Invasive Prenatal Genetic Techniques
Authors
INTRODUCTION
Advancing capabilities in the detection of genetic abnormalities and monitoring fetal development have enabled the prenatal diagnosis of a wide variety of congenital and acquired conditions. It is now possible to determine whether a fetus is affected with a specific genetic or acquired disorder rather than relying on probability assessment and statistical analyses to assess fetal risk. This chapter reviews the techniques used for invasive prenatal diagnosis and their safety, efficacy, and applications.
REQUIREMENTS AND INDICATIONS FOR INVASIVE PRENATAL DIAGNOSIS
Many clinical and historical factors can lead to prenatal diagnosis. Regardless of the specific indication, invasive prenatal testing should be performed with detailed genetic counseling performed before the procedure. Such counseling is needed to obtain critical information from the patient to assess properly fetal risk; to review personal and family histories that have an impact on this risk; to describe the risks, benefits, and limitations of the procedure(s) to be offered; and provide empathetic support to women who are considering such testing. Genetic counseling should be provided in a nondirective fashion, thus providing women with the necessary information (written and verbal) to arrive at prenatal diagnostic decisions but without coercing the woman, either overtly or covertly, into making a particular decision.
The commonest indication for consideration of invasive prenatal testing is an increased risk for fetal chromosome abnormalities. Such an increased risk can occur as result of advanced maternal age (35 years old or older at estimated date of delivery), a positive maternal analyte screen, or previous child with a chromosome abnormality or a parental chromosome rearrangement. Other indications for consideration of invasive prenatal testing include an increased risk for a detectable mendelian disorder (earlier birth of an affected child or family member, positive screening outcome), structural fetal anomalies detected by ultrasound, increased risk for polygenic/multifactorial disorder [neural tube defect]); positive screen, abnormal ultrasound, family history) or exposure to teratogens that increase the risk for detectable abnormalities (e.g., valproic acid and neural tube defects). In some cases, the indication for invasive testing may designate the type of procedure available for diagnosis. For example, in cases of increased risk for fetal neural tube defects, chorionic villus sampling is not an appropriate test as this technique cannot obtain amniotic fluid for the α-fetoprotein (AFP) and acetylcholinesterase analyses.
AMNIOCENTESIS
Traditional Amniocentesis: Gestation of 15 Weeks or Longer
Amniocentesis, the aspiration of amniotic fluid, has traditionally been performed at and after 15 to 17 weeks of gestation. At this stage of gestation, the volume of amniotic fluid is about 200 mL. The ratio of viable to nonviable cells in the amniotic fluid is relatively high,1 thus allowing timely culture and diagnosis of fetal cytogenetic abnormalities and providing women the option of pregnancy termination should an abnormality be determined.
Amniocentesis is routinely performed in an outpatient facility. An ultrasound examination should be done immediately before the procedure to evaluate fetal number and viability, perform fetal biometric measurements to confirm gestational age, establish placental location, and estimate amniotic fluid volume. In our hospitals, a fetal anatomic survey to screen for major anomalies is standard practice. In addition, ultrasonography may be useful in discovering maternal gynecologic conditions (e.g., leiomyomata) that could influence the technique and timing of the amniocentesis.
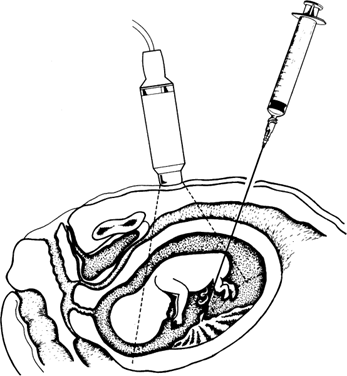
After the preoperative ultrasound examination has been completed, a needle insertion site is chosen. We prefer to insert the needle in the midline fundal area of the uterus, but this is not always the site at which the optimal pocket of amniotic fluid is located. Not infrequently, a lower uterine segment or lateral approach is required. If possible, we avoid the placenta; however, transplacental amniocentesis has not been shown to reduce the safety of amniocentesis.2,3,4 If tapping the optimal pocket of fluid requires traversing the placenta, we select the thinnest portion of the placenta possible through which the needle can be directed. The umbilical cord insertion site should be identified and avoided. The maternal bowel and bladder should also be located, because these should likewise be avoided. A local subcutaneous anesthetic (e.g., 2 to 3 mL of 1% lidocaine) may be used, but we usually find this unnecessary. After the maternal skin is cleaned with an iodine-based solution, sterile drapes are placed around the needle insertion site to help maintain an aseptic field. We prefer a 22-gauge spinal needle and recommend no larger than a 20-gauge needle.
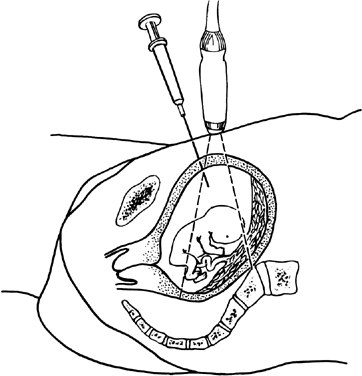
Ultrasonographic monitoring with continuous visualization of the needle should be used throughout the procedure. Ultrasound gel is applied adjacent to the insertion site, and a real-time ultrasound transducer is held in position such that the ultrasound beam is directed parallel to the planned needle track. Needle insertion should be performed with one smooth continuous motion until the needle tip is within the amniotic cavity (Fig. 1). Some practitioners locate the needle tip within the subcutaneous fat and assess the distance from tip to amniotic cavity as well as the appropriate needle angle required to successfully enter the amnion and avoid maternal structures and the fetus.
The first several milliliters of amniotic fluid are aspirated into a syringe. These first few milliliters are theoretically most likely to contain maternal cells; therefore, this initial sample is usually discarded or set aside for an AFP assay. For a second-trimester amniocentesis performed at 14 to 20 weeks inclusive, 20 to 30 mL of amniotic fluid is usually aspirated. After obtaining the amniotic fluid, the specimen should be clearly labeled and transported at ambient temperature to the laboratory.
The amniotic fluid aspirated is bloody in approximately 1% to 2% of amniocenteses. The blood, which is almost always maternal in origin, does not adversely affect amniotic cell growth. Indeed, the performance of a transplacental amniocentesis may increase the risk for a bloody tap; this has not been shown to have an adverse affect on the safety or accuracy of the amniocentesis. By contrast, brown or dark red or wine-colored amniotic fluid is associated with an increased likelihood of adverse pregnancy outcome. This color indicates earlier intra-amniotic bleeding, with hemoglobin breakdown products accounting for the pigment. Pregnancy loss eventually occurs in about one third of such cases.5 If the abnormally colored fluid is also characterized by an elevated AFP level, the risk for adverse perinatal outcome (fetal death, anencephaly, spontaneous abortion, or fetal abnormality) is usually further increased. Green amniotic fluid, presumably due to meconium staining, is apparently not associated with poor pregnancy outcome.6,7,8 By contrast, brown amniotic fluid has been associated with an increased risk of fetal aneuploidy.8
The propriety of administering Rh immunoglobulin (RhIG) to prevent Rh immunization in unsensitized women with Rh-positive fetuses remains controversial although widely done. Fetomaternal transfusion by disruption of the fetoplacental circulation logically might have an immunizing effect; however, the magnitude of the risk has not been determined. The task is difficult because one must consider such variables as ABD compatibility, number of needle insertions, placental location, and the amount of fetal blood transfused into the maternal circulation. Early investigators considered that prophylactic RhIG should not be administered following genetic amniocentesis,9 but almost all now advocate its routine use.10,11,12,13 The dose to be administered is controversial. The American College of Obstetricians and Gynecologists recommends that 300 μg of RhIG be administered for an exposure of 30 mL of fetal blood.14 In the United Kingdom, the recommended dose of RhIG is 50 μg before 20 weeks of gestation and 100 μg thereafter.15 We routinely administer 300 μg of RhIG following genetic amniocentesis, regardless of whether the needle may have traversed the placenta.
Following amniocentesis, fetal heart motion should be documented by ultrasonographic visualization. The patient is observed briefly following the procedure and is instructed to report any vaginal fluid loss or bleeding, uterine cramping, or fever. Reasonably normal activities may be resumed following the procedure; however, we recommend that strenuous exercise (e.g., jogging or aerobic exercises) and coitus be avoided for 1 day.
Multiple Gestations
In multiple gestations, amniocentesis can usually be performed on all fetuses, provided amniotic fluid volume is adequate.16 Following aspiration of amniotic fluid from the first sac, 2 to 3 mL of indigo carmine, diluted 1:10 in bacteriostatic water, is injected before the needle is taken out. A second amniocentesis is then performed. The second needle is then inserted into the sac of the second fetus, preferably determined after visualizing the membranes separating the two sacs. Aspiration of clear fluid confirms that the second sac has truly been entered. In experienced hands, amniocentesis is performed successfully in more than 95% of twin pregnancies with ostensibly no increased risks over that of amniocentesis in singleton pregnancies.16,17 Anderson and Goldberg17 observed a postprocedure twin-loss rate of 3.57% up to 28 weeks, a rate interpreted as not increased over the sum of background twin-loss rate plus the loss rate associated with singleton amniocentesis. Triplets and higher-order multiple gestations can be managed similarly by sequentially injecting dye into successive sacs. If the aspirated fluid remains clear, the clinician can be reassured that a new amniotic sac has been entered. However, the overall safety of amniocentesis in triplets and higher-order multiple gestations have not yet been formally determined.
Other techniques for sampling multiple gestations have been reported, including a single puncture technique and an air bubble infusion procedure. Despite advances in ultrasonography, we have concerns that single-puncture techniques could lead to cross-contamination between sacs, resulting in diagnostic inaccuracies. In addition, we believe it unwise to instill any substance into the amniotic cavity unless the instillation is performed in a strict, aseptic fashion and serves to improve safety or accuracy considerably and consistently. More recently, some operators have not used dye instillation to distinguish separate sacs, instead relying on ultrasonographic visualization alone.18,19,20 In view of the considerable positive experience with dye instillation techniques during the past 20 years with regard to safety and accuracy, we still use the dye instillation procedure.
Safety
Any procedure that involves passing a device into an organ, especially the pregnant uterus, entails risk; amniocentesis is no exception. Indeed, amniocentesis carries potential danger to both mother and fetus. Maternal risks are quite low, with symptomatic amnionitis occurring only rarely. Minor maternal complications such as transient vaginal spotting and minimal amniotic fluid leakage occur in 1% or fewer cases, but these are almost always self-limited. Other very rare complications include intra-abdominal organic injury or hemorrhage.
The safety of traditional amniocentesis has been addressed by several large collaborative studies. The US National Institute of Child Health and Human Development21 conducted the first major prospective study of genetic amniocentesis that comprised 1,040 study subjects and 992 matched controls. Of the 1,040 women undergoing amniocentesis, 950 (91.3%) had the procedure performed for cytogenetic analysis and 90 (8.7%) to evaluate for the possible presence of an inborn error of metabolism. Of all women who underwent amniocentesis, 3.5% experienced fetal loss between the time of the procedure and delivery compared with 3.2% of controls; the slight difference was not statistically significant and disappeared completely when corrected for maternal age. In Canada, a collaborative group conducted a cohort study but did not include a concurrent control group.22,23 Analysis was based on 1,223 amniocenteses performed during 1,020 pregnancies in 900 women. The pregnancy loss rate was 3.2%, a frequency similar to that reported in the US collaborative study.
A later British collaborative study found that the rate of fetal loss following amniocentesis was significantly greater than in controls (2.6% versus 1%).24 In the British study, however, one common indication for amniocentesis was elevated maternal serum AFP (MSAFP) now itself recognized as a factor associated with fetal loss and adverse perinatal outcome. Analysis after excluding subjects undergoing amniocentesis for that indication lowered the loss rates between study subject and control groups to less than 1%, albeit still a significant difference.25
None of the collaborative studies cited above was conducted with high-quality ultrasonography as defined by today's standards, nor was concurrent ultrasonography universally applied. More relevant recent data are from a Danish randomized controlled study of amniocentesis that involved 4,606 women aged between 25 and 34 years who were without known risk factors for fetal genetic abnormalities.26 Women with three or more previous spontaneous abortions, diabetes mellitus, multiple gestation, uterine anomalies, or intrauterine contraceptive devices were excluded. Maternal age, social group, smoking history, number of previous induced and spontaneous abortions, stillbirths, livebirths, and low-birthweight infants were comparable in the study and control groups, as was gestational age at time of entry into the study. Amniocentesis was performed under real-time ultrasound guidance with a 20-gauge needle by experienced operators. Followup evaluation was available for all but 3 women. The spontaneous abortion rate after 16 weeks was 1.7% in amniocentesis patients compared with 0.7% in controls (p < .01), with a 2.6-fold relative risk of spontaneously abortion if the placenta was traversed. Transplacental needle passage is a risk factor that has more recently been shown to not increase the risk for postprocedure loss (see previous discussion). The frequency of postural malformations in the infants in the two groups did not differ. However, respiratory distress syndrome was diagnosed more often (relative risk [RR], 2.1) in the study group, and more infants were treated for pneumonia (RR, 2.5). A more recent assessment of the safety of second-trimester amniocentesis can be found in the CEMAT study that compared conventional second-trimester amniocentesis to early amniocentesis.27 In this multicenter trial performed by experienced operators using concurrent ultrasonography, the total loss rate in the second trimester amniocentesis cohort was 5.9%.
In a study from British Columbia, Baird and colleagues28 considered the question of whether children delivered of women who had midtrimester amniocentesis can be identified by a population-based database of congenital anomalies and disabilities at a different rate from that of matched controls (i.e., children of women who had not undergone amniocentesis). The authors studied 1,296 cases (651 boys and 645 girls) and 3,704 matched controls (1,867 boys and 1,837 girls) among live births (1972 to 1983) from the Health Surveillance Registry with data collected to 1990 to allow followup of 7- to 18-years' duration. Cases were children of mothers who had midtrimester amniocentesis for advanced maternal age (35 years or older) and whose examination results were normal for chromosomal disorders and neural tube defects. When possible, three controls per case were matched for age of mother, gender, date of birth, and state of health from provincial birth records. In all 128 (9.9%) of the cases and 308 (8.3%) of the controls were registered (RR, 1.23); this RR was not significantly different from 1. The likelihood of having disabilities was examined for cases compared with controls, and no difference was found except for an increased ABO isoimmunization associated with amniocentesis. Overall, this study provides reassuring data for patients considering midtrimester amniocentesis with respect to long-term outcome.
In conclusion, we believe it wise to continue to counsel that the risk of pregnancy loss secondary to amniocentesis is 0.5% over baseline, or perhaps slightly less at centers with experienced operators. At our centers, serious maternal complications and fetal injuries are stated to be “remote” risks.
Early Amniocentesis: Gestation of 14 Weeks or Less
With the advent of high-resolution ultrasound equipment, some physicians began offering amniocentesis before 15 weeks of gestation. Some programs not offering chorionic villus sampling (CVS) viewed early amniocentesis as an attractive alternative for those women who desired prenatal diagnosis before the time in pregnancy when traditional amniocentesis is performed (i.e., 15 or more weeks of gestation). In other medical centers, early amniocentesis was explored to lessen the inconvenience of patients' having to be rescheduled if they came in for CVS and were determined to be beyond 12 weeks but under 15 weeks of gestation.
Several programs, including those at our own centers, reported experiences suggesting early amniocentesis was a promising technique. In a series of 936 amniocenteses at 12.8 weeks of gestation or less reported by Hanson and colleagues,29 loss rates were 0.7% (7 of 936) within 2 weeks of amniocentesis, with an additional 2.2% before 28 weeks and an additional 0.5% stillbirths or neonatal deaths. Total losses (32 of 936, or 3.4%) were considered comparable with the 2.1% to 3.2% in ultrasonographically normal pregnancies not undergoing a procedure; however, lack of corrections for maternal age and gestational age render comparisons less than exact. Other series reported include those conducted by Elejalde,30 Penso,31 Stripparo,32 Hackett,33 Assel,34 Djalali,35 Henry,36 Yang,37 Eiben,38 and in all cases, by their respective coworkers, and by Kerber and Held.39 Our group, then at the University of Tennessee, Memphis,40 compared our initial experience with 250 early amniocenteses (14 weeks or less) to that of our first 250 cases of transabdominal CVS (9.5 to 12.9 weeks), finding loss rates for early amniocentesis and transabdominal CVS to be 3.8% and 2.1%, respectively. Our group also reported early amniocentesis in 6 twin gestations (mean 11.9 weeks; range 10.5 to 13.6 weeks), using a similar dye injection technique as described for traditional amniocentesis (see earlier discussion).41 We successfully tapped both amniotic sacs in each of 6 cases (5 requiring two needle insertions, 1 requiring three needle insertions); all cultures yielded normal cytogenetic results, and all six pregnancies resulted in the delivery of health infants.
However, these studies represented observational reports of amniocentesis with most procedures being performed at or after 13 weeks of gestation. More rigorous comparative studies evaluating the safety and efficacy of early amniocentesis have, in general, failed to corroborate the generally favorable outcomes reported in the observational studies. Nicolaides and colleagues42 reported a comparison of amniocentesis and CVS at 10 to 13 weeks of gestation. Early amniocentesis was performed in 731 patients (493 by choice and 238 by randomization) and CVS in 570 (320 by choice and 250 by randomization). Both procedures were performed by transabdominal ultrasound-guided insertion of a 20-gauge needle. The spontaneous loss (intrauterine or neonatal death) was significantly higher after early amniocentesis (total group mean, 5.3%; 95% confidence interval [CI] 3.8 to 7.2; randomized subgroup mean, 5.9%; CI, 3.3 to 9.7) than after CVS (total group mean, 2.3%, CI 1.2 to 3.9; randomized subgroup, mean, 1.2%; CI, 0.3 to 3.5). Subsequently, Nicolaides and colleagues43 reported further findings of this study. Again, postprocedure loss rates were significantly higher in the total early amniocentesis group (4.9%) and the randomized early amniocentesis subgroup (5.8%) than in the total transabdominal CVS group (2.1%) and the randomized transabdominal CVS subgroup (1.8%). The frequency of talipes equinovarus was higher in the early amniocentesis group, but this difference did not attain statistical significance.
Sundberg and colleagues44 performed a similar type of randomized trial comparing early amniocentesis and CVS. In this study, the rate of talipes equinovarus increased significantly in the early amniocentesis group, even though investigators used a filter system to reduce the amount of fluid removed. This finding led the authors to terminate their study for safety concerns prematurely.
The results of the Canadian Early Amniocentesis versus Mid-Trimester Amniocentesis Trial,27,45 a multicenter, randomized trial of early amniocentesis and conventional second-trimester amniocentesis have been most revealing. This multicenter trial randomized 4,374 women into an early amniocentesis cohort (n = 2,183) and a conventional mid-trimester amniocentesis cohort (n = 2,185). In the early amniocentesis cohort, 1,916 women (87.8%) underwent amniocentesis before 13 weeks of gestation.
Loss rates were 7.6% for the early amniocentesis cohort and 5.9% for the midtrimester cohort (p = .012). Talipes equinovarus occurred in 1.3% of infants delivered of women in the early amniocentesis group compared to 0.1% in the midtrimester cohort (p = .0001). In addition, postprocedure amniotic fluid leakage occurred more frequently in the early amniocentesis group (3.5%) than in the midtrimester group (1.7%; p = .0007). Failed procedures, multiple needle insertions, and culture failures also occurred more frequently in the early amniocentesis group.27,45
In view of these recent studies, the ongoing United States multicenter trial evaluating the safety and efficacy of early amniocentesis with the EATA (Early Amniocentesis-Transabdominal CVS) trial ceased offering early amniocentesis to women who had sustained less than 14 weeks of gestation. These more recent prospective studies strongly suggest that second trimester amniocentesis and first-trimester chorionic villus sampling should remain, for the foreseeable future, as the invasive procedures of choice for the detection of fetal cytogenetic and Mendelian abnormalities.
CHORIONIC VILLUS SAMPLING
Because amniocentesis is most commonly performed in the midsecond trimester (15 to 16 weeks), fetal diagnosis cannot usually be established before 17 to 18 weeks of gestation. A technique that could be performed during the first trimester would be highly desirable to reduce the psychological stress of awaiting results until midpregnancy and to allow a safer method of pregnancy termination, should an abnormality be detected. CVS is such a technique.
Techniques for Chorionic Villus Sampling
TRANSCERVICAL CHRONIC VILLUS SAMPLING.
Transcervical CVS is now usually performed at 10 to 12 completed gestational weeks. Absolute contraindications to transcervical CVS include active cervical or vaginal pathology (e.g., herpetic, chlamydial, or gonorrheal infection) or maternal blood group sensitization. Relative contraindications include leiomyoma obstructing the cervical canal, bleeding from the vagina within 2 weeks of planned CVS, and a markedly retroverted, retroflexed uterus.46 Before CVS, fetal viability and normal fetal growth must be confirmed by ultrasound. The procedure is performed with a device that consists of a plastic cannula enclosing a metal obturator extending just beyond the catheter tip; the diameter of most catheters is approximately 1.5 mm.
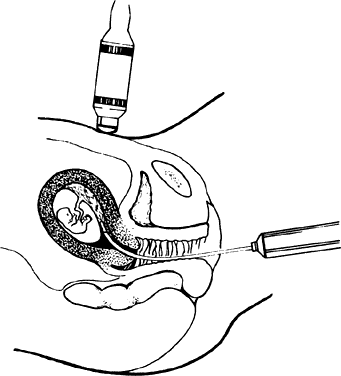
At our institutions, CVS is performed in an ultrasound suite. The patient is positioned in the dorsal lithotomy position. The vagina is cleaned with an iodine preparation, and the perineum draped with sterile towels. After insertion of a sterile vaginal speculum, placement of a tenaculum on the anterior lip of the cervix may occasionally be needed to help correct uterine anteflexion or retroflexion. The CVS catheter is introduced transcervically under simultaneous ultrasonographic visualization, with optimal catheter placement being parallel to the long access of the placenta (Fig. 2). The obturator is then withdrawn and the catheter Luer-lok attached to a 20- or 30-mL syringe. Chorionic villi are then aspirated by multiple, rapid aspirations of the syringe plunger to 20 to 30 mL negative pressure. The catheter is withdrawn under continuous maximum negative pressure. Although adequate sample is contained in at least 5 mg of villi, a sample of 10 to 25 mg is preferable.
Following the procedure, fetal heart activity is verified by ultrasonography. Patients are monitored for any untoward effects for approximately 30 minutes. Unsensitized Rhnegative patients are given RhIg. Maternal serum AFP screening for fetal neural tube defects is recommended at 16 to 18 weeks gestation.
TRANSABDOMINAL CHORIONIC VILLUS SAMPLING.
Transabdominal CVS applicable to evaluate pregnancies at the same gestational age as transcervical CVS at 10 to 12 weeks; however, this procedure also can be performed later in pregnancy,47,48,49 particularly when fetal abnormalities are visualized at ultrasound and a rapid diagnosis may influence pregnancy management. Placentas especially amenable to the transabdominal approach include those located in the fundus or those located anteriorly in an anteflexed uterus. Transabdominal CVS is also an option in certain circumstances when transcervical sampling is contraindicated (e.g., active herpes or cervical lesions).
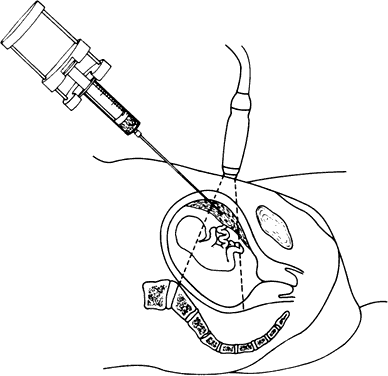
Following selection of a needle insertion site based on ultrasound findings, the overlying skin is infiltrated with a local anesthetic, cleaned with an iodine preparation, and draped with sterile towels. As performed at our centers, a 19-gauge spinal needle is inserted percutaneously through the maternal abdominal wall and myometrium under continuous ultrasound guidance. The tip is then guided into the long axis of the placenta (Fig. 3). The needle stylet is withdrawn, and next a syringe housed in an aspiration device is connected to the Luerlock of the needle. Chorionic villi are obtained by repeated (15 to 20) rapid aspirations of the syringe plunger to 20-mL negative pressure. The needle is then withdrawn under continuous negative pressure. The same postoperative protocol is used as for transcervical CVS.
Most physicians performing transabdominal CVS employ the aforementioned “freehand” technique. Alternatively, some operators still use a guide-needle or double-needle system device, which punctures the uterine wall once but permits multiple attempts at villus aspiration.50
TRANSVAGINAL CHORIONIC VILLUS SAMPLING.
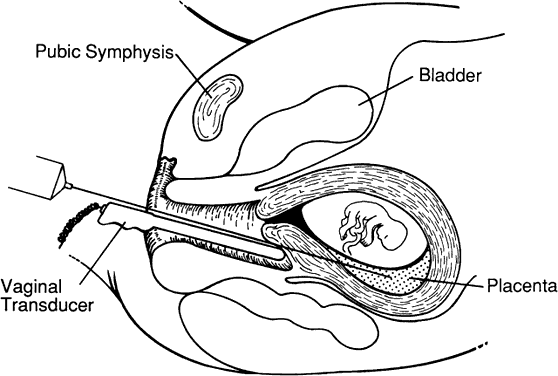
Although not commonly necessary, transvaginal CVS may be the only first-trimester option in women having a retroverted retroflexed uterus with a posterior placenta. Patients are prepared in a fashion similar to that used in transcervical CVS. We use a 35-cm 18-gauge aspiration needle to obtain chorionic villi. The wall of the vagina posterior to the cervix is infiltrated with a local anesthetic. Transabdominal ultrasound is used for needle guidance through the vaginal mucosa and myometrium into the placenta. After the needle is within the placenta, the needle stylet is removed and villi are aspirated in a similar fashion as with transabdominal CVS (Fig. 4).51
Safety of Chorionic Villus Sampling
PREGNANCY LOSS FOLLOWING CHORIONIC VILLUS SAMPLING.
The US Cooperative Clinical Comparison of Chorionic Villus Sampling and Amniocentesis study and the Canadian Collaborative CVS-Amniocentesis Trial Group study initially reported the pregnancy loss rate CVS to be 0.8% and 0.6% higher than traditional amniocentesis, respectively.52,53 Neither figure was statistically significant. In the US study, 2,278 women self-selected transcervical CVS; 671 women similarly recruited in the first trimester selected amniocentesis. Randomization did not prove possible. The Canadian study was randomized, with 1,391 subjects assigned to transcervical CVS and 1,396 to amniocentesis. Variables shown to influence fetal loss rates adversely included fundal location of the placenta, number of catheter passages, small sample size, and prior bleeding during the current pregnancy.52,54 Almost all the above invariably reflect technical difficulty. Other obstetric complications (e.g., intrauterine growth restriction [IUGR], placental abruption, premature delivery) did not exceed those in women not undergoing CVS.
Transcervical CVS and transabdominal CVS appear to be equally safe procedures for first-trimester diagnosis. In a later US NICHD collaborative study, in which 1,844 patients were randomized to transcervical CVS and 1,929 patients randomized to transabdominal CVS, the loss rates of cytogenetically normal pregnancies through 28 weeks were 2.5% and 2.3%, respectively.55 Of note, the overall loss rate (i.e., background plus procedure related) following CVS decreased by about 0.8% during this 1988 to 1990 randomization trial, in comparison with rates observed during the transcervical versus amniocentesis self-selection study (1985 to 1987). This decrease in procedure-related loss rate probably reflects increasing operator experience, as well as availability of both transcervical and transabdominal approaches. In a small Italian randomized trial, Brambati and partners56 also found no difference between transabdominal and transcervical CVS. By contrast, in a randomized comparison of amniocentesis, transabdominal CVS, and transcervical CVS in Denmark, Smidt-Jensen and associates57 found similar fetal loss rates after transabdominal CVS and amniocentesis but a significantly increased loss rate associated with transcervical CVS. These results are not surprising, however, given that the Danish experience with transabdominal CVS is far greater than with transcervical CVS.
The one major study that differs substantively from the US, Canadian, and Italian collaborative trials is the Medical Research Council (MRC) Study.58 In this multicenter randomized comparison between first-trimester CVS performed in whatever fashion deemed suitable by the obstetrician and second-trimester amniocentesis, the ultimate variable measured was completed pregnancies. The 4.4% fewer completed pregnancies in the CVS cohort reflected both unintended and intended pregnancy terminations. The latter, in turn, probably reflected inexperience in cytogenetic interpretation, given some terminations seemingly arguable in retrospect (i.e., confined placental mosaicism). Experience with CVS by the MRC study operators was also considerably less than in the US operators. For example, the only requirement for participation in the MRC study was 30 “practice” CVS procedures. Some centers also contributed few cases.
No formal attempts to assess the safety of transvaginal CVS have as yet been attempted. In our own experience,51 as well as that of Sidranski and colleagues,59 neither major complications nor obvious excessive fetal loss rates have been observed.
We conclude that clinical judgment and patient individualization in choosing the optimal approach for CVS increases safety. For example, some difficult transcervical CVS procedures (e.g., those involving fundal placentas) should be avoided in favor of a more straightforward transabdominal approach. The converse is also true.
CHORIONIC VILLUS SAMPLING IN MULTIFETAL PREGNANCY.
CVS in multifetal pregnancies has become more widely used with increasing use of fetal reduction resulting from assisted reproductive technologies. Many women requiring assisted reproductive technologies to become pregnant are also at increased risk for fetal chromosome or Mendelian abnormalities. CVS prior to fetal reduction allows for the detection of chromosomal or DNA abnormalities, thus allowing for the reduction of only abnormal gestations.60,61,62
Few data exist for safety of CVS in multiple gestations. In a major US study involving four centers, the total loss rate of chromosomally normal fetuses (spontaneous abortions, stillborns, neonatal deaths) was 5.0%,63 only slightly higher than the 4.0% observed for singleton pregnancies.52 We now use CVS in multiple gestations when both placentas can reliably be sampled as determined by ultrasonography. When placental locations preclude the reasonable procurement of separate and distinct samples, amniocentesis is preferred.
Limb Reduction Deformities (LRD)
Evaluation of the safety of CVS has recently shifted focus from concerns about the risk of fetal loss to its being the possible cause for congenital abnormalities. In 1991, Firth and colleagues64,65 reported that 5 of 289 (1.7%, or 17 in 1,000) infants exposed to CVS at 56 to 66 days of gestation (i.e., 42 to 50 days after fertilization) had severe limb-reduction deformities (LRD). Of the 5 infants, 4 had oromandibular limb hypogenesis (all transabdominal CVS); the fifth had a terminal transverse limb reduction alone (transcervical CVS). Subsequently, a number of reports followed, both supporting and refuting such an association.66,67,68,69,70,71 In the United States, Burton and partners72 reported a second cluster among 394 infants whose mothers had undergone CVS. Thirteen (3.3%) had major congenital abnormalities, including four with transverse LRD (10 of 1,000 or 1%). All four LRDs were transverse distal defects involving hypoplasia or absence of the fingers and toes. Of these cases, 3 followed transcervical sampling, using a device that, in the hands of the reporting physicians, was associated with an 11% fetal loss rate.
Teratogenic mechanisms whereby CVS might cause LRD have been hypothesized.73 These include hypoperfusion due to fetomaternal hemorrhage or pressor substances released by disturbance of villi or the chorion; embolization of chorionic villus material or maternal clots into the fetal circulation; and amniotic puncture and limb entrapment in exocoelic gel.
The potential association of LRD with CVS has been explored through various registries. Based on data from the Italian Multicenter Birth Defects Registry, Mastroiacovo and Batto74 reported that 8 cases of oromandibular-limb hypogenesis complex were entered into the registry from January 1988 through December 1991, 4 of which had been exposed to CVS, compared with 36 exposed subjects among 8,445 controls. There were 166 cases of transverse limb defects alone, 4 having been exposed to CVS, compared with 36 cases among 8,445 controls. A 1994 update of this study, based on 11 CVS-exposed cases, continued to indicate an association with transverse limb defects.75 The highest risk was associated with procedures performed at less than 70 days gestation (odds ratio [OR], 23.2; CI, 1.31 to 41.0); a lower, but still increased risk with procedures at 70 to 76 days (OR, 17.1; CI, 6.7 to 44.0); over 44 days there were no exposed cases and the risk interpreted as considerably lower. By contrast, analysis of other European registries in aggregate involving more than 600,000 births showed that only 4 of 336 cases (1.2%) with limb reduction abnormalities had been exposed to CVS, compared with 78 of 11,883 (0.66%) cases with other malformations (OR, 1.8; CI, 0.7 to 5.0).76
Firth and coworkers77 summarized LRD in 75 infants exposed to CVS by combining their own cases with those reported in the literature. The median gestational age at CVS ranged from 56 (range, 49 to 65) postmenstrual days for the most severe defects to 72 (range, 51 to 98) for the least severe. They concluded that there was a correlation between the severity of the defects and the duration of gestation when CVS was performed.
In 1994, the US Centers for Disease Control (CDC) held an open forum in Atlanta where data were presented from a US multistate case control study in an effort to quantify the risk of LDR associated with CVS.78 Case subjects were 131 infants with nonsyndromic limb deficiency from seven population-based birth defects surveillance programs born to women 34 years or older from 1988 through 1992. Controls were 131 infants with other birth defects matched to case subjects by the infant's year of birth, mother's age, race, and state of residence. Overall, the OR for limb deficiency after CVS from 8 to 12 weeks gestation was 1.7 (CI, 0.4 to 6.3). However, when analyzed for specific anatomic subtypes, there was an association for transverse digital deficiency (OR, 6.4; CI, 1.1 to 38.6). They concluded that the absolute risk for such defects was approximately 1 in 3,000. It should be noted that such transverse digital deficiencies occurred in only seven exposed cases.
Finally, the World Health Organization Committee on Chronic Villus Sampling recently analyzed data collected through an international voluntary registry.79,80 In total, 77 cases with LRD among 138,996 infants born after CVS were reported from 63 registering centers. Pattern analysis of the types of limb defects and overall frequencies of specific LRD were compared with a background population study from British Columbia, Canada.81 The pattern of defects showed the upper limb to be affected in 65%, 13% in the lower limb, and 23% in both upper and lower limbs compared with frequencies in the general population of 68%, 23%, and 9%, respectively. Transverse limb defects occurred in 41% of infants in the cohort exposed to CVS, compared with 43% in the general population and longitudinal limb deficiencies were found in 59% of cases, compared with 57% in the general population. It was concluded that the pattern analysis of the types of limb defects and calculation of overall incidences failed to find a difference between the CVS and background populations.
A more recent review of CVS safety sponsored by the World Health Organization82 evaluated the clinical outcomes of 216,381 CVS cases performed worldwide. In this review, 115 LRDs were observed with an incidence of 1 in 1,881, similar to the rate of 1 in 1,642 observed in the general population. The distribution of LRD (upper limbs 71.3%, lower limbs 11.3%, both limbs 17.4%) was also similar to that observed in the general population. The authors concluded the data demonstrate that “… CVS carries no increased risk for fetal loss or congenital malformation, including limb reduction defect …” compared with conventional mid-trimester amniocentesis.
CVS remains the only established method for first trimester prenatal diagnosis and carries minimal, if any, increased risk for adverse pregnancy outcomes when performed by experienced operators. To maximize the safety of CVS, we avoid performing CVS before 10 weeks gestation except in cases of profound risk for fetal abnormalities (e.g., fetal hydrops). We continue to counsel patients about the LRD controversy and inform them that the absolute risk at 10 to 12 weeks is believed to be very low, approximately 1 in 2,000, a rate similar to that observed in the general population.82 We also stress that this issue be placed in proper perspective and weighed against the substantial advantages that first trimester prenatal diagnosis that CVS offers.
FETAL BLOOD SAMPLING
Access to the fetal circulation was initially accomplished by fetoscopy, a method of directly visualizing the fetus, umbilical cord, and chorionic surface of the placenta, using endoscopic instruments.83,84 Fetoscopy for this purpose has now been replaced by ultrasound-directed percutaneous umbilical blood sampling (PUBS), also termed cordocentesis or funipuncture.
Fetal blood chromosome analysis has been used to help clarify purported chromosome mosaicism detected in cultured amniotic fluid cells85 or chorionic villi. Rapid assessment of fetal chromosome complement has been accomplished by “direct” cytogenetic analysis of uncultured nucleated blood cells.86 Short-term fetal lymphocyte cultures can provide a cytogenetic result within 72 hours; direct analysis of spontaneously dividing fetal cells (probably nucleated red blood cells) can provide a karyotype result within 24 hours. This proves particularly useful for patients presenting late in the second trimester, when results from amniocentesis would be available only after pregnancy termination would no longer be available. Also, in cases of fetal structural abnormalities or intrauterine growth retardation (IUGR) presenting in the third trimester, rapid results may prove useful for decision making concerning the mode of delivery.87,88 More recently, fluorescent in situ hybridization (FISH) with chromosome-specific DNA probes has also been used for rapid prenatal diagnosis of aneuploidy using nucleated fetal blood cells from umbilical cord blood, as well as amniotic fluid cells.89
Fetal blood sampling is used in the prenatal evaluation of many fetal hematologic abnormalities.90 Daffos91 reported normal hematologic values for second-trimester fetuses, and Forestier and separate colleagues92,93 reported normal blood chemistry values for second-trimester fetuses. Fetal blood hematocrit can be directly measured to assess hemolysis resulting from Rh or other blood antigen isoimmunization states.94 Before this, obstetricians had to rely on indirect evidence of fetal hemolysis, such as maternal antibody titers, past obstetric history, ultrasound findings, and spectrophotometry of bilirubin in amniotic fluid; the need for subsequent transfusions was based on somewhat arbitrary guidelines. Now the decisions about who, when, how much, and how often to transfuse can be made more rationally on the basis of actual fetal blood analyses such as hemoglobin level, hematocrit level, blood group, direct antibody titer, and reticulocyte count. Fetal hemoglobin can be directly evaluated to diagnose sickle cell disease, α- or β-thalassemia or other hemoglobinopathies,83 although these disorders are now usually diagnosed by DNA analysis of chorionic villi or amniotic fluid cells. Fetal blood sampling can be used to assess both the quantity of platelets and the quality of function.95 The maternal PLA2 (platelet antigen) alloimmunization PUBS is not only useful for diagnosis, but access to the fetal circulation also allows therapeutic alternatives including in utero platelet transfusion or maternal immunotherapy with gammaglobulin or steroids.96
Fetal blood has been used for the diagnosis of blood factor abnormalities such as hemophilia A, hemophilia B, or von Willebrand disease.93,97 In addition to hematologic studies, fetal blood samples can be used to diagnose autosomal recessive or X-linked immunologic deficiencies, including severe combined immunodeficiency (SCID), ChédiakHigashi syndrome, Wiskott-Aldrich syndrome, and chronic granulomatous disease.98,99,100
Recovery of fetal blood permits assessment of viral, bacterial, or parasitic infections of the fetus. Detection of fetal viral or parasitic infection is usually made on the basis of maternal antibody titers or ultrasound-directed fetal structural abnormalities. Serum studies of fetal blood allow for quantification of antibody titers.101, 102 In addition to antibody studies, PUBS can be used for direct analysis of viral, bacterial, and parasitic infections by culture of fetal blood.102,103,104
Although many indications for detecting fetal abnormalities that previously required fetal blood sampling are now performed by amniocentesis or CVS using DNA analysis,89,105,106 the continuing value of fetal blood sampling lies not only with those few remaining diagnostic indications but also with providing the potential for pharmacotherapy. For example, fetal arrhythmias have been treated with direct administration of antiarrhythmic medications and fetal paralysis may be induced to facilitate invasive procedures such as fetal transfusions or for magnetic resonance imaging (MRI).107
Technique
The technique most commonly used for fetal blood sampling is ultrasound-directed PUBS. The procedure can be safely undertaken from 18 weeks onward, although successful procedures have been reported as early as 12 weeks.108,109 PUBS is usually performed as an outpatient procedure. Maternal sedation is usually unnecessary, but oral benzodiazepine 1 or 2 hours before the procedure may be of benefit to the anxious patient and usually results in a transient decrease in fetal movement that can facilitate the procedure. A preliminary ultrasonographic examination of the fetus is performed to assess fetal viability, placental and umbilical cord location, fetal or placental anomalies, and fetal position. A suitable site for needle insertion is then selected; the skin over this site is anesthetized with 5 mL of 1% lidocaine. A sterile field is established; the skin is cleansed with an iodine-based solution and sterile drapes applied. The ultrasound transducer is placed on the abdomen away from the sterile insertion site, but at a location that permits visualization of the complete path of the needle from skin to fetal blood vessel.
Several potential sampling sites are available. Owing to its fixed position, the placental cord root is usually the site of choice whenever it is clearly visible (Fig. 5). Alternatively, free loops of cord or the intrahepatic vein are possibilities.90,110,111
Following percutaneous insertion of the spinal needle into the fetal blood vessel under direct ultrasound guidance, a small amount of blood is aspirated. The presence of fetal blood in this initial sample is confirmed using a model ZBI Coulter counter and channelizer to differentiate fetal from maternal blood on the basis of erythrocyte volume. The amount of blood aspirated for diagnosis depends on the indication for PUBS; rarely does the procedure require more than 5 mL.
On completion of the fetal blood sampling, the spinal needle is withdrawn and an ultrasound examination is performed to evaluate fetal status. The woman and her fetus are monitored for about 1 hour following PUBS. At our centers, all women at risk of Rh isoimmunization receive 300 μg of RhIg following the procedure.
Safety
Fetal blood sampling appears to be a relatively safe procedure when performed by experienced physicians, but it does carry more risk than CVS or amniocentesis. Maternal complications are rare but include amnionitis and transplacental hemorrhage.97,112 Data from large perinatal centers estimate the risk of in utero death or spontaneous abortion to be 3% or less following PUBS.91,101,104,113,114,115,116 Collaborative data from 14 North American centers, sampling 1,600 patients at varying gestational ages and for various indications, revealed an uncorrected fetal loss rate of 1.6%.117
A more recent assessment of fetal blood sampling loss risk was performed by Antsaklis and colleagues,118 who reviewed their clinical outcomes based on indication for fetal blood sampling. They divided their cohort into five main indication subgroups and found highly significant differences in procedure-related loss rates among the five groups. The highest loss rates were observed in the two groups characterized by fetal abnormalities and growth restriction, thus demonstrating that indication for fetal blood sampling has a major impact on risk for the procedure. Unfortunately, no studies directly comparing loss rates in control and treated groups have yet been published.
FETAL TISSUE SAMPLING
Prenatal diagnosis of severe hereditary skin diseases (genodermatoses) used to require fetal skin biopsy for ultrastructural or immunohistochemical analysis. Such genodermatoses that have been amenable to diagnosis by fetal skin sampling have included anhidrotic ectodermal dysplasia,119 bullous congenital ichthyosiform dystrophia (epidermolytic hyperkeratosis),120,121,122 epidermolysis bullosa dystrophia (Hallopean-Siemens),123 harlequin ichthyosis,124 hypohidrotic ectodermal dysplasia,125 epidermolysis bullosa lethalis,126,127 nonbullous ichthyosiform erythroderma,128 and Sjögren-Larsson syndrome.129
Most of these conditions can now be diagnosed prenatally with DNA analysis of chorionic villi or amniotic fluid cells.130 Indeed, one of the commoner lethal genodermatoses, Herlitz junctional epidermolysis bullosa, is now amenable to prenatal diagnosis by DNA analysis.131 However, prenatal diagnosis of harlequin ichthyosis still requires fetal skin sampling, although Akiyama and colleagues132 have reported that the diagnosis can now be made earlier than 21 to 22 weeks of gestation.
On such occasions, fetal skin sampling is still required and is best performed at 17 to 20 weeks of gestation. The procedure was initially performed under direct visualization using fetoscopy, a procedure associated with a total fetal loss rate of between 4% to 7%,133,134 or a procedure-related loss rate of perhaps 2%. From a safety standpoint, it seemed reasonable to assume that the smaller the caliber of the instrument introduced into the uterus, the safer the procedure would be. For this reason, we began to use ultrasound-directed biopsy forceps for fetal skin sampling in the 1980s.127 We reported the clinical outcomes of 17 ultrasound-guided fetal skin sampling procedures.127 In 5 cases, a fetal skin disorder was diagnosed, and these pregnancies were terminated. In the remaining 12 cases, all infants were delivered without complications at 37 weeks of gestation or later. Despite the use of biopsy forceps that take smaller fetal skin samples than the biopsy forceps used for fetoscopically directed fetal skin sampling, superficial scarring lesions have occasionally been noted.
Prenatal diagnosis of Becker-Duchenne muscular dystrophy is usually possible by DNA analysis. However, in a few families, meiotic recombination or homozygosity of multiple restriction fragment length polymorphisms (RFLPs) precludes a DNA-based diagnosis.135,136 In these cases, fetal muscle biopsies can be used for immunohistochemical analysis with a fluorescent antidystrophin antibody. Males with Duchenne muscular dystrophy lack dystrophin and with Becker muscular dystrophy lack or demonstrate a variable dystrophin pattern,137 whereas muscle biopsies from unaffected male fetuses show normal amounts of dystrophin.
Fetal muscle sampling is usually performed at around 18 weeks of gestation using a technique analogous to fetal skin sampling or fetal liver sampling. Muscle biopsies are taken from the gluteal region by directing a Klear Kut (Baxter, Los Angeles, CA) kidney forceps gun toward the fetal buttock. As with fetal skin and liver biopsy procedures, only a small number of fetal muscle sampling procedures have been reported.135, 138, 139 Thus, definitive statements cannot be made concerning the safety of this procedure.
COELOCENTESIS
Coelocentesis involves the transcervical or transabdominal aspiration of fluid from the extraembryonic coelom. This procedure has been suggested as a possible method for early first-trimester prenatal diagnosis. Ross and colleagues140 were successful in aspirating fluid from 48 of 50 (96%) pregnancies between 6 and 10 weeks of gestation. Although the authors were unable to obtain metaphase analyses from these specimens, subsequent work by Cruger and colleagues141,142 demonstrated success in culturing and karyotyping. In addition, Makrydimas and colleagues143 reported successful prenatal detection of beta-thalassemia by coelocentesis.
However, Ross and colleagues140 reported that short-term pregnancy loss rates associated with coelocentesis were markedly increased. In a comparative study of women undergoing pregnancy termination, 25% of women spontaneously aborted before their termination compared with 5% of women not undergoing coelocentesis (p < .01). These high loss rates suggest that coelocentesis may not be appropriate as a routine prenatal diagnostic test.
Of interest is the work of Santolaya-Forgas and colleagues.144 This group has investigated coelecentesis in baboons to determine its potential applicability for fetal therapy. In one series, coelocentesis was performed in 9 baboons; fluid aspiration (1 to 5 mL) was successful in 8 cases and 7 of the 8 pregnancies were continuing 140 days after the procedure.144 Coelocentesis was subsequently performed in 6 other baboons; assessment of the extracoelomic fluid osmometry and electrolyte composition in these 6 samples found that the chorion laeve behaves as a semipermeable membrane at 40 days' gestation,145 suggesting that it may be useful for maternal-fetal transfer of some substances that could be used for fetal therapy.
EMBRYOSCOPY
Embryoscopy is a relatively new and investigational technique that permits direct visualization of the fetus as early as the first trimester.146,147 Initially, a rigid fiberoptic endoscope was passed transcervically into the extracoelomic cavity, permitting inspection of fetal anatomic structures; fetal blood sampling was also feasible by this method.147 However, improvements and advancements in fiberoptic technology have led to the performance of thin-gauge transabdominal and transcervical embryoscopy,148,149 allowing visualization as early as 4 weeks after conception.150,151
Initial procedures were performed only on women who had elected pregnancy termination; however, embryoscopy has since been performed on continuing pregnancies.146,149 Ville and colleagues149 reported a procedure-related loss rate of 12% when the procedure was performed in the first trimester.
Further studies of the safety, accuracy, and applications of this new modality will be needed before embryoscopy is used as a routine prenatal diagnostic tool. However, the ability to access the embryonic circulation may have important application for therapeutic interventions such as drug, gene, and cell therapy.
CONCLUSIONS
Invasive prenatal testing remains a mainstay of obstetric practice in the United States. When provided within a framework of nondirective genetic counseling and supportive follow-up, invasive testing can provide critical information to women and couples concerning pregnancy outcomes. However, effective genetic counseling and follow-up as well as extensive clinician training and procedure evaluation is needed to best provide prenatal diagnosis to women at increased risk for detectable fetal abnormalities. It is hoped that further advances will facilitate access to the fetus and help develop safer invasive procedures. Current work at our and other centers is geared to develop noninvasive methods to screen, and eventually to diagnose, fetal abnormalities that are currently amenable to diagnosis by invasive methods. Indeed, the work evaluating the applicability of fetal cells in maternal blood holds great promise for such non-invasive screening and diagnosis. Until that and other technologies become integrated into clinical care, invasive prenatal testing will remain an important part of obstetrical care.
REFERENCES
Fuchs F, Riis P: Antenatal sex determination. Nature 117: 330, 1956 |
|
Bravo RR, Shulman LP, Phillips OP et al: Transplacental needle passage in early amniocentesis and pregnancy loss. Obstet Gynecol 86: 437, 1995 |
|
Marthin T, Liedgren S, Hammar M: Transplacental needle passage and other risk-factors associated with second trimester amniocentesis. Acta Obstet Gynecol Scand 76: 728, 1997 |
|
Tharmaratnam S, Sadek S, Streele EK et al: Transplacental early amniocentesis and pregnancy outcome. Br J Obstet Gynecol 105: 228, 1998 |
|
Milunsky A: The prenatal diagnosis of neural tube and other congenital defects. In Milunsky A (ed): Genetic Disorders and the Fetus, p 453. New York, Plenum, 1986 |
|
Karp LE, Schiller HS: Meconium staining of amniotic fluid at midtrimester amniocentesis. Obstet Gynecol 50: 475, 1977 |
|
Hess LW, Anderson RL, Golbus MS: Significance of opaque amniotic fluid at second-trimester amniocentesis. Obstet Gynecol 67: 44, 1986 |
|
Isada NB, Koppitch FC III, Johnson MP et al: Does the color of amniotic fluid still matter? Fetal Diagn Ther 5: 165, 1990 |
|
Golbus MS, Stephens JD, Cann HM et al: Rhisoimmunization following genetic amniocentesis. Prenat Diagn 2: 149, 1982 |
|
Henry G, Wexler P, Robinson A: Rhimmune globulin after amniocentesis for genetic diagnosis. Obstet Gynecol 48: 557, 1976 |
|
Bowman JM, Chown B, Pollock JM: Rh isoimmunization during pregnancy: antenatal prophylaxis. Can Med Assoc J 118: 623, 1978 |
|
Hill LM, Platt LD, Kellogg B: Rh-sensitization after genetic amniocentesis. Obstet Gynecol 56: 459, 1980 |
|
Khalil MA, Tabsh MA, Lobherz TB et al: Risks of prophylactic anti-D immunoglobulin after second-trimester amniocentesis. Am J Obstet Gynecol 149: 225, 1984 |
|
American College of Obstetricians and Gynecologists: ACOG Practice Bulletin No. 4. Prevention of Rh D Alloimmunization. Washington, DC: ACOG, 1999 |
|
Turnbull AC, MacKenzie IZ: Second-trimester amniocentesis and termination of pregnancy. Br Med Bull 39: 315, 1983 |
|
Elias S, Gerbie AB, Simpson JL et al: Genetic amniocentesis in twin gestations. Am J Obstet Gynecol 138: 169, 1980 |
|
Anderson RL, Goldberg JD: Prenatal diagnosis in multiple gestations: 20 years' experience with amniocentesis. Prenatal Diagn 11: 263, 1991 |
|
Bahado-Singh R, Schmitt R, Hobbins JC: New technique for genetic amniocentesis in twins. Obstet Gynecol 79: 304, 1992 |
|
Buscaglia M, Ghisoni L, Belloti M et al: Genetic amniocentesis in biamniotic twin pregnancies by a single transabdominal insertion of the needle. Prenat Diagn 15: 17, 1995 |
|
van Vugt JM, Nieuwint A, van Geijn HP: Single-needle insertion: An alternative technique for early second-trimester genetic twin amniocentesis. Fetal Diagn Ther 10: 178, 1995 |
|
National Institute of Child Health Development National Registry for Amniocentesis Study Group: Midtrimester amniocentesis for prenatal diagnosis: Safety and accuracy. JAMA 236:1471, 1976 |
|
Simpson NE, Dellaire L, Miller JR et al: Prenatal diagnosis of genetic disease in Canada: report of a collaborative study. Can Med Assoc J 15: 739, 1976 |
|
Medical Research Council: Diagnosis of Genetic Disease by Amniocentesis during the second trimester of pregnancy. Ottawa, On: Medical Research Council, 1977 |
|
United Kingdom Medical Research Council: Working Party on Amniocentesis. An assessment of the hazards of amniocentesis. Br J Obstet Gynaecol 85(Suppl 21):1, 1978 |
|
National Institute of Child Health and Human Development Consensus Conference on Antenatal Diagnosis December (1979) NIH Publication No. 801973. Washington, DC |
|
Tabor A, Philip J, Madsen MI et al: Randomized controlled trial of genetic amniocentesis in 4,606 low-risk women. Lancet 1: 1287, 1986 |
|
CEMAT Group: Randomised trial to assess safety and fetal outcome of early and midtrimester amniocentesis. The Canadian Early and Mid-Trimester Amniocentesis Trial (CEMAT) Group. Lancet 351:242, 1998 |
|
Baird PA, Yee IML, Sadnovnick AD: Population-based study of long-term outcomes after amniocentesis. Lancet 334: 1134, 1994 |
|
Hanson FW, Tennant F, Hune S et al: Early amniocentesis: Outcome, risks, and technical problems at 12.8 weeks. Am J Obstet Gynecol 166: 1707, 1992 |
|
Elejalde BR, de Elejadle MM, Acuña JB: Prospective study of amniocentesis performed between weeks 9 and 16 of gestation: Its feasibility, risks, complications and use in early genetic prenatal diagnosis. Am J Med Genet 35: 188, 1990 |
|
Penso CA, Sandstrom MM, Garber MF et al: Early amniocentesis: Report of 407 cases with neonatal followup. Obstet Gynecol 76: 1032, 1990 |
|
Stripparo L, Buscaglia M, Longatti L: Genetic amniocentesis: 505 cases performed before the sixteenth week of gestation. Prenat Diagn 10: 359, 1990 |
|
Hackett GA, Smith JH, Rebello CTH et al: Early amniocentesis at 11–14 weeks gestation for the diagnosis of fetal chromosomal abnormality—a clinical evaluation. Prenat Diagn 11: 311, 1991 |
|
Assel BG, Lewis SM, Mickerman LH et al: Single-operator comparison of early and midsecond trimester amniocentesis. Obstet Gynecol 79: 940, 1992 |
|
Djalali M, Barbi G, Kennerknecht I et al: Introduction of early amniocentesis to routine prenatal diagnosis. Prenat Diagn 12: 661, 1992 |
|
Henry GP, Miller WA: Early amniocentesis. J Reprod Med 37: 396, 1992 |
|
Yang CH, ChuHo ES, Liu CC et al: Prenatal cytogenetic diagnosis by amniocentesis before 15 weeks gestation. Chung Hua I Hueh Tsa Chih Chinese Med J 52: 81, 1993 |
|
Eiben B, Goebel R, Rutt G et al: Early amniocentesis between 12th–14th week of pregnancy. Clinical experience with 1,100 cases. Geburtshilfe Frauenheilkd 53: 554, 1993 |
|
Kerber S, Held KR: Early genetic amniocentesis—4 years' experience. Prenat Diagn 13: 21, 1993 |
|
Shulman LP, Elias S, Phillips OP et al: Amniocentesis performed at 14 weeks gestation or earlier: Comparison with first-trimester chorionic villus sampling. Obstet Gynecol 83: 543, 1994 |
|
Shulman LP, Elias S, Phillips OP et al: Early twin amniocentesis prior to 14 weeks gestation. Prenat Diagn 12: 625, 1992 |
|
Nicolaides K, Brizot ML, Patel F et al: Comparison of chorionic villus sampling and amniocentesis for fetal karyotyping at 1013 weeks gestation. Lancet 344: 435, 1994 |
|
Nicolaides K, Brizot ML, Patel F et al: Comparison of chorionic villus sampling and early amniocentesis for karyotyping in 1,492 singleton pregnancies. Fetal Diagn Ther 11: 9, 1996 |
|
Sundberg K, Bang J, Smidt-Jensen S et al: Randomised study of risk of fetal loss related to early amniocentesis versus chorionic villus sampling. Lancet 350: 697, 1997 |
|
Johnson JM, Wilson RD, Singer J et al: Technical factors in early amniocentesis predict adverse outcome. Results of the Canadian Early (EA) Versus Mid-trimester (MA) Amniocentesis Trial. Prenat Diagn 19: 732, 1999 |
|
Elias S, Simpson JL, Martin AO et al: Chorionic villus sampling for first trimester prenatal diagnosis: Northwestern University Program. Am J Obstet Gynecol 152: 204, 1985 |
|
Holzgreve W, Miny P, Basarans S et al: Safety of placental biopsy in the second and third trimester. N Engl J Med 317: 1159, 1987 |
|
Shulman LP, Tharapel AT, Meyers CM, et al: Direct analysis of cytotrophoblastic cells from second and third trimester placentas: An accurate and very-rapid method-for detecting fetal chromosome abnormalities. Am J Obstet Gynecol 163: 1606, 1990 |
|
Podobnik M, Ciglar S, Singer Z et al: Transabdominal chorionic villus sampling in the second and third trimesters of high-risk pregnancies. Prenat Diagn 17: 125, 1997 |
|
Smidt-Jensen S, Hahnemann N: Transabdominal fine needle biopsy from chorionic villi in the first trimester. Prenat Diagn 4: 163, 1984 |
|
Shulman LP, Simpson JL, Elias S et al: Transvaginal chorionic villus sampling using transabdominal ultrasound guidance: A new technique for first-trimester prenatal diagnosis. Fetal Diagn Ther 8: 144, 1993 |
|
Rhoads GG, Jackson LG, Schlesselman SE et al: The safety and efficacy of chorionic villus sampling for early prenatal diagnosis of cytogenetic abnormalities. N Engl J Med 320: 609, 1989 |
|
Canadian Collaborative CVS—Amniocentesis Clinical Trial Group: Multicentre randomized clinical trial of chorion villus sampling. Lancet 337:1491, 1991 |
|
Golbus MS, Simpson JL, Fowler SE et al: Risk factors associated with transcervical CVS losses. Prenat Diagn 12: 373, 1992 |
|
Jackson LG, Zachary JM, Desnik RJ et al: A randomized comparison of transcervical and transabdominal chorionic villus sampling. N Engl J Med 327: 594, 1992 |
|
Brambati B, Lanzani A, Tului L: Transabdominal and transcervical chorionic villus sampling: Efficiency and risk evaluation of 2,411 cases. Am J Med Genet 35: 160, 1990 |
|
Smidt-Jensen S, Permin M, Philip J et al: Randomized comparison of amniocentesis and transabdominal and transcervical chorionic villus sampling. Lancet 340: 1237, 1992 |
|
Medical Research Council Working Party on the Evaluation of Chorionic Villus Sampling: Medical Research Council European Trial of Chorion Villus Sampling. Lancet 337:1491, 1991 |
|
Sidransky E, Black SH, Soenksen DM et al: Transvaginal chorionic villus sampling. Prenat Diagn 10: 583, 1990 |
|
Brambati B, Tului L, Baldi M, et al: Genetic analysis prior to selective fetal reduction in multiple pregnancy: Technical aspects and clinical outcomes. Hum Reprod 1995; 10: 818, 1995 |
|
De Catte L, Camus M, Bonduelle M et al: Prenatal diagnosis by chorionic villus sampling in multiple pregnancies prior to fetal reduction. Am J Perinatol 15: 339, 1998 |
|
van den Berg C, Braat APG, Van Opstal D et al: Amniocentesis or chorionic villus sampling in multiple gestations? Experience with 500 cases. Prenat Diagn 19: 234, 1999 |
|
Pergament E, Schulman JD, Copeland K et al: The risk and efficacy of chorionic villus sampling in multiple gestations. Prenat Diagn 12: 377, 1992 |
|
Firth HV, Boyd PA, Chamberlin P et al: Severe limb abnormalities after chorion villus sampling at 5566 days' gestation. Lancet 337: 762, 1991 |
|
Firth HV, Boyd PA, Chamberlain P et al: Limb abnormalities and chorion villus sampling. Lancet 338: 51, 1991 |
|
Mahoney MJ, USNICHD collaborators: Limb abnormalities and chorionic villus sampling. Lancet 337:1422, 1991 |
|
Monni G, Ibba RM, Lai R et al: Limb-reduction defects and chorion villus sampling. Lancet 337: 1091, 1991 |
|
Scott R: Limb abnormalities after chorionic villus sampling. Lancet 337: 103, 1991 |
|
Jackson LG, Wapner RJ, Brambai B: Limb abnormalities and chorionic villus sampling. Lancet 337: 1423, 1991 |
|
Mastroiacovo P, Cavalcanti DP: Limb reduction defects and chorion villus sampling. Lancet 337: 1091, 1991 |
|
Hsieh FJ, Chen D, Tseng LH et al: Limb-reduction defects and chorion villus sampling. Lancet 337: 1091, 1991 |
|
Burton BK, Schulz CJ, Burd L: Limb anomalies associated with chorionic villus sampling. Obstet Gynecol 79: 726, 1992 |
|
Simpson JL, Elias S: Prenatal diagnosis of genetic disorders. In Creasy RK, Resnik R (eds): Maternal-Fetal Medicine: Principles and Practice, p 61. Philadelphia, WB Saunders, 1994 |
|
Mastroiacova P, Botto LD, Cavalcanti DP et al: Limb anomalies following chorionic villus sampling: A registry based case-control study. Am J Med Genet 44: 856, 1992 |
|
Mastroiacovo P, Botto LD: Chorionic villus sampling and limb deficiencies. Review of case control and cohort studies, p 71. In Zakut H (ed): Seventh International Conference on Early Prenatal Diagnosis of Genetic Disease. Jerusalem, Israel, May 22–27, 1994 |
|
Dolk H, Beatrand F, Lechat MF (Eurocat): Chorionic villus sampling and limb abnormalities. Lancet 339: 876, 1992 |
|
Firth HV, Boyd PA, Chamberlain P et al: Analysis of limb reduction defects in babies exposed to chorion villus sampling. Lancet 343: 1069, 1994 |
|
Olney RS, Khoury MJ, Alo CJ et al: Increased risk transverse digital deficiency after chorionic villus sampling: Results of the United States multistate case-control study, 1988-1992. Teratology 51: 2029, 1995 |
|
Fishman RHB: WHO on CVS Safety. Lancet 443: 1420, 1994 |
|
Froster UG, Jackson L: Limb defects and chorionic villous sampling: Results from an international registry 1992 to 1994. Lancet 347: 489, 1996 |
|
Froster-Ikenius UG, Baird PA: Limb reduction defects in over one million consecutive live births. Teratology 39: 127, 1989 |
|
WHO/PAHO Consultation on CVS: Evaluation of chorionic villus sampling safety. Prenat Diagn 19:97, 1999 |
|
Hobbins JC, Mahoney MJ: In utero diagnosis of hemoglobinopathies: technique for obtaining fetal blood. N Engl J Med 290: 1065, 1974 |
|
Elias S, Mazur M, Sabbagha R et al: Prenatal diagnosis of harlequin ichthyosis. Clin Genet 17: 275, 1980 |
|
Gosden C, Nicolaides KH, Rodeck CH: Fetal blood sampling in investigation of chromosome mosaicism in amniotic fluid culture. Lancet 2: 613, 1988 |
|
Tipton RE, Tharapel AT, Change HT et al: Rapid chromosome analysis using spontaneously dividing cells from umbilical cord blood (fetal and neonatal) Am J Obstet Gynecol 161:1546, 1990 |
|
Porreco RP, Harshbarger B, McGavran L: Rapid cytogenetic assessment of fetal blood samples. Obstet Gynecol 82: 242, 1993 |
|
Liou JD, Chen CP, Breg WR et al: Fetal blood sampling and cytogenetic abnormalities. Prenat Diagn 13: 1, 1993 |
|
Bryndorf T, Lundsteen C, Lamb A, et al: Rapid prenatal diagnosis of chromosome aneuploidies by interphase in situ hybridization: A one-year clinical experience with high-risk and urgent fetal and postnatal samples. Acta Obstet Gynecol Scand 79: 8, 2000 |
|
Ryan G, Rodeck CH: Fetal blood sampling. In Simpson JL, Elias S (eds): Essentials of Prenatal Diagnosis, p 63. New York, Churchill Livingstone, 1993 |
|
Daffos F: Fetal blood sampling. Annu Rev Med 40: 319, 1989 |
|
Forestier F, Daffos F, Rainaut M et al: Blood chemistry of normal human fetuses at midtrimester of pregnancy. Pediatr Res 21: 579, 1987 |
|
Forestier F, Cox WL, Daffos F et al: The assessment of fetal blood samples. Am J Obstet Gynecol 158: 1184, 1988 |
|
Nicolaides KH, Clewel WH, Rodeck CH: Measurement of human fetoplacental blood volume in erythroblastosis fetalis. Am J Obstet Gynecol 157: 50, 1987 |
|
Donnenfeld AE, Wiseman B, Lavi E et al: Prenatal diagnosis of thrombocytopenia absent radius syndrome by ultrasound and cordocentesis. Prenat Diagn 10: 29, 1990 |
|
Bussel JB, Berkowitz RL, McFarland JG et al: Antenatal treatment of neonatal alloimmune thrombocytopenia. N Engl J Med 319: 1374, 1988 |
|
Weiner CP, Grant SS, Huson J et al: Effect of diagnostic and therapeutic cordocentesis upon maternal serum alpha fetoprotein concentration. Am J Obstet Gynecol 161: 706, 1989 |
|
Durandy A, Dumez Y, Guy-Grand D et al: Prenatal diagnosis of severe combined immunodeficiency. J Pediatr 101: 995, 1982 |
|
Holmberg L, Gustavii B, Jonsson A: A prenatal study of fetal platelet count and size with application to fetus at risk for Wiskott-Aldrich syndrome. J Pediatr 102: 773, 1983 |
|
Diukman, R. Tanigawara S, Cowan MJ et al: Prenatal diagnosis of Chediak-Higashi syndrome. Prenat Diagn 12: 1877, 1992 |
|
Rodeck CH, Nicolini U: Fetal blood sampling. Eur J Obstet Gynecol Reprod Biol 28: 85, 1988 |
|
Daffos F, Forestier F, Capella-Pavlovsky M et al: Prenatal management of 746 pregnancies at risk for congenital toxoplasmosis. N Engl J Med 318: 271, 1988 |
|
Peters MT, Nicolaides KH: Cordocentesis for the diagnosis and treatment of human fetal Parvovirus infection. Obstet Gynecol 75: 501, 1990 |
|
Hsieh FJ, Ko TM, Chang FM et al: Percutaneous ultrasound-guided fetal blood sampling: Experience in the first 100 cases. Taiwan I Hsueh Hui Tsa Chih 88: 137, 1989 |
|
Newton ER: Diagnosis of perinatal TORCH infections. Clin Obstet Gynecol 42: 59, 1999 |
|
Robert-Gangneux F, Gavinet MF, Ancelle T, et al: Value of prenatal diagnosis and early postnatal diagnosis of congenital toxoplasmosis: Retrospective study of 110 cases. J Clin Microbiol 37: 2893, 1999 |
|
Moise KJ, Deter RL, Kishorn B et al: Intravenous pancuronium bromide for fetal neuromuscular blockade during intrauterine transfusion for red cell alloimmunization. Obstet Gynecol 74: 905, 1989 |
|
Orlandi F, Damiani G, Jakil C et al: Clinical results and fetal biochemical data on 140 early second trimester diagnostic cordocentesis. Acta Eur Fertil 18: 329, 1987 |
|
Orlandi F, Damiani G, Jakil C et al: The risks of early cordocentesis (1221 weeks): Analysis of 500 procedures. Prenat Diagn 10: 425, 1990 |
|
Nicolini U, Nicolaidis P, Fisk NM et al: Fetal blood sampling from the intrahepatic vein: Analysis of safety and clinical experience with 214 procedures. Obstet Gynecol 76: 47, 1990 |
|
Nicolaidis P, Nicolini U, Fisk NM, et al: Fetal blood sampling from the intrahepatic vein for rapid karyotyping in the second and third trimesters. Br J Radiol 64: 505, 1991 |
|
Nicolini U, Kochenour NK, Greco P et al: Consequences of fetomaternal haemorrhage after intrauterine transfusion. BMJ 297: 1379, 1988 |
|
Weiner CP: Cordocentesis for diagnostic indications: Two years experience. Obstet Gynecol 70: 664, 1987 |
|
Ghidini A, Sepulvada W, Lockwood CJ et al: Complications of fetal blood sampling. Am J Obstet Gynecol 168: 1339, 1993 |
|
Wilson RD, Farquharson DF, Wittmann BK et al: Cordocentesis: Overall pregnancy loss rate as important as procedure loss rate. Fetal Diagn Ther 9: 142, 1994 |
|
Buscaglia M, Ghisoni L, Belloti M et al: Percutaneous umbilical blood sampling: Indication changes and procedure loss rates in a nine years' experience. Fetal Diagn Ther 11: 106, 1996 |
|
Nicolaides KH, Thorpe-Beeston JG, Noble P: Cordocentesis in assessment and care of the fetus. In Eden RD, Boehm FH (eds): Assessment and Care of the Fetus, p 291. Norwalk, CT, Appleton & Lange, 1990 |
|
Antsaklis A, Daskalakis G, Papantoniou N et al: Fetal blood sampling—indication-related losses. Prenat Diagn 18: 934, 1998 |
|
Arnold ML, Rauskolb R, Anton-Lamprecht I et al: Prenatal diagnosis of anhidrotic ectodermal dysplasia. Prenat Diagn 4: 85, 1984 |
|
Golbus MS, Sagebiel RW, Filly RA et al: Prenatal diagnosis of congenital bullous ichthyosiform erythroderma (epidermolytic hyperkeratosis) by fetal skin biopsy. N Engl J Med 302: 93, 1980 |
|
Anton-Lamprecht I: Genetically induced abnormalities of epidermal differentiation and ultrastructure in ichthyoses and epidermolyses: Pathogenesis, heterogeneity, fetal manifestation and prenatal diagnosis. J Invest Dermatol 81: 149S, 1983 |
|
Jurkovic D, Kurjak A: Prenatina dijagnostika epidermolysis bullosa hereditaria utravuenco vodenum fetalne koze. Lijec Vjesn 111: 60, 1989 |
|
Anton-Lamprecht I, Ruskolb R, Jovanovic V et al: Prenatal diagnosis of epidermolysis bullosa dystrophica Hallopean-Siemens with electron microscopy of fetal skin. Lancet 2: 1077, 1981 |
|
Blanchet I-I, Bardon C, Dumez Y, Labée F et al: Prenatal diagnosis of harlequin fetus [letter]. Lancet 1: 132, 1983 |
|
Gilgenkrantz S, Blanchet-Bardon C, Nazzaro V et al: Hypohidrotic ectodermal dysplasia: Clinical study of a family of 30 over three generations. Hum Genet 81: 120, 1989 |
|
Rodeck CH, Eady RAJ, Gosden CJ: Prenatal diagnosis of epidermolysis bullosa lethalis. Lancet 1: 949, 1980 |
|
Elias S, Emerson DS, Simpson JL et al: Ultrasound-directed fetal skin sampling for prenatal diagnosis of genodermatoses. Obstet Gynecol 83: 337, 1994 |
|
Perry TB, Holbrook KA, Hoff MS et al: Prenatal diagnosis of congenital nonbullous ichthyohemotoxin form erythroderm (lamellar ichthyosis). Prenat Diagn 7: 145, 1987 |
|
Kouseff BG, Matsuoka LY, Stenn KS et al: Prenatal diagnosis of Sjögren-Larsson syndrome. Pediatrics 101: 998, 1982 |
|
Shimizu H, Suzumori K: Prenatal diagnosis as a test for genodermatoses: its past, present and future. J Dermatol Sci 19: 1, 1999 |
|
Christiano AM, Pulkkinen L, McGrath JA et al: Mutation-based prenatal diagnosis of Herlitz junctional epidermolysis bullosa. Prenat Diagn 17: 343, 1997 |
|
Akiyama M, Suzumori K, Shimizu H: Prenatal diagnosis of harlequin ichthyosis by the examination of keratinized hair canals and amniotic fluid cells at 19 weeks' estimated gestational age. Prenat Diagn 19: 167, 1999 |
|
Elias S, Esterly NB: Prenatal diagnosis of hereditary skin disorders. Clin Obstet Gynecol 24: 1069, 1981 |
|
Golbus MS, for the International Fetoscopy Group: Special report: The status of fetoscopy and fetal tissue sampling. Prenat Diagn 4:79, 1984 |
|
Evans MI, Hoffman EP, Cadrin C et al: Fetal muscle biopsy: collaborative experience with varied indications. Obstet Gynecol 84: 913, 1994 |
|
Nevo Y, Shomrat R, Yaron Y et al: Fetal muscle biopsy as a diagnostic tool in Duchenne muscular dystrophy. Prenat Diagn 19: 921, 1999 |
|
Evans MI, Krivchenia EL, Johnson MP et al: In utero fetal muscle biopsy alters diagnosis and carrier risks in Duchenne and Becker muscular dystrophy. Fetal Diagn Ther 10: 71, 1995 |
|
Gustavii B, Lofberg L, Henrikson KG: Fetal muscle biopsy. Acta Obstet Gynecol Scand 62: 369, 1983 |
|
Evans MI, Greg A, Kunkel LM et al:In utero fetal muscle biopsy for the diagnosis of Duchenne muscular dystrophy. Am J Obstet Gynecol 165: 728, 1991 |
|
Ross JA, Jurkovic D, Nicolaides K: Coelocentesis: A study of short-term safety. Prenat Diagn 17: 913, 1997 |
|
Cruger DG, Bruun-Petersen G, Kolvraa S: Early prenatal diagnosis: Standard cytogenetic analysis of coelomic cells obtained by coelocentesis. Prenat Diagn 16: 945, 1996 |
|
Cruger DG, Bruun-Petersen G, Kolvraa S: Turner's syndrome 45,X found by coelocentesis [letter]. Prenat Diagn 17: 588, 1997 |
|
Makrydimas G, Georgiou I, Kranas V et al: Prenatal diagnosis of beta-thalassaemia by coelocentesis. Mol Hum Reprod 3: 729, 1997 |
|
Santolaya-Forgas J, Vengalil S, Kushwaha A et al: Assessment of the risk of fetal loss after the coelocentesis procedure using a baboon model. Fetal Diagn Ther 13: 257, 1998 |
|
Santolaya-Forgas J, Duval J, Prespin C et al: Extracoelomic fluid osmometry and electrolyte composition during early gestation in the baboon. Am J Obstet Gynecol 179: 1124, 1998 |
|
Reece EA: Embryoscopy: New developments in prenatal diagnosis. Curr Opin Obstet Gynecol 4: 447, 1992 |
|
Reece EA: Embryoscopy and early prenatal diagnosis. Obstet Gynecol Clin North Am 24: 111, 1997 |
|
Quintero RA, Puder KS, Cotton DB: Embryoscopy and fetoscopy. Obstet Gynecol Clin North Am 20: 563, 1993 |
|
Ville Y, Khalil A, Homphray T et al: Diagnostic embryoscopy and fetoscopy in the first trimester of pregnancy. Prenat Diagn 17: 1237, 1997 |
|
Reece EA: First trimester prenatal diagnosis: Embryoscopy and fetoscopy. Semin Perinatol 23: 424, 1999 |
|
Elias S, Simpson JL: Techniques and safety of genetic amniocentesis and chorionic villus sampling. In Sabbagha RE (ed): Diagnostic Ultrasound Applied to Obstetrics and Gynecology, p 113. 3rd ed. Philadelphia, JB Lippincott, 1994 |