Pituitary Tumors
Authors
INTRODUCTION
Pituitary neoplasms are relatively common in the general population, the incidence ranging from 10–23%. Most of these lesions are small, even microscopic; consequently, most patients are asymptomatic. Clinically, pituitary tumors are the most common pathologic cause of pituitary dysfunction and include 10% of all intracranial neoplasms. Symptoms may result from compression of surrounding structures by a large tumor or, as in the case of a functional adenoma, hypersecretion of one or more anterior pituitary hormones. Pituitary tumors are virtually always benign and amenable to treatment, provided that mass extrasellar extension has not occurred. Current neuroradiologic techniques and the availability of radioimmunoassays for most anterior pituitary hormones have enhanced the physician's ability to diagnose and manage pituitary tumors.1
PITUITARY ANATOMY
The pituitary gland, or hypophysis, is a globular structure that measures approximately 10 × 15 × 5 mm and weighs approximately 0.5 g. It lies within the cavity of the sella turcica and is attached to the base of the brain by a pituitary stalk. The pituitary is bordered laterally by the cavernous sinus, including cranial nerves 3, 4, and 6; the first division of cranial nerve 5; and part of the internal carotid artery. Superiorly, the sellar diaphragm, which is formed by condensation of the dura, separates the gland from the optic chiasm, the hypothalamus, and other cranial structures.
Structurally, the pituitary is composed primarily of an anterior (adenohypophysis) lobe and posterior (neurohypophysis) lobe, which represent the embryonic consolidation of two ectodermal primordia. One primordium results from evagination of the embryonic pharyngeal roof (Rathke's pouch). Active sellar proliferation of the anterior wall of Rathke's pouch leads to the development of the anterior lobe of the pituitary. In humans, cells of the posterior wall are inactive and give rise to a poorly defined vestigial intermediate lobe. The pars tuberalis, a thin layer of cells that overlies the anterior surface of the pituitary stalk, also develops from Rathke' pouch. The other primordium develops as an outgrowth from the diencephalic portion of the neural tube. It extends vertically to attach to the posterior lobe of the pituitary. The remainder of this neuroectodermal derivative gives rise to the infundibulum, or pituitary stalk, and the median eminence.
HISTOPATHOLOGY
Formerly, pituitary cells were classified histologically as acidophilic, basophilic, or chromophobic. Unfortunately, because this classification is based on conventional staining techniques, it is limited and cannot account for the secretion of pituitary hormones encountered in various physiologic and pathologic conditions. By means of electron microscopy and immunocytochemistry, it has been shown that the anterior pituitary contains distinct and specialized cell types that are responsible for the synthesis and secretion of particular peptide hormones.2 These hormones are synthesized in the rough endoplasmic reticulum and packaged by the Golgi apparatus into secretory granules. Hormone release occurs through effusion of the granule membrane with that of the cell surface and exocytosis.
The largest secretory granules are found in the lactotropic cells, which are responsible for the production of prolactin. These cells are located in the lateral wings of the anterior pituitary. Adjacent to the lactotropic cells are somatotropic cells, which secrete growth hormone. The relationship between the origins of lactotropic and somatotropic cells is unknown, although prolactin and growth hormone bear close structural similarities and are thought to share an ancestral gene. Not infrequently, patients with acromegaly and a growth hormone-secreting pituitary tumor exhibit excess production of prolactin. This may be secondary to cosecretion of the two hormones or compression of vascular pathways by an expanding growth hormone-producing adenoma.
Thyrotropic cells are located in the midportion of the anterior pituitary and secrete thyroid-stimulating hormone (TSH), or thyrotropin. The gonadotropic cells appear to be distributed throughout the anterior pituitary and are responsible for the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Whether both LH and FSH are produced by a single cell type or each is produced by a separate one is unclear. For instance, both LH and FSH have been co-localized to single cell types by immunocytochemistry, yet lines may be cloned that produce FSH or LH uniquely.
Adrenocorticotropic cells are concentrated in the central part of the anterior pituitary and produce adrenocorticotropic hormone (ACTH). Some of these cells also have been found in the posterior pituitary. The cells that secrete melanocyte-stimulating hormone (MSH) appear to be located near those that secrete ACTH. Interestingly, MSH and ACTH share similar amino acid sequences, which suggests that they could be derived from a common cell; however, the existence of a distinct cell common to MSH and ACTH has not been established in humans. These data indicate that MSH, like ACTH, is secreted primarily by the cells in the anterior pituitary, not by the intermediate lobe as it is in most other vertebrates.
About one fourth of the cells in the anterior pituitary appear to be nonsecretory, as evidenced by the lack of secretory granules. According to the older classification, these cells would be termed 'chromophobic'. This term can be misleading, however, because the rapid release of hormone during conditions of high hormone output (e.g. in a functional pituitary adenoma) probably limits the formation of secretory granules.
A number of recently described tumors bear mention. The first is termed a silent subtype 3 adenoma, which is based on fine structural criteria.3 These are aggressive types of pituitary adenomas, a number of which respond to bromocriptine therapy, yet treatment with this agent fails to halt tumor growth. The second type is null cell adenoma.4 This makes up about one third of the adenomas found in the pituitary when classified by ultrastructural functional and immunologic means. These are often large neoplasms that are diagnosed late, secondary to their lack of hormonal production. Often the presence of these tumors is accompanied by modest hyperprolactinemia. Unfortunately, because of their size, a significant number of patients with null cell pituitary adenomas experience visual loss and some element of hypopituitarism. At the other end of the spectrum is the entity termed incidentialoma.5 These lesions usually are detected during radiographic evaluation of symptoms such as headache; however, when followed over time, they exhibit no change in size. It should be recalled that in 1936, when Costello6 evaluated 1000 pituitary glands obtained from unselected autopsies, he found a frequency of 22.5% and no correlation of tumors with clinical symptoms during the subjects' lifetime. Therefore, care should be taken to keep this entity in perspective in the follow-up evaluations of patients with prolactinomas. Finally, nonfunctioning pituitary adenomas, which secrete α-subunit, have been described.7, 8 These often stain positive for chromogranin-A, and thyrotropin-releasing hormone (TRH) influences chromogranin concentrations in a positive manner.9 These types of studies have been used as a means of determining the endocrine origin of clinically nonfunctioning pituitary tumors.
CAUSES OF PITUITARY TUMORS
The causes of pituitary tumor formation are unclear. Their occurrence has been attributed to multiple mechanisms, including changes in hypothalamic pituitary vascular supply, alteration of cellular genetics, deregulation of dopamine metabolism, deregulation of dopamine receptors, or malfunction of growth factor or hormonal expression. Many years ago, Page and Bergland demonstrated that the hypothalamus sends a rich blood supply into the pituitary. This tended to be a bilateral system with numerous intercommunications. Therefore, at about this time it was speculated that atheromatous changes in the vascular system might account for a differential gradient in delivery of dopamine to the lateral wings of the pituitary, therefore giving rise to the opportunity for hyperplasia and perhaps tumor formation. Morphologic evidence has been presented that arteries exist in human prolactinomas.10 These may be congenital or developed during tumor formation, and the presence of an arterial blood supply in the anterior pituitary could result in the failure of that area to perform normal hypothalamic regulation.
Signal transduction has been found to be defective in a number of types of adenomas.11 For instance, prolactinomas that have no dopamine receptors on their surface have been identified. D-2 receptors have been described on prolactin-secreting adenomas which maintain their receptor effect or coupling. Recently, however, dopamine binding has been demonstrated in growth hormone-secreting adenomas. Yet dopamine fails to exert an inhibitory activity on adenyl cyclase, indicating a failure of signal transduction.
It has been suggested that inactivation of a recessive oncogene on chromosome 11 is an important and possibly early event in the development of these four major types of pituitary adenomas. Mutations have been described in guanosine triphosphate-binding protein (Gs-α) in tumors that primarily secrete growth hormone. Further, tumors have been described with multiple autosomal losses. Likewise, T53 and rasg mutations are common events in the pathogenesis of both acromegaly and nonsecreting tumors. Klibanski and associates1 evaluated 78 tumors and found no rasg mutations identified in either prolactinomas or pituitary carcinomas. Pituitary adenomas also show no loss of heterozygosity at the retinoblastoma gene locus. Finally, an uncoupling of β-subunit gene expression in protein biosynthesis has been described as the setting of ongoing subunit biosynthesis, suggesting a potential mechanism for unbalanced synthesis and secretion of free α-subunits.12, 13
Another intriguing possible cause of prolactinomas involves the secretion of dopamine. Removal of prolactinomas still results in defective regulation of prolactin secretion, and persistent rapid growth hormone pulsatility has been described after removal of growth hormone-secreting pituitary tumors. Further, patients have been described bearing prolactin-secreting adenomas that are unresponsive to the prolactin-inhibiting properties of indirectly acting dopamine agonists. Crosignani and colleagues14 postulated a common central defect in hyperprolactinemic patients with or without radiologic signs of pituitary tumors. This hypothesis is supported by dopamine infusion studies showing normal prolactin suppressibility, but abnormal dopamine metabolism, in patients with hyperprolactinemia. These studies receive support from the observation that cell culture alters the hormonal responsiveness of the rat pituitary tumor to dynamic hormonal stimulation.
Currently, an altered balance between TRH and dopamine has been detected in prolactinomas and other pituitary tumors as compared with normal pituitary glands.14, 15, 16, 17, 18, 19, 20 Estrogen receptor expression has been demonstrated in human pituitaries and in macroadenomas. Estrogen-receptor expression is present in 2.3% of growth hormone-, 50% of prolactin-, 70% of FSH-, 83% of LH-, 4% of TSH-, and 1% of ACTH-positive tumors.21 The estrogen receptor-positive tumors may represent a subset whose growth and secretory profiles can be influenced by gonadal steroids. Using in situ hybridization techniques, Stefaneanu and associates22 treated nine lactotropic adenomas with bromocriptine, which weakened hybridization signals, suggesting suppression of the estrogen-receptor gene. Men treated with testosterone have been documented to have an exacerbation of their prolactin-secreting tumor despite continued treatment with bromocriptine therapy.23 The hypothesis would be that the testosterone enanthate is aromatized to estradiol, which would directly stimulate lactotropes.
Finally, various growth factors have been shown to influence prolactin secretion.24, 25 Fibroblastic growth factor stimulates prolactin from human anterior pituitary adenomas, but it does not appear to affect cell proliferation. Transforming growth factor-α and -β, however, are potent and effective inhibitors of pituitary tumor secretion when evaluated in GH4 lines. Therefore, all of these lines of evidence would seem to suggest that vascular, dopaminergic, genetic, receptor, and growth factor mechanisms may be involved in the expression of adenomas. It also suggests, however, that pituitary adenomas may arrive by different pathophysiologic pathways.
NON-FUNCTIONING PITUITARY TUMORS
Non-hormone-secreting pituitary tumors tend to grow slowly and insidiously. Karavitaki et al evaluated the natural history of non-functioning pituitary adenomas in 40 patients (22 women and 18 men).26 Sixteen subjects had microadenomas and 24 had macroadenomas. The mean follow-up period was 42 months (range, 8–128 months). The 48-month probabilities of tumor enlargement were 44% for macroadenomas and 19% for microadenomas.
Clinical symptoms develop when progressive tumor enlargement causes compression of adjacent pituitary tissue or surrounding vital structures. In most cases, these symptoms are recognized only after significant extrasellar tumor growth has occurred. One of the most common symptoms is visual impairment, which results from compression of the optic chiasm due to suprasellar extension.27 Characteristically, there is bilateral loss of vision in the superior temporal quadrants. Thereafter, the inferior quadrants are involved, leading to bitemporal hemianopsia. Visual acuity also may be affected. If untreated, this process leads to blindness and optic atrophy. In some cases, the visual field loss is atypical. This phenomenon is probably attributable to a variation in the anatomic relationship between the optic chiasm and the pituitary gland and to possible eccentric suprasellar extension of the tumor. Visual difficulties may be encountered with lateral tumor extension into the cavernous sinus, which contains cranial nerves 3, 4, and 6. The resultant extraocular palsy produces diplopia and visual blurring.
Headache is frequently associated with large pituitary tumors. There is no characteristic pattern because both the location and intensity of the pain vary widely. In patients who have a small pituitary adenoma without suprasellar enlargement, headache is uncommon. The cause of headache is unclear. The acute onset of severe headache and rapid visual loss may result from infarction of or hemorrhage into a pituitary tumor (pituitary apoplexy). These symptoms are caused by the sudden expansion of the intrasellar contents. Subsequent acute pituitary insufficiency resulting in hypotension and hyperthermia may develop, eventually leading to mental deterioration, coma, and death.
In rare instances, suprasellar tumor growth extends into the hypothalamus, causing diabetes insipidus and irregularities of sleep, temperature regulation, and appetite. In contrast, infrasellar extension into the sphenoidal sinus can lead to spontaneous cerebral fluid leakage.
Within the pituitary gland itself, progressive growth of a pituitary tumor often causes hypopituitarism by damaging the adjacent functional cells. In this regard, growth hormone and gonadotropin-secreting cells appear to be the most sensitive. Amenorrhea occurs in approximately 50% of women with nonfunctioning pituitary tumors, whereas a growth hormone deficiency is not clinically manifested in adults. Hypogonadotropic hypogonadism should always warrant consideration of a pituitary neoplasm. Deficiencies in pituitary TSH and ACTH secretion lead to secondary hypothyroidism and hypoadrenalism, respectively.
Because primary pituitary hypothyroidism can mimic the signs and symptoms of pituitary neoplasm, the diagnosis of primary pituitary hypothyroidism should be established by measurement of the serum TSH level.28 An increase in serum TSH and a favorable response to the administration of TRH serves to distinguish between secondary hypothalamic hypothyroidism and primary pituitary hypothyroidism. Patients with deficient ACTH secretion have decreased glucocorticoid and adrenal androgen production. Aldosterone secretion is controlled by the renin-angiotensin system, and mineralocorticoid function remains intact. Pituitary ACTH reserve can be assessed with an insulin tolerance test or measurement of the response to metyrapone administration. If, however, primary adrenal insufficiency is suggested, the capacity of the adrenal gland to respond to ACTH must be evaluated before these tests are performed. Prolactin-secreting cells are more resistant to damage. Consequently, a deficiency in prolactin secretion usually is observed only in cases of panhypopituitarism.
Diagnosis of a nonfunctioning pituitary tumor is based primarily on the radiologic evaluation. Formerly, plane cone-down viewing of the sella (hypocycloidal polytomography) was the recommended imaging technique.29 Computerized tomography (CT scanning) and magnetic resonance imaging (MRI) has since significantly improved the ability to detect small lesions and to delineate the extent of large tumors.30 High-resolution CT scanning is generally performed with an intravenous contrast medium (metrizamide), which allows direct visualization of soft tissue. The sensitivity of this technique is evident by the ability to demonstrate pituitary tumors as small as 2 mm. Most tumors appear as low-density lesions. In some instances, the lesions are hyperdense or isodense, posing diagnostic difficulty with respect to small tumors. In the case of a large, extracellular, isodense lesion, cisternography with intrathecal contrast medium may be necessary. The technique of MRI has proved to be just as sensitive as CT scanning, if not more so, in detecting small pituitary tumors. Moreover, in contrast to CT scanning, MRI does not require radiation. Therefore, MRI is usually performed when a pituitary lesion is suspected, although the cost of this procedure is approximately twice that of CT scanning.
The advantage of CT scanning and MRI also includes their ability to differentiate between an empty sella and a solid pituitary tumor and to visualize the internal carotid arteries and their branches. This latter capacity is comparable to cerebral angiography and thus may reveal a carotid aneurysm mimicking a pituitary neoplasm.31 Visual field examination is still a useful technique because it can be performed grossly at the bedside or formally during an office visit. By the time a visual field defect is discovered, however, irreparable damage and significant tumor growth may have already occurred.
Treatment of these tumors consists of surgical resection, radiation therapy, or both. The surgical approach is determined by the size, shape, and location of the lesion. As previously mentioned, most of the nonfunctioning pituitary neoplasms are large and may exhibit extensive suprasellar extension by the time diagnosis is made. Consequently, in these patients and in those with dumbbell-shaped tumors, an intracranial approach may be necessary for complete tumor removal. Improved surgical techniques have reduced morbidity and the risk of permanent sequela, although direct involvement of the surrounding vital structures by the tumor may limit the technical feasibility of complete removal.
If the lesion demonstrates minimal suprasellar extension, consists primarily of infrasellar growth, or is confined to the sella, then transnasal or trans-sphenoidal microdissection is the appropriate treatment. The incidence of complications associated with this approach appears to be low, and reports of trans-sphenoidal surgery have been favorable, particularly in the treatment of prolactin-secreting pituitary adenomas.32 The most frequent complication is transient diabetes insipidus, although more serious morbidity such as postoperative bleeding and cerebrospinal fluid leakage may occur and necessitate reoperation.
Radiation therapy is probably not indicated for a small pituitary tumor confined to the sella turcica. Irradiation of a large pituitary adenoma can be instituted primarily or secondarily after incomplete surgical resection. This form of therapy can be administered by proton-beam or heavy-particle irradiation supervoltage or by implantation of yttrium or gold within the sella. Heavy-particle therapy is the preferred method because it provides intense irradiation to small areas within the body. The major disadvantages of radiation therapy are that the total therapeutic effect may require several years, and that hypopituitarism may result in a number of cases.
FUNCTIONAL PITUITARY TUMORS
Prolactin-Secreting Pituitary Tumors
The characteristic features of prolactin-secreting pituitary tumors are galactorrhea and amenorrhea. In these patients, excessive production of prolactin is responsible for galactorrhea and may be the cause of amenorrhea. This may be a central mechanism, considering that infusion of GnRH in a pulsatile manner has been shown to induce full folliculogenesis and ovulation. This would imply that patients with hyperprolactinemia have an abnormality in GnRH pulsatility.
In reported studies, approximately 20% of patients with amenorrhea without galactorrhea exhibited elevated prolactin levels.33 These reports are generally composed of patients referred to medical centers and academic institutions. In a comparison study of screened (referred) and unscreened patients, the actual incidence of hyperprolactinemia was significantly reduced in a group of unscreened women. Similarly, the finding of galactorrhea in women with normal ovulatory function is poorly correlated with increased prolactin levels.34 Prolactin has many structural variances, which have been shown to fluctuate throughout the menstrual cycle. In addition, various forms of growth hormone have been detected in patients with galactorrhea and normal ovulatory function. Thus, this symptom alone cannot be used as a reliable marker of hyperprolactinemia. In these patients, a prior history of physiologic or pharmacologic stimulation of lactotropic cells can be elicited. It is unknown whether the transient hyperprolactinemia induced by these stimulatory influences is responsible for persistent or subsequent galactorrhea.
The diagnosis of a prolactin-secreting tumor is based primarily on the serum prolactin concentration and the radiologic appearance of the sella turcica. The upper limit of the normal range of serum prolactin is 20–30 ng/mL, depending on the individual laboratory and standards used. Prolactin values of approximately 50 ng/mL are associated with a 25% tumor incidence and values of 100 ng/mL with a 47% increased incidence; levels greater than 200 ng/mL are virtually diagnostic of a pituitary tumor. Pituitary tumors less than 1 cm, however, have been demonstrated in patients with prolactin levels less than 100 ng/mL. As with other hormonally active neoplasms, the absolute level of prolactin generally corresponds to the relative size of the lesion; however, it should be cautioned that large, inactive tumors are often associated with slightly elevated prolactin levels. Hyperprolactinemia in the absence of a demonstrable pituitary lesion suggests lactotrope hypertrophy, and these patients should be followed up at 6–12 month intervals.
The value of anterior pituitary function tests in the diagnosis of prolactin-secreting pituitary adenomas is limited and generally not used in establishing a diagnosis. In patients with a prolactin-secreting pituitary tumor, the prolactin response to TRH is blunted, probably as the result of maximal prolactin secretion by the tumor. Whether pituitary tumors function autonomously in their production of prolactin remains unclear. It appears that in most patients there is some degree of prolactin responsiveness to provocative stimulation, although not always of the same magnitude as that observed in normal persons. In addition, a blunted prolactin response occurs in hyperprolactinemic patients who have no radiologic evidence of a tumor. It is for these reasons that the predictive value of prolactin stimulation by TRH has not been established. Likewise, challenges with GnRH, levodopa infusion, and insulin-induced hypoglycemia will demonstrate altered responses in the presence of a prolactinoma, but they are not useful in establishing the diagnosis of these lesions.
The clinical management of prolactin-secreting pituitary adenomas varies according to the evaluation and the patient's needs. Treatment is directed toward eradicating aggressive pituitary tumor enlargement, which may be destructive to adjacent tissue and structures; restoring ovulation; and stopping lactation. Large pituitary tumors greater than 1 cm (macroadenomas) are usually associated with extrasellar extension. These lesions should be treated with definitive surgery or radiation therapy, or both. Dopamine agonists (e.g. bromocriptine), however, have been shown to be highly effective as a primary treatment for large prolactinomas. Surgery may also be facilitated by preoperative administration of a dopamine agonist, which has been shown to reduce the size of most prolactin-secreting microadenomas. Some neurosurgeons, however, believe this increases fibrosis and actually makes surgery more difficult.
Intrasellar lesions are usually less than 1 cm (microadenomas) and may be treated medically (Fig. 1).35 Surgery and irradiation therapy are seldom used as initial forms of treatment in these patients. Both bromocriptine administration and trans-sphenoidal resection are associated with a high degree of success.36, 37 Therapeutic effectiveness is assessed by the normalization of prolactin levels. Clinically, in approximately 70% of patients, restoration of normal endocrine function occurs within 3 months of treatment. The resolution rate of unwanted lactation, however, is less predictable. Bromocriptine administration is noninvasive and may reduce tumor size or may retard or prevent potential tumor growth; therapeutic benefits depend on its continued use. In most instances, prolactin levels return to pretreatment values within two weeks after therapy is discontinued; at times even an overshoot may occur. Whether long-term administration of bromocriptine affects permanent tumor progression is not known, but patients have been followed for as long as 17 years on therapy without evidencing a tumor regrowth. Bromocriptine is available in the United States in a short- or long-acting oral form, and in Europe in a long-acting injectable form. Surgery has an advantage of actual tumor removal and minimum morbidity, but these benefits may be offset by the significant risk of recurrent hyperprolactinemia and in some cases recurrent tumor mass necessitating long-term therapy with drugs such as bromocriptine.
Microadenomas and Pregnancy
Over the years it has become apparent that patients with intrasellar microadenomas are at minimal risk for complications during pregnancy.38 Originally there was great concern about the possibility of sudden tumor growth and its attendant complications. Fewer than 7% of reported cases, however, have manifested evidence suggestive of tumor expansion (e.g. headaches, visual disturbances, diabetes insipidus). Recognition of an intrasellar tumor is important because complications arising from expansion of a documented microadenoma during pregnancy have been exceedingly rare. Once pregnancy is confirmed in patients with a microadenoma, bromocriptine administration should be discontinued. In regard to the issue of teratogenicity, exposure of a fetus to bromocriptine either during early pregnancy in association with the induction of ovulation, or in later pregnancy during the reinstitution of drug therapy, has not been associated with an increase in congenital defects, spontaneous abortions, multiple gestations, or deficiencies in mental or physical development up to eight years of age. In the postpartum period, breastfeeding has been permitted in patients with no evidence of tumor-related difficulty. Despite the success of pregnancy outcome, a patient with an intrasellar microadenoma should be informed of the diagnosis and educated regarding potential complications. Of utmost importance is that the patient recognize visual field defects and changes in visual acuity that may accompany progressive tumor enlargement. Examination by gross confrontation may be useful. Formal evaluation of vision performed in women with microadenomas has not been productive. Measurement of serial serum prolactin levels during pregnancy to assess tumor growth has not been efficacious and should be discouraged.
Macroadenomas and Pregnancy
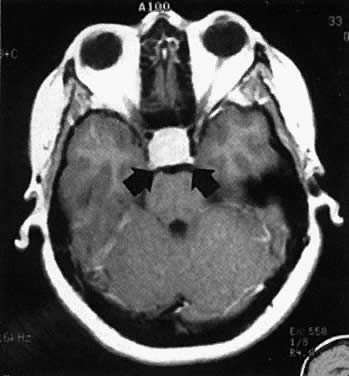
In patients with macroadenomas, however, the occurrence of pregnancy has been associated with significant risk of complications (Fig. 2). Of untreated cases, the reported incidence of complications has been reported as approximately 17% by survey. Clinical symptoms related to possible tumor growth appear to occur throughout the entire length of gestation, but the onset of symptoms tends to be most common in the first trimester. The predominant symptom is headache, although nausea and vomiting frequently occur. Often it is difficult to determine whether these symptoms are a result of pregnancy or the tumor. Once visual field defects develop or visual acuity is compromised, however, substantial tumor enlargement may have already occurred. If untreated, this process can result in blindness and optic atrophy. The problems must be attended to at once by either medical treatment with bromocriptine or more frequently with operative intervention. A growing number of patients with macroadenomas have become pregnant after primary bromocriptine therapy, and several of these patients have experienced significant side effects related to extracellular tumor growth. In nearly all of these anecdotal cases, the reinstitution of a drug resolved signs of an enlarging tumor and the remainder of the pregnancy was uneventful. Many patients with a history of a large tumor, however, have been followed throughout pregnancy without having a complication or requiring concurrent medical therapy. Those patients who have had tumor-related complications during pregnancy evidenced regression of lesions when bromocriptine administration was reinstituted, strongly suggesting that these lesions were actually prolactinomas. If significant reduction in tumor size has been demonstrated before an attempt at conception, then during pregnancy the likelihood exists that tumor-related complications would resolve by the reinstitution of bromocriptine. Therefore, it is generally recommended that patients with macroadenomas contemplating pregnancy undergo bromocriptine therapy (1) to reduce the size of their tumor, and (2) to achieve normal ovulation.
|
Therapeutic Considerations in Pregnancy
Some patients with hyperprolactinemia and microadenomas may not harbor prolactin-secreting tumors, but rather nonfunctional neoplasms that do not respond to bromocriptine. In the absence of any measurable reduction of tumor size, the therapeutic advantage of bromocriptine may not be realized in women with an extrasellar lesion. Thus, for bromocriptine to serve as a noninvasive therapeutic alternative or an adjunct in patients with microadenomas, should they become pregnant, the efficacy of the drug on tumor size must be demonstrated. In the absence of any significant effect of tumor shrinkage, then definitive therapy should be performed.
Growth Hormone-Secreting Pituitary Tumors
In children, when the epiphyseal plate is not yet closed, growth hormone excess results in gigantism. These subjects display marked increases in height, but remain eunuchoidal due to hypogonadism. Excessive secretion of growth hormone in adults causes acromegaly, the stigmata of which are usually mild (Table 1).
Table 1. Features of acromegaly
Serum growth hormone levels > 10 ng/mL
Bone growth and soft tissue proliferation
- Nose
- Jaw
- Suborbital ridge
- Hands and feet
Carpal tunnel syndrome
Parasthesias of hands
Sweating
Thickening of skin
Increased mortality in patients more than 45 years of age
- Congestive heart failure
- Hypertension
Lethargy and weight gain
Organomegaly
- Liver
- Heart
- Kidney
Acromegalic changes characteristically occur in the face and extremities. Bone growth and soft tissue proliferation resulting from inappropriate secretion of growth hormone lead to enlargement of the nose, jaw, and suborbital ridges. In addition, the tongue may enlarge, and the teeth may become widely spread. Overgrowth of bone and soft tissue about the joints causes deformity of the hands and feet, which may hasten the onset of osteoarthritis. Carpal tunnel syndrome as well as weakness and paresthesias in the hands may also occur. The voice deepens as a result of vocal cord thickening. Excessive sweating, overproduction of sebum, and thickening of the skin are early subtle changes associated with acromegaly.
The manifestations of acromegaly are significant. After age 45, mortality from cardiovascular cerebrovascular and respiratory disease in these patients is twice that as of the normal population.39 Lethargy and weight gain occur in more than one half of these patients and hypertension in about one fourth. Enlargement of the heart, liver, and kidneys is a common finding. The cardiomegaly may be associated with congestive heart failure. Because acromegalic changes develop insidiously, significant neoplastic growth may occur before the disease is recognized. Consequently, in earlier reported series, the incidence of visual disturbance was high. Visual defects are currently encountered in approximately 20% of cases.40
The diagnosis of a growth hormone-secreting pituitary tumor is based on the basal level of serum growth hormone, the growth hormone response to hypoglycemia, and the radiologic appearance of the pituitary. A normal fasting serum growth hormone level is less than 5 ng/mL. Exercise and stress tend to increase growth hormone secretion, however, and should be considered in the interpretation of basal levels. Concentrations greater than 10 ng/mL are abnormal and occur in more than 90% of patients with acromegaly. Growth hormone levels between 5 and 10 ng/mL are inconclusive and require further evaluation. To test growth hormone response to hyperglycemia, a standard (100-g) glucose tolerance test is performed. In normal subjects, circulating levels of growth hormone are suppressed to less than 5 ng/mL by 1–2 hours after oral administration of glucose. If the level of growth hormone is not suppressed to less than 5 ng/mL, the diagnosis of acromegaly is established.41 This test is particularly useful in patients with borderline normal or indeterminate levels of serum growth hormone.
As mentioned above, the development of acromegaly is subtle and may go unnoticed for years. Therefore, it is not surprising that the pituitary is enlarged in nearly all patients with growth hormone-secreting tumors. Recently, immunocytochemical studies have demonstrated the presence of growth hormone and prolactin in the same secretory granule type.42, 43 Apparently, growth factors are secreted by these tumors and have been isolated by gel filtration.44 The normal pituitary gland secretes insulin-like growth factor-1 and -2, epidermal growth factor-2, basic fibroblast growth factor, transforming growth factor-α, and nerve growth factor, and some adenomas have been demonstrated to secrete chondrocyte growth factor. In 55 tumors taken from acromegalic patients, immunocytochemical analysis showed 45.5% to contain prolactin.45 Human pituitary tumors also have been shown to produce bioactive and immunoactive interleukin-6 and to express interleukin-6 messenger RNA.46
Growth hormone-secreting tumors have been shown to be influenced by other hormonal activities. For instance, somatostatin inhibits the secretion from these adenomas, and secretion is stimulated by growth hormone-releasing factor. Solution hybridization techniques to evaluate RNA have demonstrated two of the somatostatin-receptor subtypes (SSTR-3 and SSTR-4) in these tumors. Recently a third somatostatin receptor has been described, SSTR-5, which shows preference for mammosomatotrophic lineage. Finally, estrogen has been shown to induce anterior pituitary enlargement and arteriogenesis in Fisher 344 rats. This effect can be inhibited by treatment with bromocriptine.47, 48, 49, 50
The management of growth hormone-secreting pituitary tumors is directed toward suppressing excess growth hormone secretion and eliminating sequelae of gross tumor enlargement. Reduction of serum growth hormone levels to less than 10 ng/mL arrests progressive symptoms and in most instances results in the regression of acromegalic manifestations. Acromegalic changes caused by tissue proliferation appear to be the most responsive to therapy. Excess sweating is usually entirely eliminated, but bone and cartilage changes are irreversible.
Medical treatment may be used prior to surgery or as an adjunct to other treatment modalities. Medications include dopamine agonists and somatostatin agonists, such as ostreotide and lanreotide. Studies have shown the long-term efficacy of somatostatin agonists as either primary or adjuvant therapy in acromegaly.51, 52
Definitive therapy may be accomplished by surgery or irradiation. In properly selected cases, trans-sphenoidal removal has afforded good results with minimal complications.53 Conventional external and proton-beam irradiation have had similar success in eradicating these neoplasms.54 Radiation therapy may require 3–6 years before the total effect is realized, however. Evidence for hypopituitarism is low, but partial impairment of pituitary hormone reserve has been increasingly demonstrated with more sophisticated means of evaluating pituitary function. The use of bromocriptine mesylate to reduce growth hormone levels has met with good success, but whether it exerts an inhibitory regressive effect on tumor growth is unknown. This form of therapy may be helpful when patients respond poorly to other methods or when other methods are contraindicated.
Adrenocorticotropic Hormone-Secreting Pituitary Tumors
Pituitary ACTH secretion that arises from a neoplasm produces hypercortisolism (Cushing's syndrome). The clinical manifestations of hypercortisolism are listed in Table 2. A detailed description of these symptoms is given elsewhere in these volumes.
Table 2. Clinical features of Cushing's syndrome
Feature | Frequency (%) |
Obesity | 92 |
Moon facies | 90 |
Hypertension | 85 |
Glucose intolerance | 80 |
Menstrual and sexual dysfunction | 74 |
Hirustism and acne | 70 |
Striae | 70 |
Proximal muscle weakness | 67 |
Osteoporosis | 55 |
Easy bruising | 50 |
Emotional changes | 50 |
Edema | 45 |
ACTH-secreting pituitary tumors are characteristically small. In this regard, CT scanning or MRI has proved to be extremely useful: abnormalities consistent with a small pituitary tumor have been detected in as many as 75% of patients with Cushing's disease.55 An ACTH-secreting pituitary tumor is found occasionally in patients without a discernible pituitary lesion. Recently Wilson and co-workers56 described an extracellular intercavernous sinus ACTH-releasing adenoma causing Cushing's disease.
The diagnosis is established by the lack of cortisol suppression after administration of low-dose dexamethasone and partial suppression after administration of high-dose dexamethasone, normal or moderately elevated plasma ACTH levels, and an abnormal CT scan.57, 58 In normal subjects, plasma ACTH levels are less than 100 pg/mL. In patients with an ACTH-secreting pituitary tumor, the plasma ACTH level can range from 50–200 pg/mL.
Because ACTH-secreting pituitary tumors are small, selective removal of these lesions may be readily accomplished by trans-sphenoidal microdissection. In the hands of experienced neurosurgeons the risk of complications is low, and hypopituitarism is minimal. Another means of treatment is radiation therapy. Again, however, the total therapeutic effect may require several years.59 To achieve a reliable prediction of the integrity of the hypothalamic-pituitary axis, it has been recommended that close observation and careful monitoring with serum cortisol levels be performed after this type of surgery; routine glucocorticoid therapy is not needed.60
Nelson's syndrome is a specific clinical entity in which patients exhibit hypercortisolism and Cushing's syndrome. Nelson's syndrome occurs in 10–20% of patients who have Cushing's disease and who have been previously treated with a bilateral adrenalectomy. In most cases of Cushing's disease, serum ACTH levels are normal or moderately elevated, which results in hypercortisolism. Apparently the feedback from hypercortisolism partially inhibits potential pituitary ACTH secretion. When the hypercortisolism is eliminated with a bilateral adrenalectomy, negative feedback by cortisol is lost; pituitary ACTH secretion is then unrestricted. Nelson's syndrome is characterized by exceedingly high post-treatment levels of serum ACTH and MSH, which causes severe depigmentation of the skin and rapid enlargement of the pituitary. Because most cases of Cushing's disease result from a pituitary neoplasm, it is believed that these patients have a preexisting pituitary adenoma that sustains rapid growth after bilateral adrenalectomy. In fact, the growth rate often leads to impingement on vital surrounding structures. Hypopituitarism as a result of invasive tumor growth is common, and malignant transformation has been reported.61 Therefore, aggressive management is imperative. If the lesion is not amenable to surgical resection, radiation therapy should be used.
Thyroid-Stimulating Hormone-Secreting Pituitary Tumors
Pituitary tumors that actively secrete TSH are rare. The genesis of these tumors in humans in unknown, although longstanding hypothyroidism has been associated with the development of TSH-secreting pituitary tumors. In contrast, several isolated cases of TSH-secreting tumors have been reported in which patients had clinical evidence of hyperthyroidism.62 These patients exhibited elevated serum TSH levels, blunted TSH response to TRF, and an abnormal sella turcica x-ray. Of a total of 800 patients observed for a period of 15 years, Beckers and associates63 reported seven patients with hyperthyroidism due to TSH-secreting macroadenomas. Serum TSH varied between 1.1 and 36.3 mIU/L. The serum α-subunit level was low in one case, whereas four others had elevated concentrations.64 Hyperthyroidism also has been reported due to pituitary adenomas composed of two different cell types, one apparently secreting the α-subunit alone and the other cosecreting the α-subunit along with TSH.65 These were evaluated with double-goal particle immunostaining, which showed that all cells contained secretory granules positive for the α-subunit, whereas very few were positive for β-TSH and the α-subunit.
Follicle-Stimulating Hormone-Secreting Pituitary Tumors
Like TSH-secreting tumors, those that release gonadotropins are extremely rare.66 In the few isolated cases reported, increased serum FSH or LH levels, or both, were not consistently associated with hypogonadism. Gonadotropin-secreting tumors have been misdiagnosed previously as 'nonsecreting macroadenomas'. The majority of these could be recognized even in postmenopausal patients by the response of serum LH-β to TRH challenge. For instance, Daneshdoost and colleagues67 evaluated 16 women with apparent nonsecreting adenomas and treated them with TRH; 11 had significant responses. The administration of a GnRH agonist increased rather than decreased LH in α-subunit levels in a single patient with an LH-secreting pituitary tumor, and the administration of GnRH agonists have likewise been shown to reduce prolactin secretion when chronically administered to patients with prolactinomas.68, 69 Human pituitary tumors that secrete chorionic gonadotropin have been described as have glycoprotein-producing pituitary tumors that exhibit pulsatile glycoprotein hormone secretion.70, 71 Gonadotropin-secreting tumors have been suppressed with the administration of dopamine agonists such as bromocriptine, and cabergoline has been found to decrease both FSH and prolactin secretion in macroadenomas.21, 72, 73, 74
Craniopharyngioma
Craniopharyngioma is the most common sellar neoplasm of childhood and adolescence; only one third of cases involve adult patients. Craniopharyngiomas have also been found in two siblings.75 These tumors arise from remnants of Rathke's pouch and are more commonly seen as suprasellar cystic lesions than as intrasellar tumors. The latter may be radiographically indistinguishable from nonfunctioning pituitary adenomas. The finding of suprasellar calcification in more than one half of patients suggests the diagnosis. Unlike nonfunctional tumors, craniopharyngiomas often demonstrate aggressive growth extending into the optic chiasm, hypothalamus, and third ventricle, causing visual defects, diabetes insipidus, and signs of increased intracranial pressure. Primary therapy consists of surgical resection of the tumor and drainage of the cystic lesions.76 The tumor occasionally invades surrounding vital structures and adheres to them so that complete removal of the tumor is not technically feasible. In these cases, postoperative radiation therapy is indicated and may decrease the rate of tumor recurrence.
Empty Sella Syndrome
The primary empty sella syndrome is a frequent cause of sellar enlargement, particularly in middle-aged, obese women who are often hypertensive.77 Headache is a common symptom. Associated conditions include those that result in increased intracranial pressure, such as pseudotumor cerebri. The primary empty sella develops from a congenital defect of the sellar diaphragm through which herniation of the arachnoid membrane occurs. Consequently, cerebrospinal fluid pressure is transmitted into the sellar space, thus enlarging it. In general, the sellar enlargement is uniform and symmetric, although asymmetry suggestive of an intracellular neoplasm may be observed. It has been speculated further that pituitary tumors may exist for an extended period of time, undergo infarction, and then give rise to the empty sella syndrome.78 Antipituitary antibodies also have been described in patients with empty sella syndrome and may be part of the pathogenesis of this problem.79
The diagnosis of empty sella syndrome is usually made at the time of CT scanning or MRI by the demonstration of air in the sella. The sella may be partially or totally empty. In either situation, pituitary function is usually completely normal. Management of these patients is conservative and expectant, and surgical intervention is not indicated. Many of these patients, however, will have hyperprolactinemia with concurrent signs of hypoestrogenism, and these patients are generally treated with either replacement hormonal therapy or bromocriptine. As with patients who have a microadenoma of the pituitary, estrogen replacement therapy or oral contraceptives are not contraindicated in patients with empty sella syndrome. Prospective trials have demonstrated no effect of oral contraceptives on the growth of microadenomas, and the slight increase in prolactin secretion produced by oral contraceptives is not clinically significant.80, 81
Pituitary carcinoma
Pituitary carcinoma may be defined as a tumor of adenohypophyseal cells exhibiting cerebrospinal and/or systemic metastases.82 Primary pituitary carcinomas are extremely rare with only about 140 published cases. The clinical features are due to the pressure effects to surrounding tissues and/or to the effects of excessive hormonal secretion. Most cases have arisen from malignant transformation of tumors initially considered to be benign macroadenomas. Treatment options include surgery (transsphenoidal whenever possible), radiation (conventional and stereotactic), and chemotherapy.83 Unfortunately, the prognosis is poor, with life expectancy of less than one year in most cases.84
REFERENCES
Klibanski A, Zervas NT: Diagnosis and management of hormone-secreting pituitary adenomas. N Engl J Med 324: 822, 1991 |
|
Ezrin C, Kovacs K, Horvath E: A functional anatomy of the endocrine hypothalamus and hypophysis. Med Clin North Am 62: 229, 1978 |
|
Horvath E, Kovacs K, Smyth HS et al: A novel type of pituitary adenoma: Morphological features and clinical correlations. J Clin Endocrinol Metab 66: 1111, 1988 |
|
Chao JC, Reyes CV, Chinoy M: Null cell adenoma of the pituitary gland. South Med J 84: 1239, 1991 |
|
Reincke M, Allolio B, Saeger W et al: The “incidentaloma” of the pituitary gland: Is neurosurgery required? JAMA 263: 2772, 1990 |
|
Costello RT. Subclinical adenoma of the pituitary gland. Am J Path 12: 205, 1936 |
|
Oppenheim DS, Kana AR, Sangha JS et al: Prevalence of α-subunit hypersecretion in patients with pituitary tumors: Clinically nonfunctioning and somatroph adenomas. J Clin Endocrinol Metab 70: 859, 1990 |
|
Beck-Peccoz P, Persani L, Faglia G: Glycoprotein hormone α-subunit in pituitary adenomas. Trends Endocrinol Metab 3: 41, 1992 |
|
Nobels FRE, Kwekkeboom DJ, Coopmans W et al: A comparison between the diagnostic value of gonadotropins, α-subunit, and chromogranin-A and their response to thyrotropin-releasing hormone in clinically nonfunctioning, α-subunit-secreting, and gonadotroph pituitary adenomas. J Clin Endocrinol Metab 77: 784, 1993 |
|
Schechter J, Goldsmith P, Wilson C et al: Morphological evidence for the presence of arteries in human prolactinomas. J Clin Endocrinol Metab 67: 713, 1988 |
|
Spada A, Bassetti M, Reza-Elahi F et al: Differential transduction of dopamine signal in different subtypes of human growth hormone-secreting adenomas. J Clin Endocrinol Metab 78: 411, 1994 |
|
Boggild MD, Jenkinson S, Pistorello M et al: Molecular genetic studies of sporadic pituitary tumors. J Clin Endocrinol Metab 78: 387, 1994 |
|
Jameson JL, Lindell CM, Hsu DW et al: Expression of chorionic gonadotropin-β-like messenger ribonucleic acid in an α-subunit-secreting pituitary adenoma. J Clin Endocrinol Metab 62: 1271, 1986 |
|
Crosignani PG, Ferrari C, Malinverni A et al: Effect of central nervous system dopaminergic activation on prolactin secretion in man: Evidence for a common central defect in hyperprolactinemic patients with and without radiological signs of pituitary tumors. J Clin Endocrinol Metab 51: 1068, 1990 |
|
Camanni F, Ghigo E, Ciccarelli E et al: Defective regulation of prolactin secretion after successful removal of prolactinomas. J Clin Endocrinol Metab 57: 1270, 1983 |
|
Ho PJ, Jaffe CA, Friberg RD et al: Persistence of rapid growth hormone (GH) pulsatility after successful removal of GH-producing pituitary tumors. J Clin Endocrinol Metab 78: 1403, 1994 |
|
Genazzani AR, de Leo V, Murru S et al: Dynamic tests of prolactin secretion in hyperprolactinemic states: Carbidopa-L-Dopa and indirectly acting dopamine agonists. J Clin Endocrinol Metab 54: 429, 1982 |
|
Ho KY, Smythe GA, Duncan M et al: Dopamine infusion studies in patients with pathological hyperprolactinemia: Evidence of normal prolactin suppressibility but abnormal dopamine metabolism. J Clin Endocrinol Metab 58: 128, 1984 |
|
Melmed S, Carlson HE, Briggs J et al: Cell culture alters the hormonal response of rat pituitary tumors in dynamic stimulation. Endocrinology 107: 789, 1980 |
|
Le Dafniet M, Blumberg-Tick J, Gozlan H et al: Altered balance between thyrotropin-releasing hormone and dopamine in prolactinomas and other pituitary tumors compared to normal pituitaries. J Clin Endocrinol Metab 69: 267, 1989 |
|
Friend KE, Chiou YK, Lopes MBS et al: Estrogen receptor expression in human pituitary: Correlation with immunohistochemistry in normal tissue, and immunohistochemistry and morphology in macroadenomas. J Clin Endocrinol Metab 78: 1497, 1994 |
|
Stefaneanu L, Kovacs K, Horvath E et al: In situ hybridization study of estrogen receptor messenger ribonucleic acid in human adenohypophysial cells and pituitary adenomas. J Clin Endocrinol Metab 78: 83, 1994 |
|
Prior JC, Cox TA, Fairholm D et al: Testosterone-related exacerbation of a prolactin-producing macroadenoma: Possible role for estrogen. J Clin Endocrinol Metab 64: 391, 1987 |
|
Ramsdell JS: Transforming growth factor-α and -β are potent and effective inhibitors of GH4 pituitary tumor cell proliferation. Endocrinology 128: 1981, 1991 |
|
Atkin SL, Landolt AM, Jeffreys RV et al: Basic fibroblastic growth factor stimulates prolactin secretion from human anterior pituitary adenomas without affecting adenoma cell proliferation. J Clin Endocrinol Metab 77: 831, 1993 |
|
Karavitaki N, Collison K, Halliday J, et al. What is the natural history of nonoperated non-functioning pituitary adenomas? Clin Endocrinol 67; 938-943, 2007. |
|
Hollenborst RW, Younge BR: Ocular manifestations produced by adenomas of the pituitary gland: Analysis of 1,000 cases. In Kohler PO, Ross GT (eds): Diagnosis and Treatment of Pituitary Tumors, p 53. Amsterdam, Excerpta Medica, 1973 |
|
Keye WR Jr, Yuen BS, Knopf RF et al: Amenorrhea, hyperprolactinemia, and pituitary enlargement secondary to primary hypothyroidism. Obstet Gynecol 48: 697, 1976 |
|
Vezina JL, Sutton TJ: Prolactin-secretion pituitary microadenomas. Am J Roentgenol 120: 46, 1974 |
|
Valenta LJ, Sostrin RD, Eisenberg H et al: Diagnosis of pituitary tumors by hormone assays and computerized tomography. Am J Med 72: 861, 1982 |
|
Anderson RD: Tortuosity of the cavernous carotid arteries causing sellar expansion stimulating pituitary adenomas. Am J Radiol 126: 1203, 1976 |
|
Chang RJ, Keye WR Jr, Young JR et al: Detection, evaluation, and treatment of pituitary microadenomas in patients with galactorrhea. Am J Obstet Gynecol 128: 356, 1977 |
|
Franks S, Murray MAF, Jequier AM et al: Incidence and significance of hyperprolactinemia in women with amenorrhea. Clin Endocrinol 4: 597, 1975 |
|
Sinha YN: Structural variants of prolactin: Occurrence and physiological significance. Endocr Rev 16: 354, 1995 |
|
Chiodini P, Luizzi A, Cozzi R et al: Size reduction of macroprolactinomas by bromocriptine or lisuride treatment. J Clin Endocrinol Metab 53: 737, 1981 |
|
Keye WR Jr, Chang RJ, Monroe SE et al: Prolactin secreting pituitary adenomas in women: II. Menstrual function, pituitary reserves and prolactin production following microsurgical removal. Am J Obstet Gynecol 134: 360, 1979 |
|
Thorner MO, Besser GM: Hyperprolactinemia and gonadal function: Results of bromocriptine treatment. In Crosignani PG, Robyn C (eds): Prolactin and Human Production, p 285. London, Academic Press, 1977 |
|
Mornex R, Orgiazzi J, Hugues B et al: Normal pregnancies after treatment of hyperprolactinemia with bromoergocryptine, despite suspected pituitary tumors. J Clin Endocrinol Metab 47: 290, 1978 |
|
Wright AD, Hill DM, Lowy C et al: Mortality in acromegaly. Q J Med 39: 1, 1970 |
|
Wilson CB, Dempsey LC: Transsphenoidal microsurgical removal of 250 pituitary adenomas. J Neurosurg 48: 13, 1978 |
|
Nelson JC, Kollar DJ, Lewis JE: Growth hormone secretion in pituitary disease. Arch Intern Med 133: 459, 1974 |
|
Lloyd RV, Anagnostou D, Cano M et al: Analysis of mammosomatotropic cells in normal and neoplastic human pituitary tissues by the reverse hemolytic plaque assay and immunocytochemistry. J Clin Endocrinol Metab 66: 1103, 1988 |
|
Bassetti M, Spada A, Arosio M et al: Morphological studies on mixed growth hormone (GH)- and prolactin (PRL)-secreting human pituitary adenomas: Coexistence of GH and PRL in the same secretory granule. J Clin Endocrinol Metab 62: 1093, 1986 |
|
Webster J, Ham J, Bevan JS et al: Preliminary characterization of growth factors secreted by human pituitary tumors. J Clin Endocrinol Metab 72: 687, 1991 |
|
Kanie N, Kageyama N, Kuwayama A et al: Pituitary adenomas in acromegalic patients: An immunohistochemical and endocrinological study with special reference to prolactin-secreting adenoma. J Clin Endocrinol Metab 57: 1093, 1983 |
|
Jones TH, Daniels M, James RA et al: Production of bioactive and immunoreactive interleukin-6 (IL-6) and expression of IL-6 messenger ribonucleic acid by human pituitary adenomas. J Clin Endocrinol Metab 78: 180, 1994 |
|
Lamberts SW, Verleun T, Oosterom R: The interrelationship between the effects of somatostatin and human pancreatic growth hormone-releasing factor on growth hormone release by cultured pituitary tumor cells from patients with acromegaly. J Clin Endocrinol Metab 58: 250, 1984 |
|
Greenman Y, Melmed S: Heterogeneous expression of two somatostatin receptor subtypes in pituitary tumors. J Clin Endocrinol Metab 78: 398, 1994 |
|
Greenman Y, Melmed S: Expression of three somatostatin receptor subtypes in pituitary adenomas: Evidence for preferential SSTR5 expression in the mammosomatotroph lineage. J Clin Endocrinol Metab 79: 724, 1994 |
|
Elias KA, Weiner RI: Inhibition of estrogen-induced anterior pituitary enlargement and arteriogenesis by bromocriptine in Fischer 344 rats. Endocrinology 120: 617, 1987 |
|
Colao A, Ferone D, Marzullo P, et al. Long term effects of depot long-acting somatostatin analog octreotide on hormone levels and tumor mass in acromegaly. J Clin Endocrinol Metab 86: 2779-2786, 2001. |
|
Ayuk J, Stewart SE, Stewart PM, Sheppard MC, et al. Long-term safety and efficacy of depot long-acting somatostatin analogs for the treatment of acromegaly. J Clin Endocrinol Metab 87: 4142-4146, 2002. |
|
Sang H, Wilson CB, Tyrell JB: Transsphenoidal microhypophysectomy in acromegaly. J Neurosurg 47: 480, 1977 |
|
Gorden P, Roth J: The treatment of acromegaly by conventional pituitary irradiation. In Kohler PO, Ross GT (eds): Diagnosis and Treatment of Pituitary Tumors, p 230. Amsterdam, Excerpta Medica, 1973 |
|
Hardy J: Transsphenoidal surgery of hypersecreting pituitary tumors. In Kohler PO, Ross GT (eds): Diagnosis and Treatment of Pituitary Tumors, p 179. Amsterdam, Excerpta Medica, 1973 |
|
Wilson CB, Mindermann T, Tyrrell JB: Extrasellar, intracavernous sinus adrenocorticotropin-releasing adenoma causing Cushing's disease. J Clin Endocrinol Metab 80: 1774, 1995 |
|
Pavlatos Smilo RP, Forsham PH: A rapid screening test for Cushing's syndrome. JAMA 193: 720, 1965 |
|
Liddle GW: Tests for pituitary-adrenal suppressibility in the diagnosis of Cushing's syndrome. J Clin Endocrinol Metab 20: 1539, 1960 |
|
Guilhaume B, Bertagna X, Thomsen M et al: Transsphenoidal pituitary surgery for the treatment of Cushing's disease: Results in 64 patients and long term follow-up studies. J Clin Endocrinol Metab 66: 1056, 1988 |
|
Hout WM, Arafah BM, Salazar R et al: Evaluation of the hypothalamic-pituitary-adrenal axis immediately after pituitary adenomectomy: Is perioperative steroid therapy necessary? J Clin Endocrinol Metab 66: 1208, 1988 |
|
Rovit RL, Berry R: Cushing's syndrome and the hypophysis: A reevaluation of pituitary tumors and hyperadrenalism. J Neurosurg 23: 270, 1965 |
|
Leong A, Chawla JC, Teh EC: Pituitary thyrotropic tumor secondary to long-standing primary hypothyroidism. Pathol Eur 11: 49, 1976 |
|
Beckers A, Abs R, Mahler C et al: Thyrotropin-secreting pituitary adenomas: Report of seven cases. J Clin Endocrinol Metab 72: 477, 1991 |
|
Kourides LA, Ridgeweay EC, Weintraub BD et al: Thyrotropin-induced hyperthyroidism: Use of alpha and beta subunit levels to identify patients with pituitary tumors. J Clin Endocrinol Metab 45: 534, 1977 |
|
Terzolo M, Orlandi F, Bassetti M et al: Hyperthyroidism due to a pituitary adenoma composed of two different cell types, one secreting α-subunit alone and another cosecreting α-subunit and thyrotropin. J Clin Endocrinol Metab 72: 415, 1991 |
|
Demura R, Kubo O, Demura H et al: FSH and LH secreting pituitary adenomas. J Clin Endocrinol Metab 45: 653, 1977 |
|
Daneshdoost L, Gennarelli A, Hildegarde M et al: Recognition of gonadotroph adenomas in women. N Engl J Med 324: 589, 1991 |
|
Roman SH, Goldstein M, Kourides IA et al: The luteinizing hormone-releasing hormone (LHRH) agonist [D-Trp6 -Pro9 -NEt] LHRH increased rather than lowered LH and α-subunit levels in a patient with an LH-secreting pituitary tumor. J Clin Endocrinol Metab 58: 313, 1984 |
|
Rubio MA, Cabranes JA, Schally AV et al: Prolactin-lowering effect of luteinizing hormone-releasing hormone agonist administration in prolactinoma patients. J Clin Endocrinol Metab 69: 444, 1989 |
|
Hammond E, Griffin J, Odell WD: A chorionic gonadotropin-secreting human pituitary cell. J Clin Endocrinol Metab 72: 747, 1991 |
|
Samuels MH, Henry P, Kleinschmidt-Demasters BK et al: Pulsatile glycoprotein hormone secretion in glycoprotein-producing pituitary tumors. J Clin Endocrinol Metab 73: 1281, 1991 |
|
Paoletti AM, Depau GF, Mais V et al: Effectiveness of cabergoline in reducing follicle-stimulating hormone and prolactin hypersecretion from pituitary macroadenoma in an infertile woman. Fertil Steril 62: 882, 1994 |
|
Comtois R, Bouchard J, Robert F: Hypersecretion of gonadotropins by a pituitary adenoma: Pituitary dynamic studies and treatment with bromocriptine in one patient. Fertil Steril 52: 569, 1989 |
|
Kwekkeboom DJ, de Jong FH, Lamberts SWJ: Gonadotropin release by clinically nonfunctioning and gonadotroph pituitary adenomas in vivo and in vitro: Relation to sex and effects of thyrotropin-releasing hormone, gonadotropin-releasing hormone, and bromocriptine. J Clin Endocrinol Metab 68: 1128, 1989 |
|
Vargas JR, Pino JA, Murad TM: Craniopharyngioma in two siblings. JAMA 246: 1807, 1981 |
|
Kahn EA, Gosch HH, Seeger JF et al: Forty-five years experience with the craniopharyngiomas. Surg Neurol 1: 5, 1973 |
|
Neelon FA, Goree JA, Lebovitz HE: The primary empty sella: Clinical and radiographic characteristics and endocrine function. Medicine 52: 73, 1983 |
|
Bjerre P, Lindholm J, Videbaek H: The spontaneous course of pituitary adenomas and occurrence of an empty sella in untreated acromegaly. J Clin Endocrinol Metab 63: 287, 1986 |
|
Komatsu M, Kondo T, Yamauchi K et al: Antipituitary antibodies in patients with the primary empty sella syndrome. J Clin Endocrinol Metab 67: 633, 1988 |
|
Pituitary Adenoma Study Group: University of CA at San Francisco, Clinical Center; University of Southern CA, Clinical Center, Los Angeles; University of Manitoba, Clinical Center; The Johns Hopkins University, Clinical Center and Coordinating Center: Pituitary adenomas and oral contraceptives: A multicenter case-control study. Fertil Steril 39:753, 1983 |
|
Maheux R, Jenicek M, Cleroux R et al: Oral contraceptives and prolactinomas: A case-control study. Am J Obstet Gynecol 143: 134, 1982 |
|
Scheithauer BW, Kurtkaya-Yapicier O, Kovacs K, et al. Pituitary carcinoma: a clinicopathological review. Neurosurgery 56: 1066-1074, 2005. |
|
Kaltsas GA, Nomikos P, Kontogeorgos G, et al. Diagnosis and management of pituitary carcinomas. J Clin Endocrinol Metab 90: 3089-3099, 2005. |
|
Pernicone PJ, Scheithauer BW, Sebo TJ, et al. Pituitary carcinoma: A clinicopathologic study of 15 cases. Cancer 79: 804-812, 1997. |