Microinvasive Carcinoma of the Cervix
Authors
INTRODUCTION
Microinvasive cancer of the uterine cervix represents a stage in the continuum of cervical carcinogenesis that begins with persistent infection with the human papillomavirus (HPV) and ends with frankly invasive cancer. Mestwerdt used the term “microcarcinoma” to describe cases of early invasive cancer of the cervix,1 describing it as a lesion invading the stroma to a depth of ≤5 mm, without specification of cell type.2 Since then various terms have been used to describe this pathologic entity including some of the following: microinvasive carcinoma, covert invasive carcinoma, incipient invasion, early invasive carcinoma, microscopic foci of stromal invasion, very small carcinoma, superficial invasion, minimal invasion, intraepithelial carcinoma with microinvasive foci, and early invasive preclinical carcinoma.
The most commonly used definitions are those of the International Federation of Gynecology and Obstetrics (FIGO) and the Society of Gynecologic Oncologists (SGO), neither of which specifies cell type. In 1974, SGO defined microinvasive cancer as any lesion in which neoplastic cells invade the stroma, in one or more sites, to a depth of ≤3 mm below the base of the epithelium, without lymphatic or blood vessel involvement. The SGO definition does not comment on width. FIGO has changed its definition on multiple occasions. Currently, it considers microinvasive cancer as one that can only be identified microscopically, as opposed to grossly, with a maximum depth of 5.0 mm and maximum width of 7.0 mm.3 The depth of invasion should be measured from the base of the epithelium, either surface or glandular, from which it originates. Staging is not altered by vascular invasion. FIGO further subdivides microinvasive cancer (stage IA) into stages IA1 and IA2. Stage IA1 encompasses stromal invasion ≤3.0 mm in depth and ≤7.0 mm in width, while stage IA2 encompasses stromal invasion >3.0–5.0 mm in depth and ≤7.0 mm in width. Lesions which invade up to a depth of 5 mm are associated with varying degrees of horizontal spread. Simon et al. reported that of 125 patients with squamous cell carcinomas invading to a depth of 5 mm, horizontal spread extended up to 20 mm, the median being 4.8 mm.4 Sedlis et al. found that 22% of 133 specimens with less than 4 mm of invasion had evidence of horizontal spread ranging from 8 to 22 mm.5 Consequently, any definition of microinvasive cancer must consider other dimensions of the lesion besides its depth, as is done in FIGO’s bidimensional definition. The choice of 5 and 7 mm as the maximum depths and horizontal spread, respectively, was based on the correlation between the volume of a tumor and its potential to metastasize. A three dimensional volumetric analysis of cervical carcinomas by Burghardt6 revealed that tumor volumes less than 400 mm3 were unassociated with lymph node metastasis. When determining tumor volume the depth and width are measured and it is assumed that the third dimension (length), although not measured, usually does not exceed the width by more than 50%. Thus, for a lesion that is 5 mm deep and 7 mm wide, the assumed maximum length would be 10.5 mm. The volume would thus equal 5 mm x 7 mm x 10.5 mm = 367.5 mm3.
Currently there is no consensus regarding the definition of microinvasive adenocarcinoma, perhaps in part because accurate measurement of depth of invasion poses a challenge due to the geometric complexity of endocervical glands.7 Qizilbash described microinvasive adenocarcinoma as a lesion <5 mm thick with glandular budding and an associated inflammatory reaction, a cribriform epithelial proliferation, confluent glands in a complex pattern with little or no intervening stroma or intraluminal tufting resulting in a papillary pattern.8 Christopherson and associates and Noda and associates similarly defined microinvasive adenocarcinoma as a lesion <5 mm in depth, measured from the basement membrane of the surface epithelium,9, 10 while Kurian and al-Nafussi measured from the nearest abnormal glandular epithelium.11 Schorge and associates followed FIGO staging and measured depth of invasion from the surface of the tumor or overlying benign epithelium down to the deepest point using a calibrated 40x magnification field. Tumor length was measured as the maximal horizontal spread on any one slide.12 Östör used the following histologic criteria for microinvasive adenocarcinoma: obvious invasion ≤5 mm in depth, complete obliteration of normal endocervical crypts, extension beyond the normal glandular field, and stromal response (edema, inflammation or desmoplasia) characteristic of invasion. Östör reports that microinvasive adenocarcinoma behaves similarly to microinvasive squamous cell carcinoma; however, he concedes that in about 20% of cases it is impossible to distinguish adenocarcinoma in situ from early invasive adenocarcinoma.13
CARCINOGENESIS
Certain viral types of the family of human papillomaviruses (HPVs) are thought to be necessary agents in the development of most cases of cervical cancer;14 other co-factors are as yet ill-defined. Under optimal testing, HPV DNA can be identified in most invasive cervical cancer tissue specimens.15 High-risk HPV types 16, 18, 31, 33, and 35 are associated with invasive cervical cancer, as well as the more aneuploid dysplasias, while low-risk HPV types 6, 11, 42, and 44 occur in the lesser dysplasias and benign condylomas that have a polyploid DNA distribution. Since aneuploidy confers a substantial risk of progression and since polyploidy is associated with regression, oncogenic risk of HPV infection is dependent on the infecting viral strain.1617, 18, 19, 20, 21, 22 In an International Agency for Research on Cancer (IARC) meta-analysis of 10,058 cervical cancer cases from 85 published studies, the most common HPV types identified were, in order of decreasing prevalence, HPV 16, 18, 45, 31, 33, 58, 52, 35, 59, 56, 6, 51, 68, 39, 82, 73, 66, and 70. Over two-thirds of the cases were associated with an infection of either HPV 16 (51.0%) or 18 (16.2%).23 The IARC Working Group concluded that human papillomavirus types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66 are carcinogenic to human beings.24
Prevalence of subclinical HPV infections can reach up to 40% in the female population at ages of greatest sexual activity, with an annual infection rate of 10–15%. Prevalence decreases to 5–10% in the age groups beyond 30 years. Half-life for high-risk HPV types is estimated to be 8–10 months. More than 90% of HPV-infected women resolve infections spontaneously and resolution of infection appears to confer some degree of acquired immunity with cross-reactivity across types.25
Risk factors for HPV infection include early onset of sexual activity, multiple sexual partners, and sexual partners with multiple sexual partners. Among women with HPV infection, risk factors for cervical cancer include high parity (>7), long-term use of oral contraceptives (>5 years), cigarette smoking (ever), exposure to ionizing radiation, and co-infection with HIV or Chlamydia trachomatis. Epidemiologic studies and laboratory data have provided conflicting results for an association of herpes simplex virus-2 with cervical cancer.26, 27 Male circumcision reduces risk of genital HPV DNA prevalence and cervical cancer in the female partner.28 Systematic, strict condom use reduces without totally preventing risk of transmission of HPV between sexual partners.29 Some nutrients have been associated with a reduction in the persistence of HPV, including nutrients involved in oxidation reactions (e.g., carotenoids, vitamins C and E), methylation or one-carbon transfer reactions (e.g., folic acid, vitamin B12, vitamin B6, cysteine), and hormone-like activity (e.g., retinoic acid).30
Expression of HPV E6 and E7 proteins is essential for cellular immortalization.31, 32 The viral E6 gene product of HPV types 16 and 18 binds the normal protein, resulting in inactivation of p53 tumor suppressor.33 E7 binds to pRB (retinoblastoma), liberating E2F transcription factors, thus promoting host and viral DNA synthesis. E7 also binds and activates cyclin complexes that control progression through the cell cycle.34 In the rare instances in which both HPV DNA and a p53 mutation in the cellular genome are present, cervical cancers are particularly aggressive. Although p53 mutations are rare in cervical cancer, their occurrence has a negative effect on survival.35, 36
Butler and associates suggest that the FHIT gene may have a mechanistic role as a cofactor with HPV in triggering the development of cervical cancer and loss of Fhit protein expression could have clinical utility in stratifying preinvasive cervical lesions according to risk of progression.37 In a study of 48 tissue specimens from women with cervical intraepithelial neoplasia (CIN) 1, CIN 2, CIN3, and microinvasive squamous cell carcinoma, Torng and associates showed that expression of transforming growth factor-β1 decreased as tumor cells progressed from CIN 1, CIN2, CIN3 to microinvasive cancer (p <0.05).38 Nashimura and associates analyzed DNA from 72 cervical tissue specimens (CIN III, microinvasive, and invasive cancers) for loss of heterozygosity and microsatellite instability and showed that genomic instability is a late event during carcinogenesis that is associated with conversion of CIN to an invasive phenotype.39 Though there is increasing understanding of the role of HPV in cervical cancer, we lack complete understanding of the molecular mechanisms that support HPV persistence and progression and that induce the transition from CIN 3 to microinvasive cancer.
HISTOGENESIS
Cervical intraepithelial neoplasia (CIN) is the term used to encompass all preinvasive epithelial abnormalities of the cervix. CIN is a state in which neoplastic or potentially neoplastic epithelial cells are confined to the epithelium. Older terminology included “dysplasia” and “carcinoma in situ” to connote a two-tier disease process that, in the past, impacted choice of therapy. It was felt that if only dysplasia was present, no or limited treatment was needed. On the other hand, if carcinoma in situ was diagnosed, in many instances a hysterectomy was recommended. This concept is now felt to be inappropriate, particularly since the cervical epithelium may be no thicker than 0.25 mm.
CIN is a continuum of changes from the earliest recognizable abnormality in the deep (basal) layers of the stratified squamous epithelium (CIN I or mild dysplasia) to involvement of the middle (CIN II) and superficial (CIN III) layers before invading the underlying stroma. In a study of 1001 women with CIN 1, Bansal and associates40 showed that at 6 months 49% regressed to normal, 45% persisted as low grade, and 7% progressed to high grade lesions. Of those with negative pathology at 6 months, 80% remained negative at 12 months, 16% demonstrated low grade lesions, and 4% progressed to high grade lesions. Among those with low grade lesions at 6 months, 50% regressed, 46% had persistent low grade, and 4% progressed to high grade lesions. Östör reviewed studies conducted from 1950 to 1990 with a wide range of follow-up (0.1–19 years) and found for CIN 1 the likelihood of regression was 57%, persistence 32%, progression to CIN 3 11%, and progression to invasion 1%. For CIN 2 the likelihood of regression was 43%, persistence 35%, progression to CIN 3 22%, and progression to invasion 5%.41 In a study of 1063 women diagnosed with CIN 3, 31.3% of the 143 women managed by punch or wedge biopsy and 50.3% of the 92 women in the subset who had persistent disease within 24 months developed cancer within 30 years, while only 0.7% of 593 women receiving adequate or probably adequate initial treatment with conventional therapy for recurrent disease developed cancer within 30 years, providing the most valid direct estimate available of the rate of progression from CIN 3 to invasive cancer.42
Loss of epithelial basement membrane is the sine qua non of an invasive neoplasm. Invasion is facilitated by adhesion, proteolysis, and migration.43 Adherence to basement membrane and subsequent release and activation of proteolytic enzymes permit tumor cells to invade into the underlying stroma and create sites for entry into and exit from lymphatics and blood vessels. The actual process of translocation across the basement membrane (tumor cell locomotion) may be due to tumor cell recognition and response to basement membrane proteins, chemotactic cytokines, and autocrine motility factors.43, 44 Many physiologic cellular functions are necessary for cells to successfully traverse the barriers to invasion. These range from the autonomous ability to stimulate normal endothelium to form capillaries to feed and provide transportation for the tumor cells to the ability to enter that vascular access. Attachment to specific glycoproteins of the extracellular matrix, such as fibronectin, collagen, and laminin is mediated through tumor cell receptors.45, 46, 47, 48 In an ultrastructural and immunohistochemical study of infiltration in microinvasive carcinoma of the uterine cervix, Kudo and associates49 used transmission electron microscopy to examine features of locally infiltrating lesions. Many pseudopod-like cytoplasmic protrusions of the cancer cells and abundant microfilaments parallel to the direction of the protrusion were seen. Concomitant with the disappearance of part of the basal lamina, many vesicles 70–90 nm in diameter were observed, suggesting a role for these vesicles in cancer infiltration. With the immunoperoxidase method, the distribution of fibronectin around the microinvasive lesions was noted. Fibronectin is a component of extracellular matrices and, presumably, in view of its action on cell adhesion, is a resistant factor against cancer cell infiltration. Fibronectin decreases in the transitional area between the cancer nest and the stroma during the stage of microinvasion.
In its earliest histologic manifestations, invasion of the connective tissue stroma may be characterized by isolated cells, small circumscribed groups of cells, or irregularly shaped, finger-like processes. At this time, the invasive epithelial masses are limited in their depth of penetration and are also discrete. Many cell types may be found in invasive pegs, sometimes with keratinization. Manifestations of degeneration or retrogressive changes are encountered in some of the transected invasive cell masses. Nuclear pyknosis or chromatolysis is frequently observed along with a shrinking of the nuclear membrane. Loss of the affinity of the cytoplasmic dyes and pronounced vacuolization of the cytoplasm may be observed. Occasionally, calcification is seen.
CYTOLOGY
Microinvasive cancer is a histologic diagnosis and depends on the extent of stromal invasion. The diagnosis of microinvasive cancer cannot be made cytologically because of the inability of cytologists to judge the extent of stromal invasion simply by looking at cellular characteristics alone. However, some investigators have shown that cytological characteristics can be used to predict histologic findings of microinvasive squamous cancer of the cervix with a high degree of accuracy. Ng50 noted that 65 of 66 microinvasive cancers had abnormal surface reactions including cellular and nuclear pleomorphism, disorganized cellular polarity, circumscribed foci of differentiation, presence of nucleoli, and keratinization. All of the changes were more conspicuous in the epithelium overlying the site of microinvasion and were more pronounced with infiltration. Surface ulceration was uncommon and limited infiltrations were more conspicuous as the depth of penetration increased. Other cellular features commonly found in microinvasive cancer included:
Distribution (isolated sheets, synctia)
Cellular configuration (polyhedral, round, oval, irregular)
Chromatin pattern (uniformly finely granular, irregularly finely granular, irregularly coarsely granular, pyknotic)
Nucleolus (total number, micronucleoli, macronucleoli, single or multiple).
When cellular findings were considered in relation to the depth of invasion, certain distinctive patterns were evident. Cellular features of lesions with a depth of invasion ranging from 2.1 mm to 3 mm were the most distinctive. On the basis of cellular features alone, Ng was able to diagnose 27 of 31 (87.1%) cases of microinvasive cancers correctly. Of the four that were incorrectly diagnosed, two were microinvasive cancers with a depth of invasion less than 3 mm, erroneously classified as severely dysplastic lesions (high grade CIN). The other two were microinvasive cancers, incorrectly classified as frankly invasive to a depth greater than 3 mm. In a retrospective review, Rubio51 was unable to confirm Ng's data. Instead, he found that the numbers of abnormal cells, smears, and various nuclear and cytoplasmic characteristics were essentially the same in 103 cases of microinvasive cancer as they were for 256 cases of severe dysplasia. Nguyen,52 using Ng's criteria, could identify three of five microinvasive cancers with a depth of invasion between 1 mm and 1.2 mm and 15 of 20 with invasion from 2.1 mm to 3 mm.
The cytologic concept of microinvasive carcinoma perhaps may be valid in some laboratories under strictly controlled conditions. However, it has limited validity in the day-to-day practice of diagnostic cytology. Consequently, any serious attempt by cytopathologists to make this diagnosis is both impractical and unnecessary.
COLPOSCOPY
Invented by Hinselmann53 in 1940, colposcopy as a tool for the diagnosis of early cervical neoplasia has increased markedly in popularity since the late 1960s. Its greatest value lies in directing the site of biopsy when a smear is abnormal. Though up to 15% of CIN occurs in the endocervical canal out of range of the colposcope,54 colposcopy can be used to identify extension of intraepithelial neoplasia or microinvasion onto the vagina. Colposcopic diagnoses are based on the following features of cervical lesions: vascular pattern, intercapillary distance, color tone relative to adjacent normal tissue, surface pattern, and border between two adjacent areas. The distances between normal blood vessels vary between 50 μm and 250 μm, averaging about 150 μm. These distances increase progressively in intraepithelial neoplasia and early invasion. With advancing grades of the lesion, the vessels become larger and more bizarre in configuration and the surface pattern of the lesion becomes more uneven, granulated, papillomatous, or nodular. The first colposcopic indications of early stromal invasion may be observed as areas of irregular mosaic or punctation. The most superficial parts of the basket-like mosaic vessels proliferate into the mosaic field. Similarly, the tops of the loops of coarse punctation vessels can be found running parallel to the surface covered only by a few cell layers. These horizontal vessels are so superficial that they are easily seen. By further proliferation of these horizontal vessels, a typical area of mosaic and punctation changes into an area with definitely atypical vessels. Initially, the intercapillary distance may be reduced, but as the process continues, relatively large vascular areas are formed. These atypical branched vessels are not found in intraepithelial neoplasia, only in early and frankly invasive carcinoma.
Thus, the irregularity of the vascular pattern, the degree of increase in intercapillary distance, and the characteristics of the surface help to distinguish the severity of epithelial neoplasia. In a study of 8497 women, 375 were diagnosed with CIN 2 or worse, 364 of whom had satisfactory colposcopy. Among those 364 women, 57.1% were diagnosed using colposcopically directed biopsy (biopsy in each quadrant of the cervix in which a lesion was seen), 37.4% with random biopsy (performed in each quadrant without a colposcopically visible lesion), and 5.5% with endocervical curettage alone, supporting a role for colposcopy in directing punch biopsy though with suboptimal sensitivity for detecting areas of high grade CIN.55 In an attempt to understand the role of colposcopically directed punch biopsy in the treatment of high-grade disease, four British Society of Colposcopy and Cervical Pathology-accredited colposcopists56 took a single colposcopically directed punch biopsy immediately prior to large loop excision of the transformation zone of 170 women selected on the basis of cytology and colposcopy. Overall agreement for presence or absence of CIN was poor (ĸ = 0.21) but in terms of histologic grade agreement was fair to moderate (ĸ = 0.32). Sensitivity and specificity of colposcopically directed punch biopsy for detection of high-grade CIN was 74% and 91%, respectively, with positive and negative predictive values of 97% and 48%, respectively. In this study, two microinvasive and two intraepithelial glandular lesions were missed on punch biopsy. While colposcopy is valuable for directing the biopsy, the definitive diagnosis is best established histologically, perhaps preferentially from loop electrosurgical excision procedure (LEEP)/large loop excision of the transformation zone (LLETZ) specimens.
PATHOLOGY
A generally accepted definition of microinvasive cancer is a lesion that is predominantly intraepithelial with a focus of invasion of microscopic dimensions confined to the superficial stroma. Gland involvement does not remove the lesion from the category of intraepithelial neoplasia.
Squamous cell carcinomas account for approximately 80% of invasive cancers of the cervix, while adenocarcinomas account for 15%. Among microinvasive cancers adenocarcinomas account for 12%.57
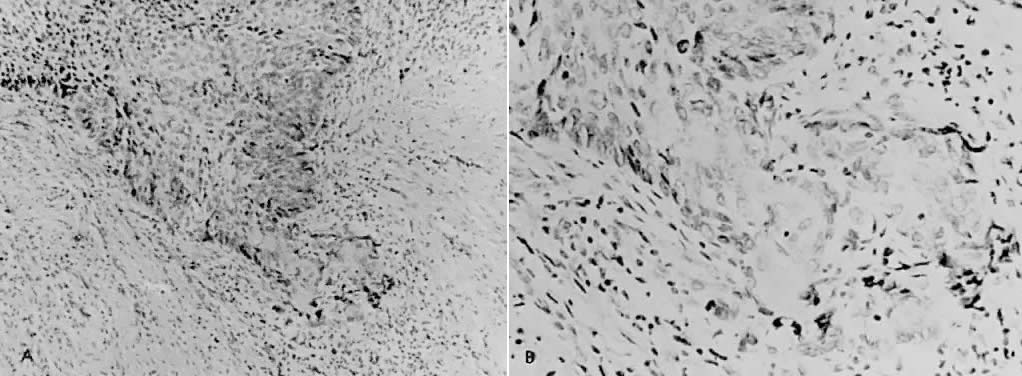
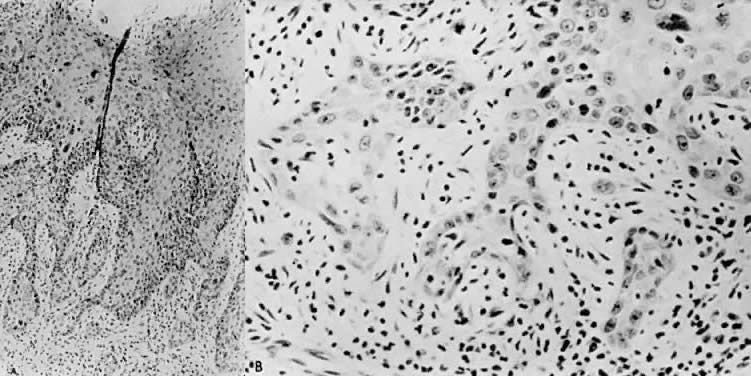
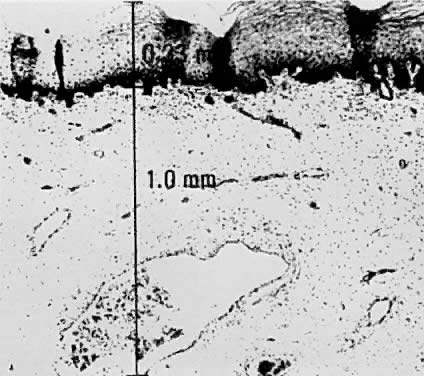
There is general agreement that disruption of the basement membrane is the criteria by which microinvasive cancer can usually be identified. Using electron microscopy to study the basement membrane, Luiebel and associates58 showed that it is usually absent or present in an imperfect form in invasive cancer. In sites where the membrane is absent, small cytoplasmic protrusions of cancer in the adjacent stroma can be seen, apparently representing invasion by malignant neoplastic cells. Ashworth and associates59 confirmed these findings. They added that invasive cell groups are partially or completely invested by a basement membrane that appears to be newly formed by cells of the invading peg. The typical histologic picture of early stromal invasion, beneath a field of CIN III, may show some differentiation of the invasive peg, characterized by a richness in cytoplasm and nuclei that are larger and clearer than those of the intraepithelial portion of the lesion. Some of the cells may be in various stages of degeneration with leukocytic infiltration. In hematoxylin-eosin preparations, pegs may appear eosinophilic and stand out from the basophilic matrix (Fig. 1), surrounded by a dense round cell infiltrate. Small tongues of tapering or branching epithelial cells, as well as the formation of narrow cell columns or the appearance of cell groups that seem to be invading the stroma, may be considered criteria of invasion (Fig. 2). On the other hand, the formation of very large epithelial pegs can only be regarded as suspicious of early invasion.60
Zaino describes two unequivocal features identifying the presence of invasion in endocervical adenocarcinoma: (1) individual cells or fragmented or incomplete glands lined by cytologically malignant-appearing cells at a stromal interface and (2) malignant-appearing glands surrounded by a desmoplastic host response. In a significant minority of microinvasive adenocarcinomas, neither of the two features will be present though architecturally complex, branching, irregular, or small glands that grow confluently and cribriform growth pattern of malignant-appearing epithelium devoid of stroma within a single gland profile may be noted.61
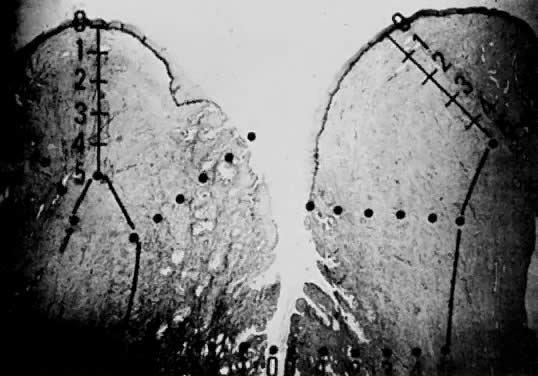
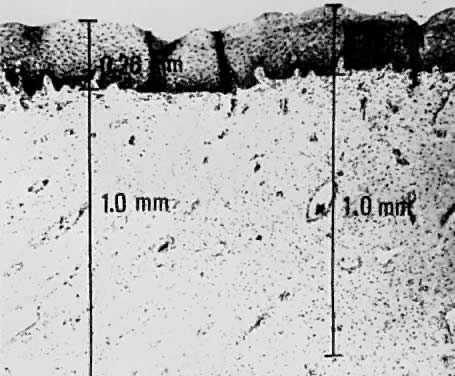
Using a hysterectomy specimen and a micrometer, Averette62 demonstrated how variations in thickness of the normal cervical epithelium impacts the actual extent to which a lesion penetrates the underlying stroma. By demonstrating variable thicknesses of the epithelium at the levels of the ectocervix, squamocolumnar junction, and distant endocervix (Fig. 3) he showed that a 1-mm point measured from the basement membrane extends to different depths into the underlying stroma (Fig. 4). In addition, vascular channels were shown to be less than 1 mm beneath the epithelium (Fig. 5). These figures suggest the following:
The depth into the stroma may vary depending on the point of measurement.
Five-millimeter depth is significant in terms of total cervical thickness.
There may be a relative difference in stromal depth when 1 mm is measured from two separate points.
Vascular channels are present within 1 mm of the stroma and are potentially a conduit for spread even in very early invasive lesions.
Duncan and Walker63 found vascular involvement in only two of 76 patients with invasion of less than 3 mm, whereas vascular involvement was apparent in eight of 15 patients who had stromal invasion in the 3–5 mm depth range. Only two of 91 patients had metastatic disease, both with vascular involvement. Vascular involvement was present in only six of 135 patients reported by Hasumi and associates:64 there was no vascular involvement in patients with invasion ≤1 mm but there was vascular involvement in three of 45 (6.7%) with invasion of 1.1–3 mm and three of 29 (11.1%) with invasion of 3.1–5 mm. None of the six cases with vascular invasion had nodal metastasis. Sedlis and associates5 reported vascular space invasion by tumor in 31 of 132 patients (23%); this increased with depth of penetration. There was a strong correlation between both residual tumor in the hysterectomy specimen and vascular space involvement with deep stromal penetration and extensive lateral spread. No positive lymph nodes were observed in the 74 patients treated by radical hysterectomy, and recurrence was noted in only two patients, both of whom had extensive vascular involvement. Although there is a strong correlation between depth of invasion and vascular space involvement, the latter is not necessarily associated with poor clinical outcomes.
Simon65 and Creasman66 and colleagues reviewed the literature with regard to lymph vascular space and lymph node involvement. In the study by Simon,65 54 (14.3%) of 378 women subjected to radical surgery and pelvic lymphadenectomy had lymphovascular space involvement but only one of the 54 (1.9%) had a node metastasis. None of the 213 women with long-term follow-up had either local recurrence or died from tumor. In Creasman's66 analysis of 267 women treated in a similar surgical fashion and with microinvasive tumors invading below the basement membrane to 3 mm or less, 39 had lymphovascular space involvement and only one (without lymphovascular involvement) had lymph node metastasis. Of 105 women with tumors invading between 3.1 and 5 mm, 49 (47%) had lymphovascular permeation, one of whom had lymph node metastasis. Of the 56 patients without lymphovascular involvement, six (11%) had positive nodes. Thus, it appears that in relationship to microinvasive carcinoma of the cervix, the presence of tumor cells in lymphovascular spaces does not imply that lymph node metastases are imminent. Additionally, there is disagreement as to what constitutes a lymphovascular space.
DIAGNOSIS
Clinical signs and symptoms of microinvasive cancer are either nonexistent or nonspecific, therefore, in the absence of a gross lesion, histologic material is usually obtained because of an abnormal Papanicolaou smear. The diagnosis is best made by colposcopically directed punch biopsy or some form of cervical conization. Random punch biopsies are inadequate for diagnosis.67, 68 Colposcopy is a safe method for the diagnosis of CIN. In the past it was thought to have an accuracy rate as high as 85%.69 More recently its accuracy has been found to be in the range of 50–60%. In the ASCUS-LSIL Triage Study, sensitivity of initial colposcopy for CIN 3 identified during 2 years of observation was 54%.70 For microinvasive cancer, specifically, colposcopy directed biopsy may have the lowest accuracy. Baldauf et al. showed global agreement between biopsy diagnosis and final diagnosis in 89.6% of cases in a study of 399 patients, however, agreement was only 31.2% for microinvasive cancers.71 Proper patient selection and physician experience are essential for the safety of this technique. When a conization is necessary, colposcopic definition of the lesion is of value in tailoring the operation to fit the lesion. Conization is necessary following initial diagnosis of microinvasive cancer by a colposcopically directed biopsy to accurately define the extent of microscopic penetration into the stroma. A conization, properly performed, should include a portion of the portio vaginalis, the external cervical os, and the endocervical canal as high up as is feasible, preferably to the internal os. The entire transformation zone should be incorporated. The inclusion of all abnormal epithelium may be facilitated by the use of the colposcope following the application of 3–5% acetic acid, or, if that is unavailable, Schiller's iodine solution. Theoretically, if an endocervical curettage is performed following the conization, all cervical lesions can be detected by proper examination of the tissue.
In order that the greatest amount of information is obtained from the cone, it is essential that the entire specimen be cut into blocks and step-serial sections be performed on each block. A preferred method of histologic evaluation of the cone uses 15 blocks from the cone and involves removal of eight to ten sections at intervals through each block.72 Another approach would be to dismantle the cone in wedge-shaped blocks and take two sections from each block. If any of the sections reveal frankly invasive carcinoma, no further study is necessary. If no malignant lesion or only CIN III is found, each block should be step-sectioned so that ten additional slides are obtained from each block, totaling 100–150 sections.73 Other methods necessitate 100–200 serial sections.74, 75, 76
More recently, the loop electrosurgical excision procedure (LEEP) has replaced cold knife conization in many areas. LEEP uses an electrical current flowing through a fine wire loop to “cut” via vaporization and coagulate via dehydration. LEEP is performed under local anesthesia and has been shown to be safe, effective, and well-tolerated. In most situations histologic specimens are excellent but charred margins can present a problem for the pathologist. To prevent their occurrence it has been recommended that the LEEP operator guides the electrode steadily through the tissue but without dragging it in order to minimize the time of contact with the tissue and thus minimize charring of the tissue specimen margins. Unfortunately, even with optimal technique, cautery artifact at the tissue specimen margins remains a troublesome entity, particularly when evidence of microinvasive cancer exists at the edge of the cauterized portion of the specimen. In this situation, there continues to be disagreement whether to recommend repeat LEEP or conization or to proceed with a more radical procedure.
TREATMENT
There are several approaches to treating of microinvasive cancer of the cervix ranging from cervical conization to radical hysterectomy with pelvic lymph node dissection and from local radium treatment to full pelvic irradiation.
Risk of recurrence
A study reviewing 370 patients with stage I cervical cancer treated by radical hysterectomy and pelvic-aortic node dissection showed that patients meeting SGO or FIGO definitions of stage I microinvasive cancer have low risk for metastasis or recurrent cancer. The literature review indicated a recurrence rate of 4.2% for stage IA2, with lymphovascular space invasion serving as a better predictor of lymph node metastasis and recurrence than surface dimension.77 Patients with stage IA1 microinvasive disease have a 1% risk for recurrence after simple hysterectomy and an overall 5-year survival of 99%. Patients with stage IA2 microinvasive disease have 3–5% risk of recurrence and an overall 5-year survival of 96%.78
Review of management options
Querleu and associates79 recommended diagnostic pelvic lymphadenectomy in the staging of early carcinoma of the cervix as a less morbid method to determine regional lymph node status. Laparoscopic radical hysterectomy with paraaortic and pelvic lymph node dissection has recently been reported as a reasonable therapeutic approach to therapy for microinvasive carcinoma of the cervix.80
Averette and associates62 reported a multicenter study of microinvasive carcinoma of the cervix. The criteria used were penetration of invasive carcinoma beneath the basement membrane of less than 1 mm, determined by calibrated optics. Additionally, the literature was reviewed for criteria of diagnosis of microinvasive cancer, as well as methods of management. In the literature, when depth of penetration of up to 5 mm was used as the criterion, the incidence of nodal metastasis was as high as 3.5%. In the institutions involved in this study, patients were treated by radical hysterectomy and pelvic lymph node dissection. There were no recurrences and no positive pelvic nodes. The conclusion was that simple hysterectomy was not adequate therapy for lesions with stromal invasion to a depth of 5 mm.
Bohm,81 Seski,82 Duncan and Walker,63 and Boronow83 reported a combined total of 165 radical hysterectomies with pelvic lymph node dissections in which there were four patients with positive pelvic nodes. The limits of invasion used by the first studies was 3 mm; however, Boronow used a previous FIGO stage IA without defining the limits of the lesion. All four patients with positive nodes were reported by Bohm.81 Two of those four patients had lymphatic or blood vessel invasion; two patients died of cancer. Roche and Norris84 found no positive nodes in 30 patients with stromal penetration up to 5 mm. Of 111 patients with microinvasive carcinoma who were studied over a 21-year period by Christopherson and Parker,67 the sole pathologic criteria for inclusion was equivocal invasion to a depth of no more than 5 mm. Eighty-four patients were treated by simple hysterectomy or less extensive surgery, 14 by conization and irradiation, three by hysterectomy and irradiation, and ten by radical hysterectomy. Ninety-one patients were followed for 5 years or until death, and 80 patients were followed for 10 years or until death; one patient was lost to follow-up at 5.5 years. Two deaths officially attributed to cervical cancer were doubtful. Both had been previously treated by cervical conization and irradiation. Eighty-four patients had been treated by either simple hysterectomy, excision of the cervical stump, or cervical conization only. There were no recurrences in this group. It was suggested that simple hysterectomy should be the maximal treatment indicated.
Hasumi and associates64 reported on 135 patients with stage I epidermoid carcinoma of the uterine cervix invading less than 5 mm below the basement membrane. These cases were studied to determine the biological behavior of early invasive carcinoma and to establish diagnostic criteria for microinvasive cancer of the cervix. None of the 135 patients had metastasis to the parametrial tissue in the final surgical specimen. One (0.9%) of 106 patients with invasion up to 3 mm had lymph node metastasis, while four (13.9%) of 29 patients with invasion of 3.1–5 mm had nodal metastasis. Of the 106 patients with invasion up to 3 mm, 25 had confluent invasion. None of the 25 patients had lymph node metastasis. In view of these results, it was felt that carcinomas with invasion less than 3 mm may be regarded as a separate diagnostic group because of their limited metastatic potential and may be treated by conservative methods used for CIN III, even if there was a confluent pattern. For carcinomas with invasion of 3.1–5 mm, more extensive procedures as used for frankly invasive cancers were thought to probably be necessary. Van Nagell85 reported on 177 patients with squamous cell carcinoma that invaded the cervical stroma to a depth of 5 mm or less. In 52 patients with lesions that invaded the cervical stroma to a depth of 3 mm or less, none contained metastatic tumor in their lymph nodes. Conversely, lymph node metastases were present in 10% of patients with lesions that had stromal invasion of 3.1–5 mm. Among 145 patients with lesions that invaded the stroma to a depth of 3 mm or less, only two developed recurrences, both of which were intraepithelial. Among the 32 cases of carcinoma that invaded the stroma 3.1–5 mm, there were three invasive recurrences and two deaths. Creasman66 analyzed 114 patients with stage IA carcinoma of the cervix retrospectively in regard to depth of invasion, capillary-like space involvement, stromal reaction, status of conization margins, and the incidence of lymph node metastasis. There were no lymph node metastases or recurrences in the group of patients whose depth of stromal invasion was less than 3 mm. Maiman and associates,86 reported 5% incidence of pelvic lymph node metastasis in a series of 117 women with histologically defined superficially invasive (1–5 mm) squamous cell carcinoma of the cervix. The incidence of metastasis in those patients with invasion 3 mm or less was 2% and in those with invasion 3.1–5 mm was 13%. Microscopic lymphovascular invasion and degree of lateral spread of tumor were also associated with lymph node metastasis, whereas tumor grade was not. Sevin and associates87 attempted to assess the risk of lymph node metastasis and treatment failures of microinvasive carcinoma according to FIGO and SGO definitions by retrospectively reviewing the histopathologic material on 370 patients with microinvasive carcinoma of the cervix who were treated by radical hysterectomy and pelvic-aortic lymph node dissection. Histopathologic analysis of tumors was based on a uniform format including measurement of the maximum depth of invasion, the width and length of the horizontal tumor spread, invasive growth pattern, cell type, tumor grade, and lymphatic or vascular space involvement. Of 370 patients, 110 had a depth of invasion of 5 mm or less. Of these, 54 patients fulfilled the SGO definition of microinvasive carcinoma; 42, the FIGO stage IA2 definition; and 27, both definitions. None of the patients with microinvasive carcinoma, as defined by either the SGO or the FIGO stage IA2 had lymph node metastases or tumor recurrence. These data support the conclusion that microinvasive carcinomas, defined by either the SGO or FIGO definitions, have a low risk for metastasizing to regional lymph nodes or recurring. It was noted, however, that a review of the literature indicated a recurrence rate for stage IA2 of 4.2%.
Roman and Latour88 treated 84 cases of stage IA carcinoma by simple hysterectomy alone, with an overall 5-year survival of 97.5%. Boyes and associates89 treat microinvasive carcinoma by cervical conization. The specimen was then examined by step-serial sections. If the lesion appeared to have been removed completely, no further treatment was instituted. If the cancer had not been completely removed by the cone biopsy, either a second cone biopsy or hysterectomy with an adequate vaginal cuff was recommended, depending on the lesion and other clinical considerations. If lymphatic involvement was seen in the biopsy, the patient was treated by irradiation or radical hysterectomy. Way and associates90 treated microinvasive lesions by modified radical hysterectomy including at least 3 cm of the upper vagina. They stated that (1) node dissection is unnecessary; (2) total hysterectomy is inadequate; and (3) conservation should be reserved only for those who desire further pregnancies. Nelson91 described 355 such operations with one fistula and no operative deaths.
Cervical cone biopsy has been used by several authors with good results under carefully selected circumstances. Of 113 patients added from different series in the literature,92, 63, 93, 94 there was one recurrence of CIN III in a group of patients that were followed for an adequate length of time. Mestwerdt95 thought that conization could be considered adequate therapy but only for young women who wanted to bear children, since this method has a high relapse quotient. Sedlis and associates96 reviewed 100 cases in which they had both conization and subsequent hysterectomy specimens. Residual invasive tumor on the hysterectomy specimen was deeper than in the cone in nine cases. Residual invasive tumor was less than that in the cone in six cases. Residual dysplasia was present in 31 cases, 27 of those harboring CIN III. No residual tumor was found in only 54 cases. The conization specimen of the nine cases with invasion greater in the hysterectomy specimen than in the cone had the following characteristics: depth of invasion greater than 2 mm in 77.5% (7 of 9); lateral extension greater than 4 mm in 90% (8 of 9); vascular space invasion present in 90% (8 of 9); and tumor involvement of the surgical margins present in 90% (8 of 9). Residual invasive carcinoma of the hysterectomy specimen increased from 0 in cases with invasion less than 1 mm on the cone specimen to 61% if invasion on the cone was deeper than 3 mm. Of 15 patients in whom the invasion did not exceed the 5-mm limit in the hysterectomy specimen and the surgical margins of the cone were involved, residual tumor was present in 12 (80%). In eight of these patients, the invasion was more extensive in the uterus than in the cone. One of the patients with recurrence of carcinoma had tumor involvement of the surgical margin on the cone specimen. In Seski's82 study of 54 patients, 78% had residual CIN III or microinvasive carcinoma in the hysterectomy specimen following cone biopsy.
In a retrospective review of 166 patients with microinvasive cancer of the cervix, Gadducci and associates97 reported on 143 patients with stage IA1 and 23 with stage IA2 receiving conization alone (n = 30, 18%), total hysterectomy (n = 82, 49%), or radical hysterectomy (n = 54, 33%). All of the patients receiving conization alone had stage IA1 disease. Of the 166 patients, eight (5%) had intraepithelial recurrence and four (2%) had invasive recurrence. Disease recurred in three (10%) patients treated with conization alone, four (5%) of those who underwent total hysterectomy, and five (9%) of those who underwent radical hysterectomy. Among the conization only group, none had invasive recurrence after a median follow-up of 45 months, suggesting that conization alone can represent definitive management in patients with disease-free cone margins and apex.
Greer and associates98 reported on 50 patients with early invasive squamous cell carcinoma of the cervix treated with a cervical conization followed by a radical hysterectomy and pelvic lymph node dissection. They found histologically positive margins at the time of cone biopsy in 66% of patients. Negative margins at the time of the cone biopsy were identified in 34%. Residual invasive disease at the time of radical hysterectomy was found in 24% of patients with negative margins. Four per cent had positive lymph nodes. Three patients had recurrent metastatic disease. This study of stage IA2 patients demonstrated that a preoperative diagnosis of that stage is difficult to establish and creates a therapeutic dilemma regarding treatment.
Fertility preservation
Abu-Rustum and associates99 describe radical vaginal trachelectomy with bilateral complete pelvic lymphadenectomy for stage IA1–IB1 cervical cancer with the intent to resect the cervix, upper 1–2 cm of vagina, parametrium, and paracolpos in a similar manner to a type III radical abdominal hysterectomy but sparing the uterine fundus or corpus. Eligibility criteria for radical vaginal trachelectomy include the following: women less than 40 years of age who have strong desire to preserve fertility, no clinical evidence of impaired fertility, FIGO stages IA–IB1, no involvement of the upper endocervical canal, and negative regional lymph nodes.100 The same guidelines could be adapted for radical abdominal trachelectomy, though some institutions will routinely perform a total radical abdominal hysterectomy for up to a 4cm stage IB1 lesion or even moderate (<6 cm) IB2 lesion. Abu-Rustum and associates favor radical vaginal trachelectomy with a laparascopic lymphadenectomy in selected patients with stage IA1 disease with evidence of lymphovascular invasion, stage IA2 disease, and selected stage IB1 lesions (mainly small occult lesions <2 cm in which the majority of tumor is wide with a superficially invasive pattern with satisfactory vaginal anatomy). Radical abdominal trachelectomy represents a fertility-sparing technique that targets the primary tumor and regional lymph nodes in the same manner as type III abdominal radical hysterectomy but sparing the uterine corpus. Normal menstrual function is seen in 93% of women who undergo radical trachelectomy.101 In a review of 72 cases of vaginal radical trachelectomy performed between 1991 and 2003 on women with stages IA, IB, and IIA cervical cancer, Plante showed a recurrence-free survival of 95%.102 In a large retrospective review of studies describing the results and complications of pregnancies after radical trachelectomy, Boss and associates103 summarized 63 studies including 355 radical trachelectomy procedures. Of 153 patients (43%) who tried to conceive during the follow-up period, 70% succeeded resulting in a total of 161 pregnancies, 49% of which were term deliveries. Cervical stenosis resulting in menstrual disorders or fertility problems was found in 15% of patients who tried to conceive and resolved with repeated surgical dilatation in the majority of cases. Complications during pregnancy included premature (<36 weeks) delivery (20%) and second trimester loss (8%).
Most recently, an Italian group104 enrolled 21 nulliparous women with stage IB1 cervical tumors (median tumor size 15 mm, range 10–30 mm) for three courses with cisplatin 75 mg/m2, paclitaxel 175 mg/m2, and ifosfamide 5g/m2 (replaced with epirubicin 80 mg/m2 in adenocarcinoma) followed by cold-knife conization and pelvic lymphadenectomy. They achieved complete response in five cases, CIN 3 or microinvasive residue in 12 and stromal invasion >3 mm in four. Five women underwent radical hysterectomy at that time; the remaining 16 women had no recurrence after a median follow-up of 69 months, with ten pregnancies occurring among six of those women.
Adenocarcinoma
In a report of 32 patients with microinvasive adenocarcinoma with a mean follow-up of 54 months for 31 of 32 patients, Ceballos and associates105 described 29 patients who underwent hysterectomy, two who underwent radical trachelectomy, and one who underwent cone biopsy; one patient received adjuvant radiotherapy. Twenty-seven patients had bilateral pelvic lymph node dissections and no lymph node metastases were identified. No recurrences were reported. The authors recommend less radical surgery in this low-risk patient population given the excellent prognosis of the tumor, absence of lymph node metastases, and lymph node dissection complication rate of 7% (two patients had chronic leg edema). In a retrospective review of patients diagnosed with early invasive (≤5 mm stromal invasion) adenocarcinoma of the cervix between 1992 and 1999, Poyner and associates106 described 33 patients with mean age of 41.6 years. Subsequent treatment included repeat conization (n = 3), simple hysterectomy (n = 4), radical hysterectomy with pelvic lymph node dissection (n = 25), and radical trachelectomy with pelvic lymph node dissection (n = 1). Ten patients had positive conization margins for invasive cancer, five of which had residual disease in the subsequent surgical specimen. Three patients had positive margins for adenocarcinoma in situ, 14 had negative margins, and six had margins that could not be evaluated. After a median follow-up of 30 months, all patients remained without evidence of disease. Based on absence of parametrial spread and pelvic lymph node involvement in early lesions, the authors recommend treatment with conization with negative margins when future fertility is desired or simple hysterectomy, though they recommend prospective studies to document the safety of this approach. Yahata and associates107 report on four women with stage IA1 cervical adenocarcinoma diagnosed during pregnancy. All four underwent laser conization and vaporization at 16–23 weeks’ gestation and were found to have endocervical type adenocarcinoma without lymphovascular space invasion. Two of the four had positive conization margins for invasive cancer and underwent a second conization at 20 weeks’ gestation and 5 weeks after delivery. All four patients delivered at term. One was treated with cervical conization alone and the other three received extended radical hysterectomy with pelvic lymph node dissection after delivery. None of the patients had residual invasive cancer in subsequent surgical specimens and none developed recurrent disease after 2–13-year follow-up.
Uniformity in treatment of microinvasive adenocarcinoma of the uterine cervix is lacking. In a study of 29 patients with stage IA1 and nine stage IA2, treatment modalities ranged from radical hysterectomy with pelvic lymph node dissection to conization only.108 No recurrences were noted for any of the women during a 72-month follow-up period. Based on a review of 1565 patients, Bisseling and associates concluded that conization is safe for stage IA1 and IA2 disease, with pelvic lymph node dissection recommended if lymphovascular space invasion is present.
Current recommendations
For patients with stage IA1 microinvasive cancer, the risk for nodal metastasis is very low (0.5–1.2%).109 Current recommendations from the World Health Organization, without specification of histopathologic type, are simple hysterectomy for women with microinvasive cancer stage IA1.110 Risk for recurrence after this treatment is 1% and overall 5-year survival is 99%.111 FIGO recommendations for treatment of stage IA1 include total abdominal or vaginal hysterectomy. The presence of associated vaginal intraepithelial neoplasia indicates that an appropriate vaginal cuff should be removed. When fertility is desired, FIGO recommends observation after cone biopsy with Pap smear follow-up at 4 months, 10 months, and then annually if both previous smears are negative (level of evidence B).112 For stage IA2, risk for nodal metastasis is considerably higher (5–7%) than in IA1.113 WHO states that simple hysterectomy with lymph node dissection is indicated, though modified radical hysterectomy with lymph node dissection is preferred. If fertility preservation is desired, WHO recommends radical trachelectomy with pelvic lymphadenectomy.114 FIGO recommends modified radical hysterectomy and pelvic lymphadenectomy for stage IA2. In the absence of lymphovascular space invasion, they suggest that extrafascial hysterectomy and pelvic lymphadenectomy may be considered. When fertility is desired, a large cone biopsy plus extraperitoneal or laparoscopic pelvic lymphadenectomy or a radical trachelectomy plus extraperitoneal or laparascopic pelvic lymphadenectomy is recommended.115 Risk for recurrence for IA2 tumors treated with modified radical hysterectomy and bilateral pelvic lymphadenectomy is 3–5% and overall 5-year survival is 96%.116
CONCLUSION
It appears that lesions that have no lymphatic or vascular involvement and are less than 3 mm in depth may be treated conservatively, but only when adequate tissue is submitted to and evaluated by the pathologist. The extent of early invasion must be carefully ascertained, usually by LEEP or cervical cone biopsy. The pathologist should properly orient and cut the cone into 15–18 blocks, depending on the size of the specimen. Each block should be step-serial sectioned in a manner to assess the exact extent of invasion, using a micrometer. This procedure may entail more than 100 slides. If this plan is rigidly followed, it appears that LEEP or cervical conization is adequate treatment when the lesion is less than 1 mm in depth. Total hysterectomy, conization, LEEP, or radical trachelectomy is probably safe if the lesion is less than 3 mm. For lesions that are greater than 3 mm in depth, radical hysterectomy or radiation is indicated. Unless elaborate measures are taken to establish the extent of involvement, the modified radical operation is the minimal safe surgical procedure and conforms to the operative principle of wide excision of the lesion, though recent studies suggest that radical trachelectomy has high efficacy for stage IA2 when fertility preservation is desired. The significance of confluence and lymphovascular space involvement has not been clearly established to date. It does appear that both phenomena increase as the depth of penetration increases and that neither occurs with regularity if the depth is less than 1 mm and neither occurs commonly up to a depth of 3 mm. Therefore, it seems that the depth should be used as a primary guide, but the other factors may be indicators of tumor volume and, therefore, should modify a decision regarding conservative treatment. LEEP and conization should primarily be reserved for the patient who desires fertility. If this method is chosen, a careful explanation of the risks should be given. Additionally, the pathologic specimen must be rigorously evaluated, the cone margins should be negative for invasion or CIN, there should be no evidence of lymphatic or vascular invasion, the lesion should be of squamous type, and the patient must be compliant to follow-up recommendations. If margins are positive but fertility is desired, radical trachelectomy can be considered for microinvasive disease.
The point at which the biologic nature of microinvasive carcinoma of the cervix changes and it begins to behave as a truly invasive lesion is not well-defined. The crux of the problem lies in the fact that investigators continue to use light microscopy to define what is, in essence, a molecular phenomenon.
REFERENCES
Mestwerdt G. Die Frühdiagnose des Kollumkarzinoms. Zentralbl Gynäkol 1947;69:198-202 |
|
Mestwerdt G. Atlas der Kolposkopie. Jena:Fischer, 1953 |
|
Creasman WT. Modification in the staging for Stage I vulvar and Stage I cervical cancer: Report of the FIGO Committee on Gynecologic Oncology. Int J Gynecol Obstet 1995;50:215-216 |
|
Simon NL, Gore H, Shingleton HM, Soong SJ, Orr JW Jr, Hatch KD. Study of superficially invasive carcinoma of the cervix. Obstet Gynecol 1986;68(1):19-24. |
|
Sedlis A, Sall S, Tsukada Y: Microinvasive carcinoma of the uterine cervix. A clinical-pathologic study. Am J Obstet Gynecol 1979;133(1):64-74. |
|
Burghardt E: Microinvasive carcinoma. Obstet Gynecol Surv 1979;34:836 |
|
Zaino RJ. Symposium part I: Adenocarcinoma in situ, glandular dysplasia, and early invasive adenocarcinoma of the uterine cervix. Int J Gynecol Pathol 2002;21:314-326. |
|
Qizilbash A. In situ and microinvasive adenocarcinoma of the uterine cervix. Am J Clin Pathol 1975;64:155-170. |
|
Christopherson W, Nealon N, Gray L. Noninvasive precursor lesions of adenocarcinoma and mixed adenosquamous carcinoma of the cervix uteri. Cancer 1979;44:975-983. |
|
Noda K, Kimura K, Ikeda M, Teshima K. Studies on the histogenesis of cervical adenocarcinoma. Int J Gynecol Pathol 1983;1:336-346. |
|
Kurian K, al-Nafussi A. Relation of cervical glandular intraepithelial neoplasia to microinvasive and invasive adenocarcinoma of the uterine cervix: a study of 121 cases. J Clin Pathol 1999;52:112-117. |
|
Schorge JO, Lee KR, Flynn CE, Goodman AK, Sheets EE. Stage IA1 cervical adenocarcinoma: definition and treatment. Obstet Gynecol 1999;93:219-222. |
|
Östör AG. Early invasive adenocarcinoma of the uterine cervix. Int J Gynecol Pathol 2000;19:29-38. |
|
Bosch FX, Lorincz A, Muñoz N, Meijer CJLM, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002;55:244-65. |
|
Bosch, FX and de Sanjose, S. The epidemiology of human papillomavirus infection and cervical cancer. Disease Markers 2007;23:213-227. |
|
Fu YS, Reagan JW, Townsend DE. Nuclear DNA study of vulvar intraepithelial and invasive squamous neoplasms. Obstet Gynecol 1981;57:643. |
|
Crum CP, Braun LA, Shah KV. Correlation of nuclear DNA content and the presence of human papillomavirus (HPV) structural antigen. Cancer 1982;49:468. |
|
Crum CP, Levine RU. Human papillomavirus infection and cervical neoplasia: New perspectives. Int J Gynecol Pathol 1984;3:376 |
|
Rastkar G, Okagaki T, Twiggs LB, Clark BA. Early invasive and in-situ warty carcinoma of the vulva: Clinical, histologic, and electron microscopic study with particular reference to viral association. Am J Obstet Gynecol 1982;143:814. |
|
Stanbridge C, Butler EB. Human papillomavirus infection of the lower female genital tract: Association with multicentric neoplasia. Int J Gynecol Pathol 1983;2:264. |
|
Reid R, Fu YS, Herschman BR. Genital warts and cervical. VI. The relationship between aneuploid and polyploid cervical lesions. Am J Obstet Gynecol 1984;150(2):189-199. |
|
Winkler B, Crum CP, Fujii T. Koilocytotic lesions of the cervix: The relationship of mitotic abnormalities to the presence of papillomavirus antigens and nuclear DNA content. Cancer 1984; 53(5):1081-1087. |
|
Franceschi S. The IARC commitment to cancer prevention: the example of papillomavirus and cervical cancer. Recent Results Cancer Res 2005;166:277-97. |
|
IARC Monographs on the evaluation of carcingenic risks to humans, volume 90, Human papillomaviruses. Lyon; International Agency for Research on Cancer 2007. |
|
Castellsagué X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol 2008;110:S4-S7. |
|
Dhanwada KR, Garrett L, Smith P, Thompson KD, Doster A, Jones C. Characterization of human keratinocytes transformed by high risk human papillomavirus types 16 or 18 and herpes simplex virus type 2. J Gen Virol 1993;74:955-63. |
|
Fang L, Ward MG, Welsh PA, Budgeon LR, Neely EB, Howett MK. Supression of human papillomavirus gene expression in vitro and in vivo by herpes simplex virus type 2 infection. Virology 2003;314(1):147-60. |
|
Castellsagué X, Bosch FX, Munoz N et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med 2002;346:1105-1112. |
|
Hogewoning CJ, Bleeker MC, van Den Brule AJ, Voorhorst FJ, Snijders PJ, Berkhof J, et al. Condom use promotes regression of cervical intraepithelial neoplasia and clearance of human papillomavirus: a randomized clinical trial. Int J Cancer 2003;107:811-6. |
|
IARC Monographs on the evaluation of carcingenic risks to humans, volume 90, Human papillomaviruses. Lyon; International Agency for Research on Cancer 2007. |
|
DiPaolo JA, Woodworth CD, Popescu NC: Induction of human cervical squamous cell carcinoma by sequential transfection with human papillomavirus 16 DNA and viral Harvey RAS. Oncogene 4: 395, 1989 |
|
Sedman SA, Barbosa MS, Vass WC: The full-length E6 protein of human papillomavirus type 16 has transforming and transactivating activities and cooperates with E7 to immortalize keratinocytes in cultures. J Virol 65: 4860, 1991 |
|
Scheffner M, Werness BA, Huibregtse JM et al: The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63: 1129, 1990 |
|
Bosch FX, Lorincz A, Munoz N, Meijer CJLM, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002;55:244-265. |
|
Crook T, Vousden KH: Properties of p53 mutations detected in primary and secondary cervical cancers suggest mechanisms of metastasis and involvement of environmental carcinogens. EMBO J 11: 3935, 1992 |
|
Borresen AL, Helland A, Nesland J et al: Papillomavirus and cervical cancer. Lancet 339: 1350, 1992 |
|
Butler D, Collins C, Mabruk M, Leader MB, Kay EW. Loss of Fhit expression as a potential marker of malignant progression in preinvasive squamous cell cancer. Gynecol Oncol 2002;86:144-149. |
|
Torng PL, Chan WY, Lin CT, Huang SC. Decreased expression of human papillomavirus E2 protein and transforming growth factor-â1 in human cervical neoplasia as an early marker in carcinogenesis. J Surg Oncol 2003;84:17-23. |
|
Nashimura M, Furumoto H, Kato T, Kamada M, Aono, T. Microsatellite instability is a late event in the carcinogenesis of uterine cervical cancer. Gynecol Oncol 2000;79:201-206. |
|
Bansal N, Wright JD, Cohen CJ, Herzog TJ. Natural history of established low grade cervical intraepithelial (CIN 1) lesions. Anticancer Res. 2008;28(3B):1763-6. |
|
Östör AG. Natural history of cervical intraepithelial neoplasia: A critical review. Int J Gynecol Pathol 1993;12:186-92. |
|
McCredie MRE, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, Skegg DCG. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol 2008;9:425-34. |
|
Liotta LA: Tumor invasion: Role of the extracellular matrix. Cancer Res 46: 1, 1986 |
|
McCarthy JB, Hager SJ, Furcht LT: Human fibronectin contains distinct adhesion- and motility-promoting domains for metastatic melanoma cells. J Cell Biol 102: 179, 1986 |
|
Hynes RO: Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 69: 11, 1992 |
|
Chen W-T, Wang J, Hasegawa T: Regulation of fibronectin receptor distribution by transformation, exogenous fibronectin, and synthetic peptides. J Cell Biol 103: 1649, 1986 |
|
Takeichi M: Cadherins: A molecular family important in selective cell-cell adhesion. Annu Rev Biochem 59: 237, 1990 |
|
Wewer UM, Liotta LA, Jaye M: Altered levels of laminin receptor mRNA in various human carcinoma cells that have different abilities to bind laminin. Proc Natl Acad Sci U S A 83: 7137, 1986 |
|
Kudo R, Sato T, Mizuuchi H: Ultrastructural and immunohistochemical study of infiltration in microinvasive carcinoma of the uterine cervix. Gynecol Oncol 36: 23, 1990 |
|
Ng ABP: Third tutorial on clinical cytology: Microinvasive carcinoma of the uterine cervix. Read before the Symposium of the International Academy of Cytology at the University of Chicago, January 8, 1971 |
|
Rubio CA: Cytologic studies in cases with carcinoma in-situ and microinvasive carcinoma of the cervix. Acta Pathol Microbiol Scand A82:161, 1974 |
|
Nguyen GK: Exfoliative cytology of microinvasive squamous-cell carcinoma of the uterine cervix. A retrospective study of 42 cases. Acta Cytol 28: 457, 1984 |
|
Hinselmann H: Zur Kenntnis der praekanzerösen Veränderungen der Portio. Zentralbl Gynakol 51: 901, 1927 |
|
Burghardt E and Östör AG. Colposcopy, cervical pathology. Textbook and atlas. 2nd ed. New York: Georg Thieme. 1991:p247. |
|
Pretorius RG, Zhang WH, Belinson JL, Huang MN, Wu LY, Zhang X, Qiao YL. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol 2004;191:430-4. |
|
Byrom J, Douce G, Jones PW, Tucker H, Millinship J, Dhar K, Redman CWE. Should punch biopsies be used when high-grade disease is suspected at initial colposcopic assessment? A prospective study. Int J Gynecol Cancer 2006;16:253-56. |
|
Östör AG. Early invasive adenocarcinoma of the uterine cervix. Int J Gynecol Pathol 2000;19:29-38. |
|
Luiebel FJ, Sanders E, Ashworth CT: Electron microscopic study of carcinoma in situ and invasive carcinoma of the cervix uteri. Cancer Res 20: 357, 1960 |
|
Ashworth CT, Stembridge VA, Luibel FJ: A study of basement membranes of normal epithelium, carcinoma in situ, and invasive carcinoma of the uterine cervix utilizing electron microscopy and histochemical methods. Acta Cytol 5: 369, 1961 |
|
Burghardt E, Holzer E: Diagnosis and treatment of microinvasive carcinoma of the cervix uteri. J Obstet Gynecol 49: 641, 1977 |
|
Zaino RJ. Symposium part I: Adenocarcinoma in situ, glandular dysplasia, and early invasive adenocarcinoma of the uterine cervix. Int J Gynecol Pathol 2002;21:314-26. |
|
Averette HE, Nelson JH, Ng ABP et al: Diagnosis and management of microinvasive (Stage 1A) carcinoma of the uterine cervix. Cancer 38: 414, 1976 |
|
Duncan ID, Walker J: Microinvasive squamous carcinoma of the cervix in the Tayside region of Scotland. Br J Obstet Gynecol 84: 67, 1977 |
|
Hasumi K, Atsuhiko S, Sugano H: Microinvasive carcinoma of the uterine cervix. Cancer 45: 928, 1980 |
|
Simon NL, Gore H, Shingleton HM et al: Study of superfically invasive carcinoma of the cervix. Obstet Gynecol 68: 19, 1986 |
|
Creasman WT, Fetter BF, Clarke-Pearson DL et al: Management of Stage 1A carcinoma of the cervix. Am J Obstet Gynecol 153: 164, 1985 |
|
Christopherson WM, Parker JE: Microinvasive carcinoma of the uterine cervix. Cancer 17: 1125, 1964 |
|
Bickenbach W, Soost HJ, Campos J: What percentage of cervices show early invasion in serial histological sections in uteri which were removed under the biopsy diagnosis of "carcinoma in situ.” Acta Cytol 5: 340, 1961 |
|
Savage EW: Correlation of colposcopically directed biopsy and conization with histologic diagnosis of cervical lesions. J Reprod Med 15: 211, 1975 |
|
The ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol 2003;188:1383-92. |
|
Baldauf JJ, Dreyfus M, Ritter J, Philippe E. An analysis of the factors involved in the diagnostic accuracy of colposcopically directed biopsy. Acta Obstet Gynecol Scand 1997;76:468-73. |
|
Fidler Boyes DA, Lock DR: Intraepithelial carcinoma of the cervix: 214 cases with emphasis on investigation by cytology and cone biopsy. Can Med Assoc J 77: 79, 1957 |
|
Bryans FE, Boyes DA, Boyd JR et al: The cytology program in British Columbia. Can Med Assoc J 90: 62, 1964 |
|
Johannisson E, Kolstad P, Soderberg G: Cytologic, vascular and histologic patterns of dysplasia, carcinoma in situ and early invasive carcinoma of the cervix. Acta Radiol Suppl 258, 1966 |
|
Foushee JHS, Greiss FC, Lock FR: Stage 1A squamous cell carcinoma of the uterine cervix. Am J Obstet Gynecol 105: 46, 1969 |
|
Parker RT, Cuyler WK, Kauffman LA et al: Intraepithelial (Stage O) cancer of the cervix. Am J Obstet Gynecol 80: 693, 1960 |
|
Sevin BU, Nadji M, Averette HE, Hilsenbeck S, Smith D, Lampe B. Microinvasive carcinoma of the cervix. Cancer 1992;70(8):2121-8. |
|
Gray HJ. Primary management of early stage cervical cancer (IA1-IB) and appropriate selection of adjuvant therapy. JNCCN 2008;6:47-52. |
|
Querleu D, Leblanc E, Castelain B: Laparoscopic pelvic lymphadenectomy in the staging of early carcinoma of the cervix. Am J Obstet Gynecol 164: 579, 1991 |
|
Nezhat CR, Burrell MO, Nezhat FR et al: Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. |
|
Bohm JW, Krupp PJ, Lee FYL: Lymph node metastasis in microinvasive epidermoid cancer of the cervix. Obstet Gynecol 48: 65, 1976 |
|
Seski JC, Abell MR, Morley GW: Microinvasive squamous carcinoma of the cervix: Definition, histologic analysis, late results of treatment. Obstet Gynecol 50: 4, 1977 |
|
Boronow RC: Stage I cervix cancer and pelvic node metastasis: Special reference to the implications of the new and the recently replaced FIGO classification on Stage 1A. Am J Obstet Gynecol 127: 2, 1977 |
|
Roche WD, Norris HJ: Microinvasive carcinoma of the cervix: The significance of lymphatic invasion and confluent patterns of stromal growth. Cancer 36: 180, 1975 |
|
van Nagell Jr, Greewell N, Powel DF et al: Microinvasive carcinoma of the cervix. Am J Obstet Gynecol 145:981, 1983 |
|
Maiman MA, Fruchter RG, DiMaio TM, Boyce JG: Superficially invasive squamous cell carcinoma of the cervix. Obstet Gynecol 72: 339, 1988 |
|
Sevin BU, Nadji M, Averette HE et al: Microinvasive carcinoma of the cervix. Cancer 70: 2121, 1992 |
|
Roman TN, Latour JPA: The effect of early diagnosis on survival statistics in carcinoma of the uterine cervix. Am J Obstet Gynecol 97: 739, 1967 |
|
Boyes DA, Worth AJ, Fidler HK: The result of treatment of 4389 cases of preclinical cervical squamous carcinoma. J Obstet Gynecol Br Common 7: 769, 1970 |
|
Way S, Hennigan M, Wright VC: Some experiences with preinvasive and microinvasive carcinoma of the cervix. J Obstet Gynecol Br Common 75: 593, 1968 |
|
Nelson JH: Atlas of Radical Pelvic Surgery. New York, Appleton-Century-Crofts, 1969 |
|
Burghardt E: Microinvasive carcinoma. Obstet Gynecol Surv 34: 836, 1979 |
|
Wilkinson E, Komorowski R: Borderline microinvasive carcinoma of the cervix. Obstet Gynecol 51: 472, 1978 |
|
Popkin D, Pilgorge R, Latour J: The treatment of microinvasive squamous cell carcinoma of the uterine cervix. Gynecol Oncol 8: 84, 1979 |
|
Mestwerdt G: Fruhdiagnose des Kollumkarzinoms. Zentralbl Gynakol 69: 326, 1947 |
|
Sedlis A, Sall S, Tsukada Y: Microinvasive carcinoma of the uterine cervix. A clinical-pathologic study. Am J Obstet Gynecol 133: 64, 1979 |
|
Gadducci A, Sartori E, Maggino T, Landoni F, Zola P, Cosio S, Pasinetti B, Alessi C, Maneo A, Ferrero A. The clinical outcome of patients with stage IA1 and IA2 squamous cell carcinoma of the uterine cervix: a Cooperation Task Force (CTF) study. Eur J Gynaecol Oncol. 2003;24(6):513-6. |
|
Greer BE, Figge DC, Tamimi HK et al: Stage IA2 squamous carcinoma of the cervix: Difficult diagnosis and therapeutic dilemma. Am J Obstet Gynecol 162: 1406, 1990 |
|
Abu-Rustum NR, Sonoda Y, Black D, Levine DA, Chi DS, Barakat RR. Fertility-sparing radical abdominal trachelectomy for cervical carcinoma: technique and review of the literature. Gynecol Oncol 2006 103:807-813. |
|
Roy M, Plante M. Pregnancies after radical vaginal trachelectomy for early-stage cervical cancer. Am J Obstet Gynecol 1998 (Dec) 179:1491-6. |
|
Ungar L, Palfalvi L, Hogg RS et al. Abdominal radical trachelectomy: a fertility-preserving option for women with early cervical cancer. BJOG 2005;112(3):366-9. |
|
Plante M, Renaud MC, Francois H, Roy M: Vaginal radical trachelectomy: an oncologically safe fertility-preserving surgery. An updated series of 72 cases and review of the literature. Gynecol Oncol 2004; 94: 614-623 |
|
Boss EA, van Golde RJ, Beerendonk CC, Massuger LF. Pregnancy after radical trachelectomy: a real option? Gynecol Oncol 2005 (May);97(2):707-9. |
|
Maneo A, Chiara S, Bonazzi C, Mangioni C. Neoadjuvant chemotherapy and conservative surgery for stage IB1 cervical cancer. Gynecol Oncol. 2008 Oct 2. (Epub ahead of print). |
|
Ceballos KM, Shaw D, Daya D. Microinvasive cervical adenocarcinoma (FIGO stage IA tumors): results of surgical staging and outcome analysis. Am J Surg Pathol 2006;30(3):370-4. |
|
Poyner EA, Marshall D, Sonoda Y, Slomovitz BM, Barakat RR, Soslow RA. Clinicopathologic features of early adenocarcinoma of the cervix initially managed with cervical conoziation. Gynecol Oncol 2006; 103:960-5. |
|
Yahata T, Numata M, Kashima K, Sekine M, Fujita K, Yamamoto T, Tanaka K. Conservative treatment of stage IA1 hysterecetomy. In adenocarcinoma of the cervix during pregnancy. Gynecol Oncol 2008;109:49-52. |
|
Bisseling KCHM, Bekkers RLM, Rome RM, Quinn, MA. Treatment of microinvasive adenocarcinoma of the uterine cervix: a retrospective study and review of the literature. Gynecologic Oncology 107(2007):424-430. |
|
Sevin BU, Nadji M, Averette HE, et al. Microinvasive carcinoma of the cervix. Cancer 1992;70:2121-28. |
|
Comprehensive cervical cancer control: a guide to essential practice. WHO Press. Geneva, Switzerland. 2006. pp179-181. |
|
Benedet JL, Odicino F, Maisonneuve P, et al. Carcinoma of the cervix uteri. Int J Gynaecol Obstst 2003;83(Suppl 1):41-78. |
|
Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynaecologic cancers. FIGO committee on gynecologic oncology. International Journal of Gynecology and Obstetrics 2000;70(2):209-62. |
|
Sevin BU, Nadji M, Averette HE, et al. Microinvasive carcinoma of the cervix. Cancer 1992;70:2121-28. |
|
Comprehensive cervical cancer control: a guide to essential practice. WHO Press. Geneva, Switzerland. 2006. pp179-181. |
|
Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynaecologic cancers. FIGO committee on gynecologic oncology. International Journal of Gynecology and Obstetrics 2000;70(2):209-62. |
|
Benedet JL, Odicino F, Maisonneuve P, et al. Carcinoma of the cervix uteri. Int J Gynaecol Obstst 2003;83(Suppl 1):41-78. |