138 These data indicate that women with BV may have a higher rate of developing HIV infection. Treatment trials of BV are planned to determine whether treatment of BV influences the rate of HIV acquisition. These studies need to be watched carefully, because they could lead to treatment of asymptomatic nonpregnant women with BV.
Therapy
FIRST-LINE THERAPY.
Several antimicrobial regimens provide cure rates of 85% to 95%. These regimens include metronidazole (500 mg) taken orally twice daily for 7 days,98,139 2% clindamycin cream monthly for 7 days,140 and 0.75% metronidazole gel inserted twice daily for 5 days141 (Table 5). Local regimens are associated with fewer side effects because the dose of antibiotic is low compared with oral therapy.
TABLE 5. Treatment of Bacterial Vaginosis
Treatment | Regimen |
Recommended | Metronidazole, 500 mg bid for 7 days |
| 2% Clindamycin cream hs for 7 days |
| 0.75% Metronidazole gel bid for 5 days |
Alternative | Metronidazole, 2 g in a single dose |
| Clindamycin, 300 mg orally bid for 7 days |
OTHER CHOICES.
The two regimens that provide cure rates of 80% to 85% include metronidazole (2 g) taken orally at one time,98,139 300 mg of clindamycin taken orally for 7 days,142 and amoxicillin/clavulanic acid (500 mg) three times daily for 7 days.143
Third-line regimens have minimal efficacy in the treatment of BV. Ampicillin or amoxicillin (500 mg) taken orally in three to four times daily cures BV in only 30% to 45% of patients.143,144 Ciprofloxacin (500 mg) taken orally three times daily145 and triple sulfa vaginal cream116 used twice daily for 7 days provide cure rates of only 20% to 50%. Generally, these regimens should not be used.
Several drugs have been ineffective in treating BV. These agents include erythromycin,147 tetracycline, doxycycline,94 and several intravaginal products, including povidone-iodine,148 acetic acid gel, and Lactobacillus.149
MALE THERAPY.
BV has frequently been suspected of being a sexually transmitted condition, although no direct proof exists. Because recurrent infection is common, some investigators advocate treatment of male partners. There are four randomized trials in which metronidazole therapy of male sexual partners of women with BV had no effect on the subsequent recurrence of BV in the female.98,99,100 Male partner treatment should not be given in the usual treatment of BV.
FREQUENTLY RECURRENT BACTERIAL VAGINOSIS.
Cure rates are high when first- or second-choice drugs are used, but a small group of women have rapid or repetitive recurrent infection. The cause of recurrent infection has not been elucidated, but one possibility is resistant bacteria. Patients with rapidly recurrent infection should be empirically switched to the alternative antimicrobial agent, such as from metronidazole to clindamycin. For patients whose disease recurs after changing antimicrobials, I give an intravaginal preparation of metronidazole or clindamycin for 3 weeks, followed by intravaginal therapy every third day for 3 additional weeks. The latter part of the regimen is designed to inhibit bacteria causing BV while allowing Lactobacillus to recolonize in the vagina. A few patients who develop recurrences associated with intercourse benefit from taking a tablet of metronidazole with each episode of intercourse.
ATROPHIC VAGINITIS
Atrophic vaginitis is a symptomatic vaginal inflammatory condition caused by estrogen-deficient vaginal epithelium. Symptoms include vaginal bleeding and soreness, external dysuria, pruritus, and dyspareunia. The diagnosis of atrophic vaginitis can be confirmed by finding a smooth, pale pink vaginal surface without rugae and a predominance of parabasal cells on a vaginal smear. Moderate vulvar irritation may exist. The discharge is usually slight, with a pH higher than 5. Microscopy usually reveals an absence of organisms, including lactobacilli, although the bacteriology of atrophic vaginitis has not been well defined. Neoplasm, foreign bodies, and other infectious causes of the symptoms should be excluded. Atrophic vaginitis is treated with topical estrogens, which thicken the vaginal epithelium and reduce the symptoms. Estrogen cream can be inserted into the vagina once daily for 2 weeks and then every other day for 2 weeks. In most patients, estrogen therapy can be completely stopped without recurrence of symptoms. Because vaginal estrogens are readily absorbed, prolonged vaginal use of estrogen has the same disadvantages as systemic use in the postmenopausal woman, and therapy is not usually necessary beyond 4 weeks for most patients.
OTHER CAUSES OF VAGINAL DISCHARGE
Approximately 5% of women with vaginitis have one of a variety of heterogeneous vaginal conditions. Many women have DIV, characterized by symptoms of profuse irritation, vaginal discharge, vulvar irritation, and often pain with intercourse.17 Physical findings include a purulent vaginal discharge with a pH higher than 5 and patches of mucosal erythema in the introitus and in the top one third of the vagina.17 A large number of WBCs, parabasal cells, small bacteria, and no lactobacilli or clue cells are seen in the saline wet mount. The cause of DIV is unknown. Patients tend to have high concentrations of group B Streptococci or coliforms recovered from the vagina, but these bacteria may not be a primary cause of infection. Women with DIV may have the onset of symptoms with antibiotic therapy. They usually have normal immunity, although some have autoimmune diseases. I find that patients have responded to 2% clindamycin17 or 2% cephalexin cream nightly for 4 weeks, followed by every other night treatment for another 4 weeks. Alternatively, hydrocortisone suppositories (Anusol HC) can be alternated with the antibiotic cream intravaginally for 4 weeks in patients with suppressed immunity or autoimmune disease. Recurrence rates are moderately high with all regimens.
REFERENCES
Berg AO, Heidrich FE, Fihn SD. Establishing to cause of symptoms in women in a family practice. JAMA 1984;251:620 |
|
Hurley R, DeLorreocs J. Candida vaginitis. Postgrad Med J 1929;55:645 |
|
Nyirjesy P, Weitz V, Grody T, et al. Over-the-counter and alternative medicine in the treatment of chronic vaginal symptoms. Obstet Gynecol 1997;90:50 |
|
Eckert LO, Hawes SE, Stevens CE et al. Vulvovaginal candidiasis: clinical manifestation, risk factors, management algorithm. Obstet Gynecol 1998;92:757 |
|
McCormack WM, Sarbo KM, Zimmer SH. Symptoms associated with vaginal colonization with yeast. Am J Obstet Gynecol 1988;158:31 |
|
Redondo-Lopez V, Cook RL, Sobel J. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis 1990;12:856 |
|
Hawes SE, Hillier SL, Benedetti T et al. Hydrogen peroxide producing lactobacilli and acquisition of vaginal infection. J Infect Dis 1996;174:1058 |
|
Gupta K, Stapleton A, Hooton TM et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infection. J Infect Dis 1998;178:446 |
|
Klebanoff SJ, Coombs RW. Viricidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J Exp Med 1991;174:282 |
|
Larsson PG, Bergman B, Forsum U et al. Mobiluncus and clue cells as predictors of PID after first-trimester abortion. Acta Obstet Gynecol Scand 1989;68:217 |
|
Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol 1990;163:1016 |
|
Watts DW, Krohn M, Hillier SL, Eschenbach DA. Bacterial vaginosis as a risk factor for post cesarean endometritis. Obstet Gynecol 1990;75:52 |
|
Silver HM, Sperling RS, St. Clair PJ, Gibbs RS. Evidence relating bacterial vaginosis to intra-amniotic infection. Am J Obstet Gynecol 1989;161:808 |
|
Hillier SL, Nugent RP, Eschenbach DA et al. The association of bacterial vaginosis, Bacteroides and Mycoplasma hominis with preterm low birthweight delivery. N Engl J Med 1995;333:1737 |
|
Taka TE et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 1998;12:1699 |
|
Gardner HL, Dukes CD. Haemophilus vaginalis vaginitis. Am J Obstet Gynecol 1955;69:962 |
|
Sobel JD. Desquamative inflammatory vaginitis: a new subgroup of purulent vaginitis responsive to topical 2% clindamycin therapy. Am J Obstet Gynecol 1994;171:1215 |
|
Brunham RC, Paavonen JA, Stevens CE. Mucopurulent cervicitis: the ignored counterpart in women of urethritis in men. N Engl J Med 1984;311:1 |
|
Huggins GR, Preti G. Volatile constituents of human vaginal secretions. Am J Obstet Gynecol 1976;126:129 |
|
Eschenbach DA, Hillier S, Critchlow C et al. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol 1988;158:819 |
|
Eschenbach DA, Davick PR, Williams BL et al. Prevalence of hydrogen peroxide producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol 1989;27:251 |
|
Klebanoff SJ, Hillier SL, Eschenbach DA et al. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J Infect Dis 1991;164:94 |
|
Hillier SL, Krohn MA, Rabe LK et al. The normal vaginal flora, H2O2-producing lactobacilli and bacterial vaginosis in pregnant women. Clin Infect Dis 1993;16(Suppl 4):S273 |
|
Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol 1991;29:297 |
|
Hillier SL, Krohn MA, Nugent RP, Gibbs RS. Characteristics of three vaginal flora patterns assessed by Gram stain among pregnant women. Am J Obstet Gynecol 1992; 166:938 |
|
McLennon MT, Smith JM, McClennon CE. Diagnosis of vaginal mycosis, trichomoniasis: reliability of cytologic smear, wet smear and culture. Obstet Gynecol 1972; 40:231–34 |
|
Bergeron S, Binik YM, Khalife S et al. Vulvar vestibulitis syndrome: a critical review. Clin J Pain 1997;13:27 |
|
Wolner-Hanssen P, Krieger JN, Stevens CE et al. Clinical manifestation of vaginal trichomoniasis. JAMA 1989; 261:571 |
|
Amsel R, Totten PA, Spiegel CA et al. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiological association. Am J Med 1983;74:14 |
|
Stamm WE. Chlamydia trachomatis infection of the adults. In Holmes KK, Sparling PF, Mårdh PH, et al (eds). Sexually Transmitted Diseases, p 407. New York: McGraw-Hill, 1989 |
|
Krueger JN et al. Diagnosis of trichomoniasis: comparison of conventional wet mount examination with cytologic studies, cultures and monoclonal antibody staining, of direct specimens. JAMA 1998; 259:1223 |
|
Onderdonk AB, Kelandy NL, Henkism PL et al. Quantitative and qualitative effects of douche preparations in vaginal microflora. Obstet Gynecol 1992; 80:333 |
|
Wolner-Hanssen P, Eschenbach DA, Paavenen J et al. Association between vaginal douching and acute pelvis inflammatory disease. JAMA 1990; 263:1936 |
|
Spinillo A, Capuzzo E, Egbe TO et al. Torulopsis glabrata vaginitis. Obstet Gynecol 1995;85:993 |
|
Geiger AM, Foxman B, Gillespie BW. Epidemiology of vulvovaginal candidiasis among university students. Am J Public Health 1995;85:1146 |
|
Spinillo A, Pizzoli G, Colonna L et al. Epidemiologic characteristics of women with idiopathic recurrent vulvovaginal candidiasis. Obstet Gynecol 1993;81:721 |
|
Foxman B. Epidemiology of vulvovaginal candidiasis: risk factors. Am J Public Health 1990;80:329 |
|
Spinillo A, Capuzzo F, Nicola S et al. The impact of oral contraception on vulvovaginal candidiasis. Contraception 1995;51:293 |
|
Hooten TM, Roberts PL, Stamm WE. Effect of recent sexual activity and use of a diaphragm on vaginal flora. Clin Infect Dis 1994;19:274 |
|
Bluestein D, Rutledge C, Limsden L. Predicting the occurrence of antibiotic-induced candidal vaginitis. Fam Pract Res J 1991;11:319 |
|
Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 1985; 152:924 |
|
Reed BD. Risk factors for Candida vulvovaginitis. Obstet Gynecol Surv 1992;47:551 |
|
Ferris DG, Dekle C, Litaker MS. Women's use of over-the-counter antifungal pharmaceutical products for gynecologic symptoms. J Fam Pract 1996;42:595 |
|
Lebherz T, Guess E, Wolfson N. Efficacy of single versus multiple dose clotrimazole therapy in the management of vulvovaginal candidiasis. Am J Obstet Gynecol 1985; 153:965 |
|
Sobel JD, Brooker D, Stein GE et al. Single oral dose fluconazole compared with conventional topical therapy of Candida vaginitis. Am J Obstet Gynecol 1995;172:1263 |
|
Physicians Desk Reference, p 2379. Montvale, NJ: Medical Economics, 1999 |
|
Van Slyke KK, Michel VP, Rein MF. Treatment of vulvovaginal candidiasis with boric acid powder. Am J Obstet Gynecol 1988;95:920 |
|
Cooke CW. Vulvovaginal candidiasis. Obstet Gynecol 1976;48:631 |
|
Nyirjesy P. Seeney SM, Grody MHT et al. Chronic fungal vaginitis: the value of cultures. Am J Obstet Gynecol 1995;173:820 |
|
Sobel JD, Chaim W. Treatment of Candida glabrata vaginitis: a retrospective review of boric acid therapy. Clin Infect Dis 1997;24:649 |
|
Miles MR, Olsen L, Rigers A. Recurrent vaginal candidiasis: importance of an intestinal reservoir. JAMA 1977; 238:1836 |
|
Nystatin Multicenter Study Group. Therapy of candidal vaginitis: the effect of eliminating intestinal Candida. Am J Obstet Gynecol 1986;155:651 |
|
Hilton E, Isenbey HE, Alperstein P et al. Ingestion of yogurt containing Lactobacillus acidophilus on prophylaxis for candidal vaginitis. Ann Intern Med 1992;116 |
|
Horowitz BJ, Edelstein SW, Lippman L. Sexual transmission of Candida. Obstet Gynecol 1987;69:883 |
|
Sobel JD. Recurrent vulvovaginal candidiasis: a prospective study of the efficacy of maintenance ketoconazole therapy. N Engl J Med 1986;315:1455 |
|
Balsdon MJ, Tobin JM. Recurrent vaginal candidosis: prospective study of effectiveness of maintenance miconazole treatment. Genitourin Med 1988;64:124 |
|
Sobel JD. Treatment of recurrent vulvovaginal candidiasis with maintenance fluconazole. Int J Gynecol Obstet 1992;37:17 |
|
Vazquez F et al. Gonorrhea in women prostitutes: clinical data and auxotypes, serovars, plasmid contents of PPNG, and susceptibility profiles. Sex Transm Dis 1991;18:5 |
|
Wasserheit JN. Epidemiological synergy: interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis 1992;19:61 |
|
Centers for Disease Control and Prevention, Division of STD Prevention: Sexually Transmitted Disease Surveillance, 1995. Atlanta: U.S. Department of Health and Human Services, Public Health Services, September, 1996 |
|
Pabst KM et al. Disease prevalence among women attending a sexually transmitted disease clinic varies with reason for visit. Sex Transm Dis 1992;19:88 |
|
Cotch MF et al. Demographic and behavioral predictors of Trichomonas vaginalis infection among pregnant women. Obstet Gynecol 1991;78:1087 |
|
Whittington MJ. Epidemiology of infections with Trichomonas vaginalis in the light of improved diagnostic methods. Br J Vener Dis 1957;33:80 |
|
Watt L, Jennison RF. Incidence of Trichomonas vaginalis in marital partners. Br J Vener Dis 1960;36:163 |
|
Weston TE, Nicol CS. Natural history of trichomonal infection in males. Br J Vener Dis 1963;39:251 |
|
Krieger JN. Trichomoniasis in men: old issues and new data. Sex Transm Dis 1995;22:83 |
|
Krieger JN et al. Geographic variation among Trichomonas vaginalis isolates from women with and without colpitis macularis. J Infect Dis 1990;161:307 |
|
Cotch MF, Pastoreck JG, Nujent RP et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex Trans Dis 1997;24:353 |
|
Madico G, Quinn TC, Rompalo A et al. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swabs. J Clin Microbiol 1998;36:3206 |
|
Gelbart SM et al. Growth of Trichomonas vaginalis in commercial culture media. J Clin Microbiol 1990;28:962 |
|
Korner B, Jensen HK. Sensitivity of Trichomonas vaginalis to metronidazole, tinidazole, and nifurantel in vitro. Br J Vener Dis 1976;52:404 |
|
Robertson DH et al. Treatment failure in Trichomonas vaginalis infections in females: I. Concentrations of metronidazole in plasma and vaginal content during normal and high dosage. J Antimicrob Chemother 1988;21:373 |
|
Lossick JG. Treatment of Trichomonas vaginalis infections. Rev Infect Dis 1982;4:801 |
|
Lossick JG et al. In vitro drug susceptibility and doses of metronidazole required for cure in cases of refractory vaginal trichomoniasis. J Infect Dis 1986;153:948 |
|
Hager WD et al. Metronidazole for vaginal trichomoniasis: seven day vs. single dose regimens. JAMA 1980;244:846 |
|
Vejtorp M, Bollrup AC, Vejtorp L et al. Bacterial vaginosis: a double-blinded randomized trial of the effect of treatment of sexual partners. Br J Obstet Gynecol 1988;95:920 |
|
Kazmier FJ. A significant interaction between metronidazole and warfarin. Mayo Clin Proc 1976;51:782 |
|
Rustia M, Shobik P. Induction of lung tumors and malignant lymphomas in mice by metronidazole. J Natl Cancer Inst 1972;48:421 |
|
Beard CM, Noller KL et al. Cancer after exposure to metronidazole. Mayo Clin Proc 1988;63:147 |
|
Friedman GD. Cancer after metronidazole [Letter]. N Engl J Med 1980;302:519 |
|
Lossick JG. Single-dose metronidazole treatment for vaginal trichomoniasis. Obstet Gynecol 1980;56:508 |
|
Pereyra AJ, Lansing JD. Urogenital trichomoniasis: treatment with metronidazole in 2002 incarcerated women. Obstet Gynecol 1964;24:499 |
|
Dombrowski MP et al. Intravenous therapy of metronidazole-resistant Trichomonas vaginalis. Obstet Gynecol 1987;69:524 |
|
Frytak S, Moertel CG, Childs OS et al. Neurologic toxicity associated with high dose metronidazole. Ann Intern Med 1978;88:361 |
|
Hamed KA, Studemeister AE. Successful response of metronidazole-resistant trichomonal vaginitis to tinidazole: a case report. Sex Transm Dis 1992;19:339 |
|
Legal HP. The treatment of Trichomonas and Candida vaginitis with clotrimazole vaginal tablets. Postgrad Med J 1974;50:S81 |
|
Nyirjesy P, Sobel J, Weitz V. Difficult to treat trichomoniasis: results with paramycin cream. Clin Infect Dis 1998; 26:986 |
|
Livengood CH, Lossick JG. Resolution of resistant vaginal trichomoniasis associated with the use of intravaginal nonoxynol-9. Obstet Gynecol 1991 |
|
Burton P, Taddeo A, AriGierna O et al. Safety of metronidazole in pregnancy: a meta-analysis. Am J Obstet Gynecol 1995;172:525 |
|
Thomason JL, Gelbart SM, Scaglione NJ. Bacterial vaginosis: current review with indications for asymptomatic therapy. Am J Obstet Gynecol 1991;165:1210 |
|
Sewankambo N et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 1997;350:546 |
|
Eschenbach DA. Bacterial vaginosis. In Hitchock PJ, MacKay HT, Wasserheit JN (eds). Sexually Transmitted Diseases and Adverse Outcomes of Pregnancy, p 103. Washington, DC: American Society of Microbiology Press, 1999 |
|
Criswell BS, Ludwig CL, Gardner HL. Haemophilus vaginalis: vaginitis by inoculation from culture. Obstet Gynecol 1969;33:195 |
|
Pheifer TA, Forsyth MS, Durfee MA et al. Nonspecific vaginitis: role of Haemophilus vaginalis and treatment with metronidazole. N Engl J Med 1978;26:1429 |
|
Avonts D et al. Incidence of uncomplicated genital infections in women using oral contraception or an intrauterine device: a prospective study. Sex Transm Dis 1990;17:23 |
|
Barbone F et al. A follow-up study of methods of contraception, sexual activity, and rates of trichomoniasis, candidiasis and bacterial vaginosis. Am J Obstet Gynecol 1990;163:510 |
|
Berger BJ et al. Bacterial vaginosis and lesbians: a sexually transmitted disease. Clin Infect Dis 1995;21:1402 |
|
Swedberg J, Steiner JF, Deiss F et al. Comparison of single-dose vs one week course of metronidazole for symptomatic BV. JAMA 1985;254:1046 |
|
Vejtorp M, Bollerup AC, Vejtorp L et al. Bacterial vaginosis: a double-blinded randomized trial of the effect of treatment of sexual partners. Br J Obstet Gynecol 1988; 95:920 |
|
Mengel MB, Berg AO, Weaver CH et al. The effectiveness of single dose metronidazole therapy for patients and their partners with bacterial vaginosis. J Fam Pract 1989;28:163 |
|
Holst E et al. Bacterial vaginosis: microbiological and clinical findings. Eur J Clin Microbiol 1987;6:536 |
|
Hay PE et al. A longitudinal study of bacterial vaginosis during pregnancy. Br J Obstet Gynecol 1994;101:1048 |
|
Spiegel CA, Amsel R, Eschenbach DA et al. Anaerobic bacteria in nonspecific vaginitis. N Engl J Med 1980; 303:601 |
|
Spiegel CA, Eschenbach DA, Amsel et al. Curved anaerobic bacteria in nonspecific vaginosis and their response to antibiotic therapy. J Infect Dis 1983;148:817 |
|
Hillier SL, Holmes KK. Bacterial vaginosis. In Holmes KK, Sparling PF, Mardh PA et al (eds). Sexually Transmitted Disease, p 563. New York: McGraw-Hill, 1999 |
|
Briselden AM et al. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated micro-flora. J Clin Microbiol 1992;30:663 |
|
Sturm AW. Chemotaxis inhibition by Gardnerella vaginales and succinate producing vaginal anaerobes: composition of vaginal discharge associated with G. vaginalis. Genitourin Med 1989;65:109 |
|
Cauci S, Monte R, Driursi S et al. Impairment of the mucosal immune system: IgA and IgM cleavage detected in vaginal washings of a subgroup of patients with bacterial vaginosis. J Infect Dis 1998;178:1698 |
|
Mardh P-A et al. The grivet monkey as a model for study of vaginitis: challenge with anaerobic curved rods and Gardnerella vaginalis. In Mardh P-A, Taylor-Robinson D, eds. Bacterial Vaginosis, p 117. Stockholm: Almqvist & Wiksell, 1984 |
|
Hillier SL, Lipinshki CM, Briselden AM et al. Efficacy of intravaginal 0.75% metronidazole gel for the treatment of bacterial vaginosis. Obstet Gynecol 1993;81:963 |
|
Brand JM, Galask RP. Trimethylamine: the substance mainly responsible for the fishy odor often associated with bacterial vaginosis. Obstet Gynecol 1986;63:682 |
|
Platz-Christensen JJ et al. Endotoxin and interleukin-1α in the cervical mucus and vaginal fluid of pregnant women with bacterial vaginosis. Am J Obstet Gynecol 1993; 169:1161 |
|
McGregor JA et al. Bacterial vaginosis is associated with prematurity and vaginal fluid mucinase and sialidase: results of a controlled trial of topical clindamycin cream. Am J Obstet Gynecol 1994;170:1048 |
|
Thomason JL, Gelbart SM, Anderson RJ et al. Statistical evaluation of diagnostic criteria for bacterial vaginosis. Am J Obstet Gynecol 1990;162:155 |
|
Platz-Christensen J-J, Larsson P-G, Sundstrom D et al. Detection of bacterial vaginosis in Papanicolaou smears. Am J Obstet Gynecol 1989;160:132 |
|
Totten P, Amsel R, Hale J et al. Selective differential human blood bilayer media for isolation of Gardnerella vaginalis. J Clin Microbiol 1982;15:141 |
|
Gravett MG, Hummel D, Eschenbach DA et al. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol 1986;67:229 |
|
Hillier SL, Martius J, Krohn MA et al. A case-control study of chorioamnionic infection and chorioamnionitis in prematurity. N Engl J Med 1988;319:972 |
|
Watts DH, Krohn MA, Hillier SL et al. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol 1992;79:351 |
|
Silver HM, Sperling RS, St. Clair PJ et al. Evidence relating bacterial vaginosis to intra-amniotic infection. Am J Obstet Gynecol 1989;161:808 |
|
Morales WJ, Shorr S, Albriton J. Effect of metronidazole in patients with preterm birth in preceding pregnancy and bacterial vaginosis: a placebo-controlled, double-blind study. Am J Obstet Gynecol 1994;171:345 |
|
Hauth JC, Goldenberg RL, Andrews WW. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. N Engl J Med 1995;333:1732 |
|
McDonald HM, O'Loughlin JA, Vigneswaren R. Impact of metronidazole therapy on preterm birth in women with bacterial vaginosis flora (Gardnerella vaginalis): a randomised, placebo controlled trial. Br J Obstet Gynecol 1997;104:1391 |
|
Klebanoff M, Carey JC. Metronidazole did not prevent preterm birth in asymptomatic women with bacterial vaginosis [Abstract]. Am J Obstet Gynecol 1999;180: (Suppl)S2 |
|
Watts DW, Krohn M, Hillier SL et al. Bacterial vaginosis as a risk factor for post cesarean endometritis. Obstet Gynecol 1990;75:52 |
|
Watts H, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas and Chlamydia trachomatis. Obstet Gynecol 1989;73:52 |
|
Newton ER, Prihoda TJ, Gibbs RS. A clinical and microbiologic analysis and risk factors for puerperal endometritis. Obstet Gynecol 1990;75:402 |
|
Larsson P-G, Platz-Christensen J-J, Forsum U. Clue cells in predicting infections after abdominal hysterectomy. Obstet Gynecol 1991;77:450 |
|
Dhar KK, Dhall GI, Ayyahari A. Single dose tinidazole prophylaxis in vaginal hysterectomy. Int J Gynecol Obstet 1993;42:117 |
|
Dhar KK, Dhall GI, Ayyahari A. Tinidazole prophylaxis in elective abdominal hysterectomy. Int J Gynecol Obstet 1993;42:121 |
|
Moller BR, Ahrons S, Laurin J et al. Pelvic infection after elective abortion associated with Chlamydia trachomatis. Obstet Gynecol 1982;59:210 |
|
Larsson P-G, Platz-Christensen J-J, Thejls H et al. Incidence of pelvic inflammatory disease after first-trimester legal abortion in women with bacterial vaginosis after treatment with metronidazole: a double-blind, randomized study. Am J Obstet Gynecol 1992;166:100 |
|
Hillier SL et al. Role of bacterial vaginosis-associated microorganisms in endometritis. Am J Obstet Gynecol 1996;175:435 |
|
Sweet RL, Mills J, Hadley KW et al. Use of laparoscopy to determine the etiology of acute salpingitis. Am J Obstet Gynecol 1979;134:68 |
|
Korn AP et al. Plasma cell endometritis in women with symptomatic bacterial vaginosis. Obstet Gynecol 1995; 85:387 |
|
Korn AP et al. Risk factors for plasma cell endometritis among women with cervical Neisseria gonorrhoeae, cervical Chlamydia trachomatis, or bacterial vaginosis. Am J Obstet Gynecol 1998;178:987 |
|
Cohen CR et al. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS 1995;9:1093 |
|
Martin HL, Nyange PM, Richardson BA et al. Hormonal contraception, sexually transmitted disease and risk of heterosexual transmission of HIV-1. J Infect Dis 1998; 178:1053 |
|
Eschenbach DA, Critchlow CW, Watkins H et al. A dose-duration study of metronidazole for the treatment of nonspecific vaginosis. Scand J Infect Dis Suppl 1983;40:73 |
|
Hillier SL, Krohn MA, Watts DH et al. Microbiological efficacy of intravaginal clindamycin cream for the treatment of bacterial vaginosis. Obstet Gynecol 1990;76:407 |
|
Hillier SL, Lipinski CM, Briselden AM et al. Efficacy of intravaginal 0.75% metronidazole gel for the treatment of bacterial vaginosis. Obstet Gynecol 1993;81:963 |
|
Greaves W, Chungafung J, Morris B et al. Clindamycin versus metronidazole in the treatment of bacterial vaginosis. Obstet Gynecol 1988;72:799 |
|
Van der meijden WI, Piot P, Loriaux SM et al. Amoxicillin, amoxicillin-clavulanic acid and metronidazole in the treatment of clue cell-positive discharge: a comparative clinical and laboratory study. J Antimicrob Chemother 1987; 20:735 |
|
Duff P, Lee MC, Hillier SL et al. Amoxicillin treatment of bacterial vaginosis during pregnancy. Obstet Gynecol 1991;77:431 |
|
Carmona O, Hernandez-Gonzalez S, Kobelt R. Ciprofloxacin in the treatment of nonspecific vaginitis. Am J Med 1987;82(Suppl 4):321 |
|
Piot P, Van Dyck E, Godts P et al. A placebo-controlled, double-blind comparison of tinidazole and triple sulfonamide cream for the treatment of nonspecific vaginitis. Am J Obstet Gynecol 1983;147:85 |
|
Durfee MA, Forsyth PS, Hale J et al. Ineffectiveness of erythromycin for treatment of Haemophilus vaginalis associated vaginitis. Antimicrob Agents Chemother 1979; 16:635 |
|
Van der meijden WI, Piot P, Schmitz PIM et al. Treatment of clue cell-positive discharge with 200mg povidone-iodine pessaries: a double-blind and placebo controlled trial. Eur J Obstet Gynecol Reprod Biol 1987;24:299 |
|
Fredricsson B, Englund K, Weintraub L et al. Bacterial vaginosis is not a simple ecologic disorder. Gynecol Obstet Invest 1989;28:156 |