Influence of Pregnancy on the Oral Cavity
Authors
INTRODUCTION
Some striking observations have now been made about the role of sex hormones in the development of pathologic changes in the gingiva. It has been known for a long time that sex hormones contribute to the vascular changes in gingiva during pregnancy. Evidence now suggests that sex hormones also are capable of altering the normal sub-gingival flora and the immune response in the oral cavity, resulting in intense (pregnancy granuloma) and frequent gingivitis in pregnant women. Other problems that seem to appear in the oral cavity during pregnancy are discussed later and are for the most part unrelated to hormonal changes. These unrelated pathologic findings include periodontitis and dental caries. The special treatment and prevention needs of dental patients during pregnancy are also discussed.
ORAL PATHOLOGY IN PREGNANCY
Four oral diseases have been described as affecting pregnant women to a greater degree than their non-pregnant counterparts: gingivitis, pregnancy granuloma, periodontitis, and dental caries.
Gingivitis
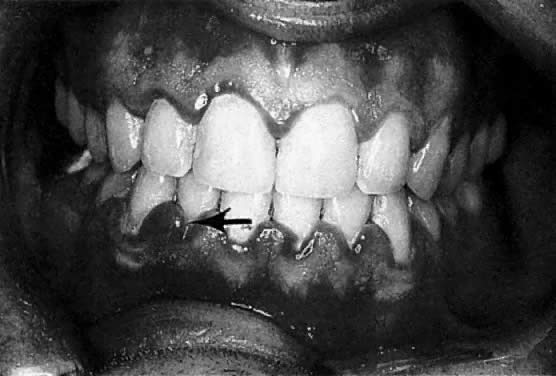
The first descriptions of “tooth pains” in pregnancy was elicited by Vermeeren in 1778.1 Gingival hyperplasia in pregnancy was discussed by Pitcarin in 1817.2 The frequently observed gingival changes that occur during pregnancy were reported in 1877.3 For many years, however, there have been questions about the reported prevalence of periodontal disease in pregnancy, the role that local and hormonal factors may have in the pathogenesis, and the implication of certain microorganisms in the etiology of this disease. Based on clinical observation, the reported frequency of so-called pregnancy gingivitis ranges from 30% to 100% 4, 5, 6, 7, 8 (Fig. 1). This variation may be a reflection of both the populations studied and the clinical parameters used.
|
According to studies using well-defined indices, gingival inflammation is a heightened or exacerbated response to dental plaque during a period of progesterone and estrogen imbalance.9 In addition, the effect of pregnancy on pre-existing gingival inflammation is first noticeable in the 2nd month of gestation and peaks in the 8th month (see Fig. 1). During the last month of gestation, a definite decrease in gingivitis generally occurs, and the gingival status immediately postpartum is found to be similar to that at the 2nd month of pregnancy. 8 The greatest relative increase in gingivitis during pregnancy is observed around the anterior teeth, although the molars demonstrate the highest gingivitis scores throughout pregnancy. The papillae (interproximal areas) are the most frequent sites of gingival inflammation both during pregnancy and after parturition.
Etiology
The causes of gingivitis in pregnancy can be separated into two general headings: host factors and microbial changes. Relative to host factors, the onset of increased gingival inflammation observed in the 2nd month of gestation coincides with an increase in the circulating levels of estrogen and progesterone. The continuous rise in these two hormone levels up to the 8th month is reflected in the greatest amount of gingival inflammation noted during pregnancy. In addition, a marked reduction in gingivitis after the 8th month correlates with an abrupt decrease of the circulating levels of these hormones. Estrogen and progesterone receptors have been demonstrated in human gingiva,10 indicating that it is a target tissue for hormones.11 Additionally, it has been demonstrated that progesterone is metabolized faster by inflamed human gingiva than by normal gingiva.12 The kinetics of progesterone in the gingiva, coupled with the clinical observations that the abnormal changes in gingiva during pregnancy parallel the circulatory levels of progesterone and estrogen, provide convincing evidence that these two hormones play a role in exacerbating gingivitis.
The mechanisms of action of progesterone- and estrogen-induced gingival changes during pregnancy have become much better understood. Increased circulating levels of progesterone in pregnancy cause dilatation of gingival capillaries, increased capillary permeability, and gingival exudate. Vittek and colleagues13 described the effect of progesterone on the gingival vasculature and the resultant increased exudation. The effects included a direct action of progesterone on the
endothelial cells, possible effects on the synthesis of prostaglandins, and suppression of the cellular immune response.
Clinical appearance is similar to that of gingivitis and may vary depending on the severity of the manifestation. Included signs may be edematous changes such as erythema, hypertrophy and hyperplasia. Additionally, an increase in bleeding on provocation may be seen.
Progesterone causes dramatic morphologic changes in the gingival microvasculature.14 The morphologic basis of the induced vascular permeability is the formation of gaps in the normally intact endothelial lining, together with channels resulting from coalescence of adjacent vesicles. The changes in both capillaries and venules, as well as the long duration of leakage from these vessels, are unlike the short action of histamine.
The keratinization of the gingiva is known to be decreased during pregnancy, and this, together with an increase in epithelial glycogen, results in a diminution in the effectiveness of the epithelial barrier.15 Estrogen also causes changes in the keratinization of the gingival epithelium and alters the degree of polymerization of ground substance.15 Because of the vascular changes caused by these hormones, there is a more florid response to the irritant effects of dental plaque.16 Increased serum levels of progesterone have been correlated with increased gingival crevicular fluid flow rate, which in periodontal diagnosis has been shown to reflect gingival inflammatory conditions.17
Physiologic levels of estrogen and progesterone in pregnancy have been shown to be stimulatory to prostaglandin synthesis.18, 19 Prostaglandins, especially PGE1 and PGE2, act as long-term mediators of inflammation.20 Prostaglandins are synthesized by activated macrophages and, to a lesser degree, by polymorphonuclear neutrophils in response to inflammatory stimuli, both of which increase in number as the gingiva becomes inflamed.21 Prostaglandin concentration within the gingiva and gingival fluid also increases dramatically, with the occurrence of gingival inflammation.22 Along with initiation of vascular changes, stimulation of prostaglandin synthesis illustrates another mechanism that raises progesterone levels in pregnancy, magnifying the clinical features of dental plaque-induced gingivitis.
Immune mechanisms have also been suggested to have an important role in the initiation and development of gingivitis and periodontitis.23, 24 Little is known about the effects of pregnancy on immune response in the oral cavity. Nevertheless, it has been demonstrated that the cell-mediated response is depressed during pregnancy, possibly contributing to the altered responsiveness of the gingival tissue to dental plaque.25
Dental plaque is the principal etiologic factor in gingivitis. In periodontitis, it is well established that the sub-gingival plaque is characterized by a shift toward a more anaerobic flora. Strong evidence supports the observation that gingival inflammation during pregnancy results from an alteration of the sub-gingival flora to a more anaerobic state. The anaerobe-to-aerobe ratio increases significantly during the 13th through 16th week of pregnancy and remains high during the third trimester. 26 It has been shown that increased proportions of Prevotella intermedia are concomitant with an increase in gingivitis and elevated serum levels of estrogen and progesterone in pregnancy.26 When the proportion of bacteroides species was monitored in the dental plaque of pregnant women, non-pregnant women, and non-pregnant women taking contraceptives, a 55-fold increase over the control group was noted in the populations of the bacteroides species in pregnant women and a 16-fold increase in women taking oral contraceptives.27 This concomitant increase in P. intermedia is most pronounced in the second trimester and correlates with increased gingivitis scores.27 Subsequent pure culture studies have shown that the marked increase in the proportion of bacteroides species during pregnancy seems to be associated with increased serum levels of circulating progesterone and estrogens. Both hormones can substitute for naphthoquinone, which is an essential growth factor for P. intermedia. The studies reported to date indicate that female sex hormones may be capable of altering the gingival vascular system, the immune response, and the normal sub-gingival flora.
Clinical manifestations
As previously noted, the marginal gingiva and interdental papillae are fiery red and the gingiva is enlarged, mostly affecting the interdental papillae. The gingiva shows an increased tendency to bleed, and in advanced cases, patients sometimes even experience slight pain. During the second and third trimester, the inflammation often becomes more severe.28, 29 It should be noted that not all women respond in this fashion: in fact, many do not have a clinically altered gingival condition. When there is no dental plaque-associated gingivitis before pregnancy and attentive oral hygiene is monitored, gingivitis usually does not develop. Preventive measures, such as more frequent dental visits for prophylaxis and meticulous plaque control, are therefore indicated for pregnant women. Recommendations include twice daily brushing along with flossing on a daily basis.
Pregnancy granuloma
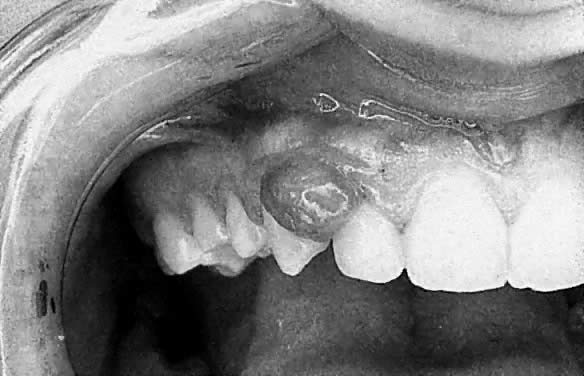
Apart from these generalized gingival changes, pregnancy may also give rise to the formation of tumor-like growths (epulides), along the gingival margin (Fig. 2). A number of terms for this lesion have been suggested, such as pregnancy tumor, epulis gravidarum, and pregnancy granuloma. Of these, pregnancy granuloma is preferred, because the histologic structure is similar to the structure in pyogenic (telangiectatic) granuloma.30 The reported frequency of pregnancy granulomas varies from 0% to 9.6%.31, 32 The granuloma occurs more frequently in the maxilla, favoring the vestibular aspect of the anterior region.
There appears to be no correlation between the appearance of this lesion and the month of pregnancy, although patients may notice the lesion more often in the second or third trimester, owing to its growth or the complications of its vascular nature.33 The lesion often shows rapid growth, although it seldom becomes larger than approximately 2 cm in diameter. A pregnancy granuloma is generally a pedunculated, soft growth of interdental origin; is fiery red; and often has small, fibrin-covered areas (see Fig. 2). Pregnancy granulomas frequently bleed readily when touched and have a tendency to recur rapidly.
The cause of these lesions appears to be an accentuation of the inflammatory reaction of gingivitis in pregnancy, including the endocrine and bacterial changes described earlier. Additionally, the dramatic enlargement in these lesions may be partly related to the hormonal effect on collagen metabolism. The sequential changes in levels of estradiol and progesterone during pregnancy and immediately after parturition suggest that these hormones are important in the regulation of collagenolytic activity. Progesterone and methylhydroxyprogesterone inhibit collagenase production in the culture of postpartum uterine explants.34, 35 The inhibition of collagenase production by these two hormones is apparently concentration dependent.
It is well known that endogenous gingival collagenase is the key enzyme involved in physiologic collagen turnover. During pregnancy, the inhibition of collagenase production ultimately results in accumulation of excess collagen within the connective tissue, thereby supplying a possible additional mechanism for the dramatic gingival enlargement of some pregnancy granulomas.
Histopathology
A pregnancy granuloma is composed of capillaries, fibrous tissue, and inflammatory cells, with marked vascularity being the most characteristic histologic feature. As such, distinguishing it from a pyogenic granuloma without other clinical data is difficult. The epithelium is generally thin and atrophic, but may be hyperplastic. If the lesion is ulcerated, it shows a fibrous exudate of varying thickness over the surface and a moderately intense infiltration of polymorphonuclear leukocytes, lymphocytes, and plasma cells. The excessive vascularity accounts for the bright red color, and the hyperemia and edema account for the enlargement.36, 37
Differential diagnosis
The differential diagnosis of a small, pedunculated hemorrhagic lesion of the marginal gingival tissue must include the following:
1. Peripheral fibroma
2. Pyogenic granuloma
3 Peripheral giant granuloma
4. Eosinophilia granuloma
5. Lymphomas or leukemic infiltrates
6. Hemangiomas
Treatment
It is prudent, if possible, to wait until parturition for surgical excision of a pregnancy granuloma, unless the lesion is creating a functional problem or appears to be having a deleterious effect on the adjacent periodontium. These lesions may regress after birth; however, surgical excision is usually warranted. The surgery can be accomplished safely throughout pregnancy with the use of local anesthesia and may be surgically excised with the use of a scalpel. An electrocautery or laser may be employed if necessary for hemostasis. Lasers have the tendency to reduce the postsurgical bleeding typically experienced after excision of a pyogenic granuloma.38 Incomplete excision results in recurrence.32 A residual fibrous mass may remain if the lesion is large and is allowed to regress postpartum without surgical intervention.32
Periodontitis
Gingivitis, or inflammation of the gingiva, is considered to be a reversible process. In contrast, periodontitis results in the loss of tooth attachment (periodontal ligament and alveolar bone) and pocket formation. Though gingivitis is often associated with periodontitis, gingivitis does not necessarily develop into periodontitis because the putative pathogenic bacteria in periodontitis differ from those associated with gingivitis and because periodontitis is believed to be dependent on different immune mechanisms.
A number of investigators have noted sex hormone-mediated alteration of the sub-gingival flora and the subsequent increase in gingival inflammation.26, 27, 39 When pregnant and non-pregnant women with periodontitis are compared, however, the differences become less obvious. Studies have shown that in contrast to subjects with gingivitis, no significant differences are noted in the total bacterial counts and the proportion of P. intermedia in periodontal pockets of pregnant versus non-pregnant women.39 Although differences exist in the degree of periodontitis between pregnant and non-pregnant female populations, these reported differences are not impressive.40, 41, 42 Therefore, non-surgical approaches for the prevention and treatment of periodontitis are indicated for pregnant patients. Surgical intervention may be considered if there is acute necessity, while elective procedures should be considered postpartum.
Dental caries
Many of the uninformed populations appear to believe that pregnancy is a direct cause of dental caries. The old wives' tale “with each child, a tooth” has been quoted in both dental and medical literature. In 1875 Coles wrote, “We have during pregnancy, an increasing liability to caries, with each generation.”43 He noted that during the first months of pregnancy, patients may have a “severe toothache” secondary to caries. He explained this as “a diminution of earthy salts” during pregnancy. This belief has been fostered and been one of the most stubborn misconceptions to appear in dental and medical literature. There is no scientifically proven evidence to support this misconception.44
The hydroxyapatite crystal, of which enamel is made, does not respond to the biochemical and metabolic changes of pregnancy, nor does it respond to changes in calcium metabolism. The thought that morning sickness and vomiting can create an acid pH and therefore increase the decay rate is highly suspect as well. The short-term alteration of the pH of the oral environment is inconsequential compared to the months needed for the development of caries.
Use of fluoride in pregnancy
Administration of fluoride supplements to pregnant patients in an effort to benefit in utero development of the teeth of the offspring has been evaluated in several clinical studies. Although the collective findings of these studies indicate a possible benefit to the primary dentition of the offspring, the evidence is not sufficiently conclusive to warrant the supplemental prescription of fluoride.45 Similarly, conflicting evidence as to the benefit of the ingestion of fluoridated water to the primary dentition in utero exists. The data have failed to show any difference in the caries resistance of the primary teeth of children born prior to the fluoridation of a water supply and those born after its introduction, who were exposed both pre- and postnatally.45
Strong evidence46 exists that shows children of those mothers with poor self-rated oral health are more likely to grow up having poorer oral health than those of mothers with good self-rated oral health. Maternal self-rated oral health when children are young appears to be a valid representation of the intricacies of the shared genetic and environmental factors that contribute to oral health throughout the course of life. Unfavorable maternal self-rated oral health should be regarded as a possible risk indicator for poor oral health among offspring later in life. Simple questions about maternal oral health should form part of a preliminary and inexpensive assessment of a child’s future oral disease risk (on both a clinical and public health basis). In addition, it is important that mothers are informed that their oral health can have an impact on their child’s as well. Based on these findings, it is incumbent on the oral health professional to encourage mothers of young children to receive dental care.
INTERCEPTIVE CARE
There has been evidence reported of an association between maternal periodontal infection and the birth of preterm, low birth weight infants.45 Chronic periodontal infections can produce both local and systemic inflammatory responses. The activation of the maternal inflammatory cell response and cytokine release could play a role in the pathophysiological process of preterm birth, low-birth-weight and pre-eclampsia.47, 48 Jeffcoat et al.’s work49 suggested that periodontal treatment consisting of scaling and root planing in pregnant women with periodontal disease may reduce the risk of preterm birth. In contrast, Michalowicz et al.50 found no significant effect of periodontal treatment on birth outcomes and concluded that non-surgical periodontal therapy does not improve birth outcomes in pregnant women with periodontitis. At this point, though it is important to sustain periodontal health in pregnant women, there is no convincing evidence to support the notion that periodontitis causes preterm or low birth weight outcomes.
DENTAL TREATMENT IN PREGNANCY
Normal pregnancy does not necessarily contraindicate dental treatment if the stage of gestation and the extent of dental procedures are taken into account. The first trimester is the period of organogenesis. In addition, 75–80% of spontaneous abortions occur before the 16th week of gestation. Fetal sensitivity to the environment is most critical after the 30th week of gestation. Prolonged chair time should be avoided because supine hypotensive syndrome may occur. Whether a pregnant woman is in a semi-reclining or a supine position, the great vessels, particularly the inferior vena cava, are compressed by the uterus. Interference with venous return, may cause hypotension, decreased cardiac output, and potential loss of consciousness. Supine hypotensive syndrome can usually be reversed by turning the patient on her left side, thereby relieving the pressure on the vena cava and allowing blood to return to the lower extremities and pelvic areas. Because of these risks, however, elective procedures, such as definitive periodontal surgery should only be considered during the 2nd trimester if necessary or postpartum if possible.51
The role of local irritants in the initiation of periodontal disease during pregnancy cannot be overemphasized based on clinical findings. Thus, it is prudent to educate and reinforce the need for dental plaque control on the part of pregnant women early on after conception.6, 41, 52 Local irritants should be removed early on prior to the amplified hormonal effects of inflammation are manifested in the gingival tissues.
In the event that emergency treatment is indicated, it should be performed anytime during gestation to eliminate any associated physical or emotional stress. The pain and anxiety precipitated by a dental emergency may be more detrimental to a fetus than the treatment itself.52
The use of routine diagnostic and dental radiographs is a sensitive subject during pregnancy. Though the amount of absorbed radiation, when proper shielding is employed, is minimal, it may be unnerving to a patient. Serious dental emergencies require radiographic evaluation for diagnosis and should judiciously taken, especially in the sensitive first trimester.
Another area of concern involves drug therapy, as many medications can affect the fetus by diffusion across the placental barrier. In most cases, it is safe practice to use a local anesthetic such as 2% lidocaine with a vasoconstrictor of epinephrine in a concentration of 1:100,000. Many analgesics, including acetaminophen and aspirin are also safe. The exception is the third trimester and partum when a potential bleeding diathesis is of concern.52
Certain drugs occasionally prescribed by dentists are known to cause complications during pregnancy and therefore should be avoided. These include the benzodiazepines as well as antibiotics which may cross the placental barrier. Some reports contraindicate the use of nitrous oxide during organogenesis (first trimester) though no confirmatory data are available. Neither general anesthesia nor intravenous sedation should be used at all during pregnancy.
SUMMARY
By far the most common dental complaint of pregnant women is bleeding gingiva (see Gingivitis and Pregnancy granuloma sections). The bleeding may be spontaneous or secondary to trauma, and it is distressing and sometimes embarrassing to patients. Fear of blood loss may prompt an expectant mother to discontinue tooth brushing and flossing to protect the gingiva from trauma; this, in turn, increases the risk of a more severe periodontal infection. Pregnant women should be alerted to this risk and should be advised to seek dental care immediately if gingival bleeding occurs, so that the cause of the hemorrhage can be eliminated or checked.
Pain is another frequent complaint if dental caries has been allowed to progress to a significant stage before or during pregnancy. A pregnant patient with dental pain should be encouraged to seek immediate consultation and treatment from a dental professional.
Visits to a dentist every 3 months during pregnancy should be encouraged, and if a patient has a prior history of treatment for gingivitis or periodontitis, visits to a dentist and hygienist for consultation and prophylaxis should occur more frequently – on a bimonthly basis.
It is strongly recommended that a dentist with proper understanding of potential dental pathology during pregnancy be in attendance to the outpatient department of maternity hospitals. Most important is that dental examination and appropriate dental care should become integral to the routine management of every pregnant woman.
REFERENCES
Newman M, Takei H, Klokkeveld P, Carranza’s Clinical Periodontology, 12th Edition, p 439, St. Louis. Saunders, Elsevier, 2015. |
|
Pitcarin J: A case of disease of the gums [that] occurred during pregnancy, Dublin Hosp Rep 2:309, 1818. |
|
Pinard A, Pinard D: Treatment of the gingivitis of puerperal women. Dental Cosmos 19: 327, 1877 |
|
Hanson L, Sobol SM, Abelson T: The otolaryngologic manifestations of pregnancy, J Fam Prac 23:151, 1986. |
|
Levin RP: Pregnancy gingivitis, Md State Dent Assoc 30:27, 1987 |
|
Loe H, Silness, J: Periodontal disease in pregnancy. 1. Prevalence and severity, Acta Odontol Scand 21:533, 1984 |
|
Samant A, Malik CP, Chabra, SK, et al: Gingivitis and periodontal disease in pregnancy, J Periodontol 47:415, 1976. |
|
Loe H, Silness J: Periodontal disease in pregnancy. Acta Odontol Scand 21: 533, 1963 |
|
Lundergren D, Lindche J: Lack of influence of female sex hormones on alveolar bone loss in hamsters. Scand J Dent Res 79: 113, 1971 |
|
Vittek J, Rappaport SC, Gordon GG et al: Metabolism of androgens by human periodontal ligament. J Dent Res 61: 1153, 1982 |
|
Ojanotko A, Neinstedt W, Harri P: Metabolism of testosterone by human healthy and inflamed gingiva (in vitro). Arch Oral Biol 25: 381, 1980 |
|
El Attar TMA: Metabolism of progesterone 7&b.alpha;3 H in vitro in human gingiva with periodontitis. J Periodontol 42: 721, 1971 |
|
Vittek J, Gordon GG, Rappaport SC et al: Cellular regulations of the metabolism of androgen in rat oral mucosa. J Dent Res 58: 624, 1979 |
|
Mohamed AH, Waterhouse JP, Friederici HHR: The microvasculature of the rabbit gingiva as affected by progesterone. J Periodontol 45: 50, 1974 |
|
Manson JD: Periodontics, p 38. London, Kimpton Medical Publications, 1986 |
|
Sooriyamoorthy M, Gower DB: Hormonal influences on gingival tissues: Relationship to periodontal disease. J Clin Periodontol 16: 201, 1989 |
|
Lamster IB, Oshrain RL, Harper SD: Enzyme activity in crevicular fluid for detection and prediction of clinical attachment loss in patients with chronic adult periodontitis: Six month results. J Periodontol 59: 516, 1988 |
|
Loe H: Periodontal changes in pregnancy. J Periodontol 36: 209, 1965 |
|
Hugoson A: Gingival inflammation and female sex hormones. J Periodontal Res 5 (suppl): 1, 1970 |
|
Vane J: Prostaglandins as mediators of inflammation. Adv Prostaglandin Thromboxane Leukot Res 2: 791, 1976 |
|
Humes J, Bonney R, Pelus L et al: Macrophages synthesize and release prostaglandins in response to inflammatory stimuli. Nature 269: 149, 1977 |
|
Goodson J, Dewhirst F, Brunetti A: Prostaglandin E levels and human periodontal disease. Prostaglandins 6: 81, 1974 |
|
Page RC, Schroeder HE: Pathogenesis of inflammatory periodontal disease. Lab Invest 3: 235, 1976 |
|
Genco R, Slots J: Host responses in periodontal diseases. J Dent Res 63: 441, 1984 |
|
O'Neal TCA: Maternal T-lymphocyte response and gingivitis in pregnancy. J Periodontol 50: 178, 1979 |
|
Kornman KS, Loesche WJ: The sub-gingival microbial flora during pregnancy. J Periodontal Res 15: 111, 1980 |
|
Jansen J, Liljemark W, Bloomquist C: The effect of female sex hormones on sub-gingival plaque. J Periodontol 52: 588, 1981 |
|
Goldman H, Cohen DW: Periodontal Therapy, 6th edn. St. Louis, CV Mosby, 1978 |
|
Lindhe J: Textbook on Clinical Periodontology, 2nd edn, p 287. Copenhagen, Munksgaard, 1989 |
|
Pindborg J: Atlas of Diseases of the Oral Mucosa, 4th edn, p 228. Philadelphia, WB Saunders, 1985 |
|
Tiilila I: Epulus Gravidarum. Thesis, Suom Hammaslaak Toim (suppl 1):58, 1962 |
|
Arafat A: The prevalence of pyogenic granuloma in pregnant women. J Balt Coll Dent Surg 29: 14, 1974 |
|
Blum T: Pregnancy tumors: A study of 16 cases. J Am Dent Assoc 18: 393, 1981 |
|
Jonsson R, Howland BE, Bowden GHW: Relationships between periodontal health, salivary steroids, and Bacteroides intermedius in males, pregnant and nonpregnant women. J Dent Res 67: 1062, 1988 |
|
Jeffrey JJ: Collagen synthesis and degradation in the uterine deciduoma: Regulation of collagenase activity by progesterone. Collagen Rel Res 1: 257, 1981 |
|
Shafer W, Hine M, Levi B: A Textbook of Oral Pathology, 4th edn, p 360. Philadelphia, WB Saunders, 1983 |
|
Robinson HBG, Miller A: Color Atlas of Oral Pathology, 5th edn, p 97. Philadelphia, JB Lippincott, 1990 |
|
Pick RM, Pecaro BC: Use of the CO2 laser in soft tissue dental surgery. Lasers Surg Med 7: 207, 1987 |
|
Jonsson R, Howland BE, Bowden GHW: Relationships between periodontal health, salivary steroids, and Bacteroides intermedius in males, pregnant and non-pregnant women. J Dent Res 67: 1062, 1988 |
|
Reteitschak KH: Tooth mobility changes in pregnancy. J Periodontal Res 2: 199, 1967 |
|
Cohen DW, Friedman L, Shapiro J et al: A longitudinal investigation of the periodontal changes during pregnancy. J Periodontol 40: 563, 1969 |
|
Hugo son A, Lind he J: Gingival tissue regeneration in non-pregnant female dogs treated with sex hormones: Clinical observations. Odontology Rev 22: 237, 1971 |
|
Coles O: On the condition of the mouth and teeth during pregnancy. Am J Dent Sic 8: 361, 1875 |
|
Barrett-Connor E: Infections and pregnancy: A review. South Med J 62: 275, 1969 |
|
Report of Council on Dental Therapeutics: Accepted Dental Therapeutics. Chicago, American Dental Association, 1982 |
|
Shearer DM, Thomson WM., Broadbent JM., and Poulson R. Maternal Oral Health Predicts Their Children’s Caries Experience in adulthood. J Dent Res 2011; 90 (5): 672-677 |
|
Shearer DM, Thomson WM., Broadbent JM., and Poulson R. Maternal Oral Health Predicts Their Children’s Caries Experience in adulthood. J Dent Res 2011; 90 (5): 672-677 |
|
von Dadelszen P, Magee LA: Could an infectious trigger explain the differential maternal response to the shared placental pathology of preeclampsia and normotensive intrauterine growth restriction? Acta Obstet Gynecol Scand. 81:642-8, 2002 |
|
Jeffcoat MK, Hauth JC, Geurs NC et al: Periodontal disease and preterm birth: results of a pilot intervention study. J Periodontol 2003 Aug; 74(8):1214-8 |
|
Michalowicz BS, Gustaffson A, Thumbigere-Math V, Buhlin K: The effects of periodontal treatment on pregnancy outcomes. J Periodontol and J Clin Periodontol 2013: 195-207. |
|
Genco RJ, Goldman HM, Cohen DW: Contemporary Periodontics, p 221. St. Louis, CV Mosby, 1990 |
|
Offenbacher S, Katz V, Fertik G et al: Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol 67: 1103-13, 1996 |